Gastric Mixed Neuroendocrine/Non- Neuroendocrine Neoplasia (MINEN) in a 48-Year-Old Female Patient: Evidence for a Common Precursor Cell Origin
by Jakse F1*, Müllauer L1, Bodlaj G2, Häfner M2, Pfeffel F2, Schima W3, Klimpfinger M1,4
1Department of Pathology, Medical University of Vienna, Vienna, Austria
2Medical Department II, Barmherzige Schwestern Krankenhaus, Vinzenzgruppe, Vienna, Austria
3Department of Diagnostic and Interventional Radiology, Göttlicher Heiland Krankenhaus, Austria
Barmherzige Schwestern Krankenhaus, and Sankt Josef Krankenhaus, Vinzenzgruppe, Vienna, Austria
4Labor Dr. Ulm GmbH, Wien, Austria
*Corresponding author: Friedrich Jakse, Department of Pathology, Medical University of Vienna, Vienna, Austria
Received Date: 25 January 2025
Accepted Date: 29 January 2025
Published Date: 31 January 2025
Citation: Jakse F, Müllauer L, Bodlaj G, Hafner M, Pfeffel F, et al (2025) Gastric Mixed Neuroendocrine/Non- Neuroendocrine Neoplasia (MINEN) in a 48-Year-Old Female Patient: Evidence for a Common Precursor Cell Origin. Ann Case Report. 10: 2172. https://doi.org/10.29011/2574-7754.102172
Abstract
A 48-year-old female patient consulted medical advice after noticing unspecific symptoms like obstipation, followed by b-symptoms. The patient underwent a CT scan and gastroscopy, during which a polypoid tumor in corpus ventriculi was observed. Subsequently an endoscopic submucosal dissection (ESD) was performed. Histological examination, along with immunohistochemistry, led to the diagnosis of a mixed neuroendocrine/non-neuroendocrine neoplasia (MINEN). The sequencing of both tumor components allows the assumption that both neoplasms derived from a common precursor cell.
Keywords: Mixed Neuroendocrine/Non-Neuroendocrine Neoplasia (MINEN); Composite tumor; Common Precursor Cell; Endoscopic Submucosal Dissection (ESD);
Case Presentation
A middle-aged female presented to the clinic with malaise, significant unintended weight loss and night sweats. Physical examination and the initial blood test revealed no abnormalities.
Radiology
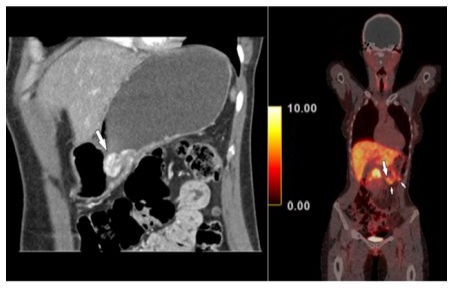
Hence, a radiological assessment was requested. Contrastenhanced hydro-multidetector computed tomography (MDCT) of the stomach after oral administration of 1500 ml of water showed a hypervascular polypoid mass in the gastric body (Figure 1) as well as two hypervascular lymph nodes close to the greater curvature. Findings were corroborated by somatostatin receptor analogue (68Ga DOTANOC) PET/CT, which demonstrated not only the hypermetabolic gastric tumor, but also two lymph nodes adjacent to the stomach with an SUVmax of 6.6 (Figure 1). MDCT and PET/CT features were consistent with the presence of a NET, without demonstrating the morphology of another tumor entity. Radiologically, there were no signs of transmural infiltration of the gastric wall.
Gastroscopy
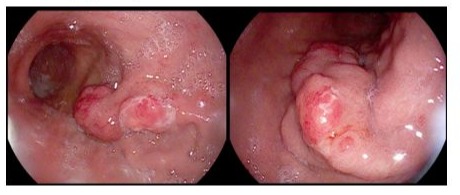
Initially, it was recommended to perform a gastroscopy with endoscopic ultrasound, during which a biopsy specimen was taken from the exophytic part of the gastric tumor.
Biopsy: Histological Report
Histological assessment classified the tumor lesion as a gastric adenoma, intestinal type, with high-grade dysplasia. The surrounding background in the biopsy specimen showed H. pylori- negative chronic atrophic gastritis of autoimmune type, with distinct atrophy of the gastric glands and intestinal metaplasia.
Further blood test examination
A subsequent blood test revealed seropositivity for anti-parietalcell antibodies at 45 U/mL [<7 U/mL]. The seropositivity for antiparietal-cell antibodies confirmed the histological diagnosis of autoimmune gastritis.
Treatment: Endoscopic submucosal dissection (ESD)
An interdisciplinary team managed the case and advised that an elective endoscopic intervention should be performed as a minimally invasive resection option. While the potentially polypoid
ESD-Specimen: Histological Report (Figure 3-7) Gastric Adenocarcinoma
Histological examination revealed a gastric adenoma (intestinal type) with high-grade dysplasia, progressing into a welldifferentiated gastric adenocarcinoma composed of loosely organized neoplastic glands infiltrating the gastric wall up to sections of submucosa. The neoplastic epithelium exhibited a high variability in nuclear size and shape, with nuclear pleomorphism and a vesicular to hyperchromatic appearance of the nuclei.
Figure 3: Gastric adenocarcinoma and its precursor lesion a gastric tubulovillous adenoma with high grade dysplasia next to formations of a neuroendocrine tumor (H&E, 40x).
Figure 5: Gastric adenocarcinoma next to formations of a neuroendocrine tumor (H&E, 200x).
Neuroendocrine Tumor (NET)
Moreover, another neoplastic cell population consisting of monomorphic neoplastic cells organized in nests and trabeculae was observed, indicating a neuroendocrine differentiation. This cell population displayed strong immunohistochemical expression of Synaptophysin and Chromogranin A. Similar to the formations of well-differentiated gastric adenocarcinoma, nests of the neuroendocrine tumor (NET) were detected in sections of the submucosa. The proliferation index (Ki67) of the neuroendocrine differentiated tumor component exhibited considerable heterogeneity, with an average value of 5-10%.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.