First in Human Study Evaluating a Novel Hyaluronic Acid Chondroitin Complex for Reducing Striae Distensae Alba
by Marco Spadafora1, Cristina Bertoli2, Laura Bigi2, Silvana Ciardo2, Alex Curti2, Rossi Elena2, Caterina Longo2, Franco Grimolizzi3, Gilberto Bellia3*, Giovanni Pellacani4
1Azienda Unità Sanitaria Locale – IRCCS di Reggio Emilia, Skin Cancer Center, Reggio Emilia, Italy
2Department of Dermatology, University of Modena and Reggio Emilia, Modena, Italy
3IBSA Farmaceutici Italia Srl, Lodi (LO), Italy
4GP Dermatology Clinic, Department of Clinical Internal, Anesthesiological and Cardiovascular Sciences, Sapienza University of Rome, Rome, Italy
*Corresponding author: Gilberto Bellia, IBSA Farmaceutici Italia Srl, Lodi (LO), Italy. Email: gilberto.bellia@ibsa.it
Received Date: 15 October 2025
Accepted Date: 20 October 2025
Published Date: 22 October 2025
Citation: Spadafora M, Bertoli C, Bigi L, Ciardo S, Bellia G, et al. (2025). First in Human Study Evaluating a Novel Hyaluronic Acid Chondroitin Complex for Reducing Striae Distensae Alba. Ann Case Report. 10: 2432. https://doi.org/10.29011/2574-7754.102432
Abstract
Background: Striae distensae (SD) can cause psychological distress/anxiety to individuals. Objectives: The safety and efficacy of a new medical device containing hybrid complex based on hyaluronic acid and biotechnological chondroitin to treat SD was investigated. Methods: Females with ≥4 abdominal, gluteus, hip, or thigh SD areas were injected with the investigated medical device at baseline, Week 4, and Week 8, and followed-up on Week 12. Incidence of treatment-emergent adverse events (TEAEs) was the primary endpoint. Secondary endpoints included local tolerability of the medical device and volume of depression, size, collagen type, epidermal thickness, and aesthetic appearance (assessed by Global Aesthetic Improvement Scale [GAIS])) of treated areas. Results: For the final analysis set (N=18), two patients (11.1%) had TEAEs (all mild). Only 4 patients at longer follow-up visits (Week 12) had only mild pain at the injection site. There was a statistically significant decrease in the mean volume of depression (P=0.0197), length (P=0.0007), and thickness (P<0.0001) of treated areas but not control areas (without treatment) at follow-up versus baseline. There was a statistically significant decrease in the size of treated areas according to two of three dermatologists at follow-up versus baseline. Coarse collagen was the predominant collagen type in most patients, and presence of collagen fibrils significantly increased with treatment (P<0.001). Post-treatment, the aesthetic appearance (by GAIS) of treated areas results improved for most patients. Conclusion: The use of hyaluronans and chondroitin hybrid complex was safe and effective in slightly decreasing the size of SD areas and improving their appearance.
Keywords: Stretch Marks; Hyaluronic Acid; Chrondroitin; Medical Device; Safety; Efficacy.
Introduction
Striae distensae (SD; also known as stretch marks or striae gravidarum) is a dermal lesion that can occur during pregnancy, adolescence, and obesity, the course of a disease (e.g., Cushing’s syndrome), or with medication (e.g., the chronic use of steroids) [1]. More specifically, SD can be described as scars with flattening of the epidermis, a reduction in melanocyte cells (HEMa-LP), thinning, and a decrease in dermal collagen and elastin [2]. Most individuals with SD are pregnant women and adolescents who have pregnancy-related SD (also known as striae gravidarum) [1]. There are two stages of SD: early-stage striae rubra (red/purple, flat, or sometimes slightly convex in appearance) and late-stage striae alba (white in appearance due to a reduction in melanin and atrophy) [1, 3, 4]. SD is typically located on the abdomen, breasts, buttocks, and thigh areas and can cause psychological distress and anxiety to individuals [1, 4]. Risk factors for developing SD include a young maternal age, a family history of stretch marks, excessive weight gain during pregnancy, and high neonatal weight [1]. It is estimated that SD occurs in 43% and 88% of women during pregnancy, 6-86% of people during puberty, and in 43% of obese individuals [3].
The pathogenesis of SD is currently unknown [1, 4, 5], but it is thought that it is related to changes in the extracellular matrix components such as fibrillin, elastin, and collagen [1]. Some treatment modalities that have been investigated for SD have attempted to produce more collagen, decrease vascularity, and increase pigmentation [1]. Topical treatment modalities (such as tretinoin and glycolic acid) are mainly used as adjunctive therapies [1]. Ablative lasers and non-ablative lasers have commonly been used to treat SD [1]. Other therapies that have been investigated include radiofrequency, micro-needling, platelet-rich plasma therapy, microdermabrasion, carboxytherapy, or combination treatments such as (radiofrequency plus microneedling or fractional CO2 laser treatment plus platelet-rich plasma therapy) [1]. However, there is currently no ‘gold standard’ treatment for SD [1, 6] due to inadequate skin color improvement or persistent skin atrophy [6]. Injectable dermal fillers, used as monotherapy or combination therapy, may be used to treat SD with minimal side effects [6]. It is suggested that hyaluronic acid stimulates fibroblast activity and collagen production [7]. Several moisturizers that include a combination of hyaluronic acid, vitamins, and fatty acids have been demonstrated to significantly reduce the incidence of striae gravidarum when applied through massage [8]. Case studies of high-velocity pneumatic injection of hyaluronic acid for SD showed an improvement from baseline in the appearance of treated areas [6].
A new medical device is here described, comprising a pre-filled syringe of a buffered physiological solution of a novel hybrid complex based on high molecular weight hyaluronic acid (2.4% sodium hyaluronate) and biotechnological chondroitin (1.6% sodium chondroitin) that is intradermally injected. The hyaluronic acid included in the device is produced by fermentation without chemical modification processes, which is then highly purified.
Then, hyaluronan and chondroitin hybrid complexes were obtained thanks to a patent protected process (EP 2 614 090 B1; WO 2012/032151) based on the NAHYCO™ technology. NAHYCO™ is a well-known technology already used to obtain thermally stabilized hybrid cooperative complexes (HCC) composed by low (80-100 kDa) and high (1100-1400 kDa) molecular weight of hyaluronic acid chains [9].
Hyaluronan and chondroitin complexes can be similarly obtained by high-temperature thermal treatment of aqueous solutions of high molecular weight hyaluronic acid with low molecular weight (35 kDa) biotechnological chondroitin at a total concentration of 40 mg/ml.
As described by D’Agostino et al. [2], the newly obtained hyaluronans and chondroitin complex results in a solution with low viscosity (G’ = 92 ± 3 Pa; G” = 116 ± 5 Pa) that can be easily extruded and intradermally injected, also maintaining high resistance to degradation compared to linear high molecular weight hyaluronic acid. Moreover, in vitro preclinical evaluations on keratinocytes and fibroblasts cultures have demonstrated the ability of the hyaluronans and chondroitin complexes to promote cell migration, wound healing processes and to reduce inflammation [2]. Finally, these newly created complexes also proved their efficacy to increase collagen (type I and III) and elastin expression and to modulate the melanin release, remodeling the extracellular matrix and potentially supporting the reduction of the stretch marks [2].
Although promising, in vivo evidence obtained by clinical studies are needed to confirm the efficacy of the hyaluronans chondroitin complexes. Therefore, the objective of this first-in-human, open-label, single-center study was to investigate the safety and efficacy of a new medical device (a pre-filled syringe of a novel hybrid complex based on hyaluronic acid and biotechnological chondroitin) injected intradermally for the treatment of SD.
Patients and Methods
Eligibility criteria
Eligible participants were females aged between ≥18 and <55 years in generally good health with at least ≥4 areas of SD alba in an area of skin that was otherwise smooth, located on the abdomen, glutes, hip, or thigh. SD regions of these patients were required to be approximately similar in their dimension, location, or color and ≥6 cm in length and ≤5 mm in thickness. Patients included in the study had Fitzpatrick skin types I-IV and a body mass index between ≥18.5 and <35.0 Kg/m2. Eligible participants with childbearing potential were required to use an appropriate contraception method to participate in the study.
Sample size has been calculated based on the probability that one or more TEAEs did not occur in a sample of 15 patients with an anticipated incidence rate of TEAEs of 20% was 5% (the power of this investigation was 95% in such situation). A sample size of 18 patients in total has therefore been accounted for the replacement of up to 20% of non-evaluable patients for any reason (drop-out).
Study design and outcomes
The study design of the prospective, open-label, first-in-human, single-center study is shown in Figure 1. Eligible patients received three separate injections of the investigated medical device (four weeks apart) and were followed-up four weeks later. The primary endpoint was the incidence of treatment-emergent adverse events (classified according to the Medical Dictionary for Regulatory Activities; MeDRA). Secondary endpoints included local tolerability of the medical device, changes in vital signs from baseline to Weeks 4-12, and changes in physical examination parameters from baseline to Weeks 4-12 (Figure 1). Physical parameters of treated SD areas examined included their volume (assessed by non-invasive tridimensional analysis [Antera 3D®, Miravex Limited, Dublin, Ireland]); size (determined via the assessment of high-resolution digital photographs by three different dermatologists); collagen types of the dermal matrix (measured by reflectance confocal microscopy; RCM); collagen fibril grading (measured by RCM); length and width; and aesthetic appearance (established by both patient- and investigator-determined Global Aesthetic Improvement Scale [GAIS] score). Collagen fibril grading was performed in accordance with previously published methods (10). Briefly, a 3 × 3 mm area was divided into 36 segments and graded based on the presence of collagen fibrils in each segment (grade 0 = collagen fibrils absent from analyzed segments; 1 = present in < 9 segments analyzed; 2 = present in 9-17 segments; 3 = present in 18-26 segments; 4 = present in >27 segments).
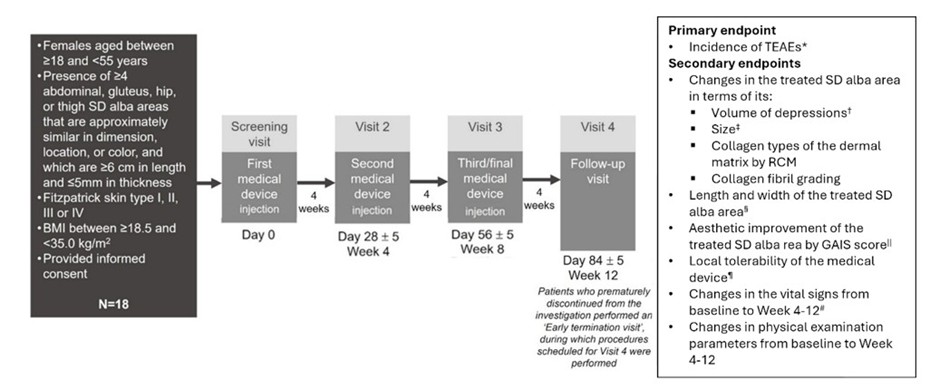
Figure 1: Participants, treatment, and study endpoints. All AEs that occurred after starting treatment. †Assessed by non-invasive tridimensional analysis (Antera 3D®). ‡Determined by the assessment of photographs of the treated area by 3 dermatologists. §Determined by clinical evaluation. ||Evaluated by both patients and investigators. ¶Incidence of pain, erythema, swelling, or skin hardening at the injection site were recorded. #Vital signs included heart rate and blood pressure evaluations. Abbreviations: AE- adverse events; GAIS- Global Aesthetic Improvement Scale- RCM, reflectance confocal microscopy; SD- striae distensae; TEAE- treatment-emergent adverse events.
The first patient was enrolled in the study on 22 September 2021, and the last patient completed the study on 21 September 2022.
Statistical analysis
The safety analysis set included all patients who received ≥1 injection dose of the investigated medical device. The final analysis set (FAS) included all patients who received ≥1 injection dose of the investigated medical device who had a valid baseline assessment and ≥1 post-baseline evaluation in ≥1 performance endpoint. Proportions of patients were assessed for categorical variables. Means, standard deviations (Std), median values, minimum and maximum, and interquartile range (IQR) (i.e., descriptive statistics) were calculated for continuous variables. For TEAEs, 95% confidence interval (CI) values of observed proportions were calculated using Poisson distribution if proportions of patients with TEAEs were <10% or the Clopper-Pearson method. The change from baseline to Weeks 4-12 in dermal matrix parameters (assessed by RCM), average epidermal thickness (assessed by OCT), and presence of collagen fibrils were evaluated using the Friedman’s test, Student’s t-test for paired data and Mann Whitney test for paired data, respectively. Statistically significant differences were determined by P-values of ≤0.05. All statistical analyses were performed using SAS System software (version 9.4; SAS Institute Inc., Cary, North Carolina, USA). Planned enrollment number was 15 patients.
Results
Patient demographics and baseline characteristics
Eighteen participants were included in the study. The mean (Std) age of females who participated in the study was 34.7 (9.6) years (Table 1). The most common skin types were Fitzpatrick scale II (44.4% of patients) and Fitzpatrick scale III (38.9%). The location of SD regions targeted for treatment or to be used as control regions (without treatment) were mainly on the right-hand side of the thighs (44.4%), glutes (22.2%), and abdomen (16.7%). The mean (Std) length of the target SD regions was 10.1 (2.5) cm, and the mean (Std) thickness of those areas were 2.4 (0.9) mm.
|
Characteristic |
All patients |
Characteristic |
All patients |
|
(N=18) |
(N=18) |
||
|
Mean (Std) age, years |
34.7 (9.6) |
Mean (Std) length of target SD, cm |
10.1 (2.5) |
|
White race, n (%) |
18 (100.0) |
Mean (Std) thickness of target SD, mm |
2.4 (0.9) |
|
Fitzpatrick scale I, n (%) |
3 (16.7) |
Mean (Std) length of control SD, cm |
9.0 (1.9) |
|
Fitzpatrick scale II, n (%) |
8 (44.4) |
Mean (Std) thickness of control SD, mm |
1.9 (0.7) |
|
Mean (Std) BMI, kg/m2 |
21.3 (2.4) |
Concomitant diseases, n (%) |
|
|
Location of target SD, n (%) |
Drug hypersensitivity |
2 (11.1) |
|
|
Abdomen (right hand side) |
3 (16.7) |
Menopause |
2 (11.1) |
|
Glutes (left hand side) |
1 (5.6) |
Allergy to metals |
1 (5.6) |
|
Glutes (right hand side) |
4 (22.2) |
Hypertension |
1 (5.6) |
|
Hip (right hand side) |
1 (5.6) |
Hypothyroidism |
1 (5.6) |
|
Thigh (left hand side) |
1 (5.6) |
Microcytic anemia |
1 (5.6) |
|
Thigh (right hand side) |
8 (44.4) |
Mite allergy |
1 (5.6) |
|
Location of control SD, n (%) |
Concomitant medications, n (%) |
||
|
Abdomen (right hand side) |
3 (16.7) |
Desogestrel |
1 (5.6) |
|
Glutes (left hand side) |
1 (5.6) |
Dienogest plus estradiol valerate |
1 (5.6) |
|
Glutes (right hand side) |
4 (22.2) |
Drospirenone plus ethinylestradiol |
1 (5.6) |
|
Hip (right hand side) |
1 (5.6) |
Ferric carboxymaltose |
1 (5.6) |
|
Thigh (left hand side) |
1 (5.6) |
Levothyroxine sodium |
1 (5.6) |
|
Thigh (right hand side) |
8 (44.4) |
Perindopril |
1 (5.6) |
Table 1: Patient demographics and baseline characteristics.
Two patients had 4 TEAEs (3 TEAEs of microcytic anemia, hypoesthesia and Lyme disease were reported in one patient, and 1 TEAE of delayed menstruation occurred in another patient). All TEAEs were of mild intensity. Abbreviations: BMI- body mass index; n- number of patients; N- total number of patients; SD- striae distensae; Std- standard deviation; TEAE- treatment-emergent adverse events.
Incidence of TEAEs and local tolerability of the medical device
TEAEs were reported in two patients (11.1%; microcytic anemia, hypoesthesia and Lyme disease were reported in one patient, and delayed menstruation occurred in another patient; Table 2). All TEAEs were of mild intensity, no serious TEAEs occurred, and none were related to the medical device or led to treatment discontinuation.
|
Number of patients, n (%) |
|||||
|
(N=18) |
|||||
|
≥ 1 TEAE |
2 (11.1)* |
||||
|
≥1 serious TEAE |
0 (0) |
||||
|
≥ 1 TEAE related to the medical device |
0 (0) |
||||
|
≥ 1 TEAE leading to treatment discontinuation |
0 (0) |
||||
|
≥ 1 TEAE leading to death |
0 (0) |
||||
|
Local tolerability at the injection site |
Baseline |
Week 4 |
Week 8 |
Week 12 |
|
|
Local erythema (faint redness†) |
16 (88.9) |
18 (100.0) |
16 (88.9) |
6 (33.3) |
|
|
Local erythema (moderate redness) |
2 (11.1) |
0 (0.0) |
1 (5.6) |
0 (0.0) |
|
|
Mild swelling |
18 (100.0) |
17 (94.4) |
17 (94.4) |
4 (22.2) |
|
|
Moderate swelling |
0 (0.0) |
1 (5.6) |
0 (0.0) |
0 (0.0) |
|
|
Mild hardening |
14 (77.8) |
16 (88.9) |
11 (61.1) |
4 (22.2) |
|
|
Mild pain |
18 (100.0) |
15 (83.3) |
15 (83.3) |
4 (22.2) |
|
Table 2: Incidence of TEAEs, local tolerability at the injection site for the entire duration of the study. Two patients had 4 TEAEs (3 TEAEs of microcytic anemia, hypoesthesia and Lyme disease were reported in one patient, and 1 TEAE of delayed menstruation occurred in another patient). All TEAEs were of mild intensity. †Not considered clinically relevant. n, number of patients; N, total number of patients; TEAE, treatment-emergent adverse events
In terms of local tolerability (assessed 30 minutes post-injection), faint redness (local erythema) that was not considered to be clinically relevant occurred in most patients at baseline, Week 4, and Week 8 (88.9%-100.0%) and fewer patients (33.3%) still had these symptoms at follow-up (Table 2). On the other hand, moderate redness occurred 30 minutes post-injection in only two (11.1%) of patients at baseline, no patients at Week 4, one (5.6%) patient at Week 8, and none of the patients at follow-up. Almost all patients had mild swelling at baseline, Week 4, and Week 8 (94.4%, 100.0%), but only 4 (22.2%) still had mild swelling at follow-up. No patients had moderate swelling at the injection site except for one (5.6%) at Week 4. Mild hardening at the injection site occurred in 14 (77.8%), 16 (88.9%), and 11 (61.1%) of patients at baseline, Week 4, and Week 8, but only 4 (22.2%) of these patients still had these symptoms at follow-up. Although all patients reported experiencing mild pain at the injection site at baseline, 15 (83.3%) patients had mild pain at Week 4 and Week 8, and only 4 (22.2%) patients still had these symptoms at Week 12.
Changes in the volume of depression in treated SD areas
There was a statistically significant decrease in the mean volume of depression in treated target SD regions post-treatment at follow-up (Week 12) versus baseline (mean change [Std], -0.19 [0.37] 3mm; P=0.0197; [Figure 2A] [Table 3], but not at Week 4 or Week 8 versus baseline [Table 3].
|
Number of patients, n (%) |
||||
|
Volume of depressions (mm3) |
Baseline |
Week 4 |
Week 8 |
Week 12 |
|
Mean (Std) |
0.52 (0.52) |
0.43 (0.50) |
0.39 (0.53) |
0.33 (0.41) |
|
Median (IQR) |
0.34 (0.26-0.71) |
0.27 (0.16-0.61) |
0.23 (0.18-0.40) |
0.16 (0.14-0.45) |
|
Minimum to maximum value |
0.08-2.26 |
0.02-2.18 |
0.09-2.42 |
0.00-1.77 |
|
Mean change from baseline |
N/A |
-0.09 |
-0.13 |
-0.19 |
|
P-value (compared with baseline) |
N/A |
0.2622 |
0.1012 |
0.0197 |
|
Size as determined by Dermatologist 1 (cm2) |
||||
|
Mean (Std) |
10.07 (2.46) |
10.03 (2.40) |
10.45 (3.13) |
9.24 (1.55) |
|
Median (IQR) |
9.75 (8.00-11.20) |
9.60 (8.00-11.00) |
9.50 (8.90-10.85) |
9.40 (7.80-10.20) |
|
Minimum to maximum value |
6.70-17.00 |
7.00-17.00 |
7.20-20.00 |
6.00-12.00 |
|
Mean change from baseline |
N/A |
-0.04 |
0.12 |
-0.83 |
|
P-value (compared with baseline) |
N/A |
0.9248 |
0.7628 |
0.0498 |
|
Size as determined by Dermatologist 2 (cm2) |
||||
|
Mean (Std) |
10.07 (2.46) |
10.00 (2.37) |
9.98 (2.15) |
9.47 (2.13) |
|
Median (IQR) |
9.75 (8.00-11.20) |
9.65 (8.00-10.80) |
9.50 (8.80-10.85) |
9.35 (8.00-10.20) |
|
Minimum to maximum value |
6.70-17.00 |
7.00-17.00 |
7.20-16.00 |
6.20-16.00 |
|
Mean change from baseline |
N/A |
-0.07 |
-0.36 |
-0.61 |
|
P-value (compared with baseline) |
N/A |
0.6077 |
0.0291 |
0.0001 |
|
Size as determined by Dermatologist 3 (cm2) |
||||
|
Mean (Std) |
10.07 (2.46) |
9.99 (2.34) |
10.06 (2.28) |
9.54 (2.22) |
|
Median (IQR) |
9.75 (8.00-11.20) |
9.65 (8.00-10.90) |
9.50 (8.95-10.90) |
9.30 (8.00-10.30) |
|
Minimum to maximum value |
6.70-17.00 |
7.10-17.00 |
7.10-16.50 |
6.30-16.50 |
|
Mean change from baseline |
N/A |
-0.08 |
-0.28 |
-0.53 |
|
P-value (compared with baseline) |
N/A |
0.5264 |
0.0774 |
0.0003 |
Table 3: Volume of depressions in target SD regions as measured by tridimensional analysis (Antera) and size of the target SD as determined by Dermatologist 1, Dermatologist 2, and Dermatologist 3 (N=18). IQR- interquartile range; n- number of patients- N, total number of patients- N/A- not applicable; SD- striae distensae; Std- standard deviation.
For comparison, the volume of depression in control (untreated) SD areas was also assessed. As expected, there was no statistically significant difference between the volume of the untreated area from baseline to Week 4, 8, or 12 (Figure 2A).
Changes in the size of treated SD areas
With regards to size of the treated SD as measured by three different dermatologists from photographs of the treated area, there was a statistically significant decrease in the mean size of the treated area from baseline to Week 12 (mean change [Std], -0.83 [1.37] cm2 [P=0.0498], -0.61 [0.80] cm2 [P=0.0001], and -0.53 [0.76] cm2 [P=0.0003], for Dermatologist 1, Dermatologist 2, and Dermatologist 3, respectively; Table 3). Although not significant at Week 4 and Week 8, a general positive trend for decrease in the mean size of the treated area versus baseline can be observed (Table 3).
Length and thickness of the treated SD area
There was a statistically significant reduction in the mean (Std) clinically evaluated length of the target SD at Week 12 versus baseline (8.89 [1.46] versus 10.07 [2.46]; P=0.0007; Table 4.
|
Number of patients, n (%) |
|||||
|
Clinically evaluated length (cm) |
Baseline |
Week 4 |
Week 8 |
Week 12 |
|
|
Mean (Std) |
10.07 (2.46) |
9.68 (2.20) |
9.13 (1.35) |
8.89 (1.46) |
|
|
Median (IQR) |
9.75 (8.00-11.20) |
9.50 (8.00-10.00) |
9.40 (8.00-10.00) |
9.00 (8.00-10.00) |
|
|
Minimum to maximum value |
6.70-17.00 |
7.00-17.00 |
6.00-11.50 |
5.50-11.50 |
|
|
Mean change from baseline |
N/A |
-0.39 |
-0.94 |
-1.18 |
|
|
P-value (compared with baseline) |
N/A |
0.2294 |
0.0054 |
0.0007 |
|
|
Clinically evaluated thickness (cm) |
|||||
|
Mean (Std) |
2.39 (0.93) |
2.11 (0.70) |
2.00 (0.75) |
1.72 (0.67) |
|
|
Median (IQR) |
2.25 (2.00-3.00) |
2.00 (2.00-3.00) |
2.00 (1.50-3.00) |
1.50 (1.50-2.00) |
|
|
Minimum to maximum value |
1.00-4.00 |
1.00-3.00 |
1.00-3.00 |
1.00-3.00 |
|
|
Mean change from baseline |
N/A |
-0.28 |
-0.39 |
-0.67 |
|
|
P-value (compared with baseline) |
N/A |
0.0612 |
0.0104 |
<0.0001 |
|
Table 4: Clinically evaluated length and clinically evaluated thickness of target SD
There was a statistically significant reduction in the mean (Std) clinically evaluated thickness of the target SD at Week 8 versus baseline (2.00 [0.75] versus 2.39 [0.93]; P=0.0104; Table 4) and Week 12 versus baseline (1.72 [0.67] versus 2.39 [0.93]; P<0.0001; Figure 2B and Table 4.
For comparison, the clinically evaluated length and thickness of control SD areas were also assessed. There was no statistically significant difference in the clinically evaluated length from baseline to Week 4, 8, or 12. There was also no statistically significant difference in the clinically evaluated thickness from baseline to Week 4 or 8 (Figure 2B).
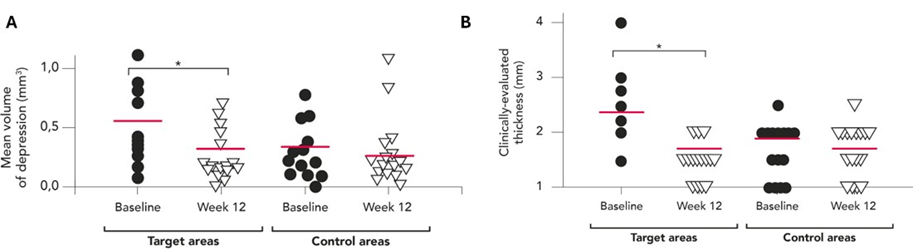
Figure 2: (A) Volume of depressions in target SD regions as measured by tridimensional analysis (Antera) and (B) clinically evaluated thickness of target SD at baseline and Week 12 (N=18). Horizontal lines within the scatter plots represent mean values. N- total number of patients; SD- striae distensae.
Changes in the treated SD area in terms of collagen types of the dermal matrix and presence of collagen fibrils
As measured by RCM to determine the different collagen fiber types in the treated area, none of the patients had huddled collagen or curled collagen-fiber at the end of the study (Figure 3A). Coarse collagen was the predominant type of collagen in 14 patients (77.8%) at baseline and 16 patients (88.9%) at Week 12. Long straight fibers were predominant in four patients at baseline and two patients at Week 12 at the end of the study. Presence of collagen fibrils, also assessed by RCM, was significantly greater in the targeted area at Week 12 compared with baseline (mean scores of 2.8 vs 1.4, respectively; P<0.001);(Figure 3B). The presence of collagen fibrils remained consistent for untreated control areas, with no increase observed.
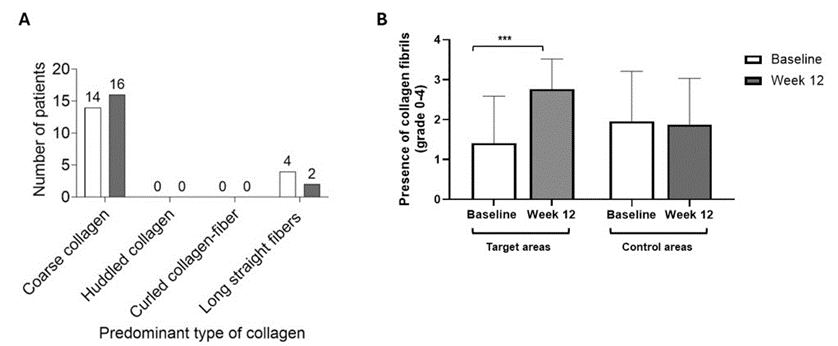
Figure 3: (A) Predominant collagen fiber types (N=18) and (B) collagen fibril grading (N=17) as measured by RCM at baseline and Week 12 *** P<0.001. RCM, reflectance confocal microscopy. Figure 3B: Graded based on the presence of collagen fibrils (0 = absent; 1 = present in < 9 segments analyzed; 2 = present in 9-17 segments; 3 = present in 18-26 segments; 4 = present in >27 segments).
Aesthetic improvement of the treated SD area by GAIS score
GAIS was assessed by investigators and patients at Weeks 4-12. As determined by investigators, the aesthetic appearance of treated SD areas was ‘somewhat improved’ for most patients at Week 4 (15 [83.3%]; Table 5), Week 8 (13 [72.2%]), and Week 12 (17 [94.4%]). As determined by patients, the aesthetic appearance of treated SD areas was also ‘somewhat improved’ for most patients at Week 4 (13 [72.2%]), Week 8 (9 [50.0%]) and Week 12 (17 [94.4%]). One patient (5.6%) had a ‘much improved’ aesthetic appearance in their treated SD areas, as determined by both the investigator and patient.
|
Investigator-assessed GAIS |
Baseline |
Week 4 |
Week 8 |
Week 12 |
|
|
Very much improved |
N/A |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
|
Much improved |
N/A |
1 (5.6) |
0 (0.0) |
0 (0.0) |
|
|
Somewhat improved |
N/A |
15 (83.3) |
13 (72.2) |
17 (94.4) |
|
|
No change |
N/A |
2 (11.1) |
5 (27.8) |
1 (5.6) |
|
|
Worsened |
N/A |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
|
Patient-assessed GAIS |
|||||
|
Very much improved |
N/A |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
|
Much improved |
N/A |
1 (5.6) |
0 (0.0) |
0 (0.0) |
|
|
Somewhat improved |
N/A |
13 (72.2) |
9 (50.0) |
17 (94.4) |
|
|
No change |
N/A |
4 (22.2) |
9 (50.0) |
1 (5.6) |
|
|
Worsened |
N/A |
0 (0.0) |
0 (0.0) |
0 (0.0) |
Table 5: Incidence of investigator-assessed and patient-assessed GAIS during the entire duration of the study. Abbreviations: GAIS- global aesthetic improvement scale; n- number of patients; N- total number of patients.
Discussion
In a first-in-human study, investigating the safety and efficacy of a novel hybrid complex based on hyaluronic acid and biotechnological chondroitin to treat SD in 18 females, two patients had TEAEs, none related to treatment. Most patients had faint redness and mild swelling, hardening at the injection site, and pain post-injection at baseline and Weeks 4 and 8, but none of them were evaluated as serious and disappeared in a short period. A statistically significant decrease in the mean volume of depression (P=0.0197) and clinically evaluated length (P=0.0007) and thickness (P<0.0001) in target SD regions at follow-up versus baseline occurred, but not in control areas (without treatment). Similarly, a statistically significant decrease in the size of the treated SD areas (by photograph assessment) occurred at follow-up versus baseline. Coarse collagen was the predominant collagen type in most patients at baseline (77.8%) and follow-up (88.9%).
By investigator- and patient-determined GAIS, the aesthetic appearance of treated SD areas was improved for most patients at followup (Week 12). Post-treatment, no substantial changes in vital signs or body system abnormalities occurred, confirming the high safety profile of the investigated medical device.
Notably, most treatments for SD are developed to increase collagen production [9]. These treatments include topical creams such as retinoic acid and hyaluronic acid, lasers, microdermabrasion, chemical peels, radiofrequency, intense pulsed light, percutaneous collagen induction therapy, platelet-rich plasma, infrared light, and galvanopuncture [9]. It is thought that hyaluronic acid enhances collagen production by stimulating fibroblasts [9].
In a randomized controlled study investigating a cream containing an onion extract, Centella asiatica, and hyaluronic acid that was applied twice daily for 12 weeks in 55 women with striae rubra in the thigh area, there was a statistically significant improvement in the proximal aspect of the striae rubra according to its overall appearance, softness, color, and texture, as evaluated by both investigators and patients [11]. However, there was no statistically significant improvement in skin elasticity between treated and untreated areas, and the study did not measure a change in the size of striae rubra areas.
In another 16-week, randomized clinical study in women with SD, 24 patients received a topical hyaluronic acid cream (also comprising vitamin C and beta glucan) twice daily and were injected with an injectable solution of hyaluronic acid (also containing vitamin C and beta glucan) twice a week, 22 patients had the topical hyaluronic acid cream twice daily, and 20 patients had a topical placebo solution [12]. By quantitative topographic measurements and clinical and histological evaluations, the hyaluronic acid injection plus topical hyaluronic acid cream provided a 57% improvement in treated areas versus topical hyaluronic acid cream alone (P<0.05), and the hyaluronic acid cream resulted in a 32% improvement versus placebo (P<0.05) [12]. Additionally, that study reported no side effects besides a slight burning sensation at the injection site.
In a case series investigating the pneumatic injection of hyaluronic acid with a jet volumetric remodeling system for two participants with SD in the breast and abdomen area, two injections eight weeks apart led to a 45.7% and 42.8% improvement in appearance (assessed using a validated scar scale) at six months and 15 months post-procedure, respectively [13]. Furthermore, in a retrospective analysis of 115 participants treated with a high-velocity pneumatic injection of non-crosslinked or crosslinked hyaluronic acid for aesthetic skin concerns, including SD, all aesthetic skin concerns improved with minimal downtime, pain, or adverse events [14]. In that analysis, 7% of participants had bruises at the injection site, 1% had local edema, and participants had a ‘much improved’ GAIS score in the neck, décolleté and perioral areas [14].
In an in vitro study of biotechnological chondroitin and a high molecular weight hyaluronic acid in an inflammation skin model aimed at resembling SD, the treatment led to an increased expression of collagen type I, collagen type III, elastin, and Fibrillin-1 in TNF-α insulated cells, indicating matrix regeneration and reduced inflammation [2]. Additionally, biotechnological chondroitin and high molecular weight hyaluronic acid formulations increased the biosynthesis of melanin [2]. In keeping with these in vitro observations, results from this study indicated a significant increase in collagen fibril presence in target areas when comparing baseline with Week 12. The presence of collagen fibrils remained consistent for untreated control areas, with no increase observed during the study. In addition, untreated areas of normal skin (i.e. areas without SD) were examined and exhibited consistently high levels of collagen fibrils throughout the study. Coarse collagen was the predominant collagen type in most patients, with long straight fibers observed in minority of patients. Although these positive results are preliminary, they are indicative of in vivo extracellular matrix remodeling due to the investigative device and further establish the applicability of RCM as a non-invasive technique for assessing treatment outcomes in dermatology [10, 15].
Our first-in-human study is limited by the small number of patients, the fact that it does not have a control arm (although control SD areas of the body that were not treated were included for assessments), and a short-term follow-up period. It is possible that greater improvements in SD areas could be achieved with longer-term treatment or follow-up. In addition, larger studies with a treatment control arm are needed to confirm initial study findings. However, overall, the study indicates that the investigated medical device, composed by hybrid complex of hyaluronans and biotechnological chondroitin, is safe and effective for slightly reducing the size of SD areas located in the abdomen, glutes, hips, or thigh regions of the body and improving their overall appearance.
Author contributions: Conceptualization, L.B., G.B., G.P.; Methodology, C.L.; Validation, C.L.; Investigation, C.L.; Data curation, C.B., S.C., A.C., R.E., C.L.; Writing-original draft preparation, F.G., C.L.; Writing-review and editing, All authors. All authors have read and agreed to the published version of the manuscript.
Data availability statement: The author confirms that the data supporting the findings of this study are available within the article. Further data can be provided upon reasonable request.
Acknowledgements: The authors are grateful to Sally Hassan, PhD, an employee of Papyrus Medical Communications Ltd, for medical writing support with developing the first draft of the manuscript.
Funding: The study described in this article and medical writing support for the first draft of this publication was funded by IBSA Farmaceutici Italia Srl.
Conflict of interest statement: F.G., G.B. are currently employees of IBSA Farmaceutici Italia Srl. The other authors declare no conflict of interest.
Ethics and informed consent: The study was conducted according to the Declaration of Helsinki, International Conference on Harmonization Good Clinical Practices, ISO 14155, European Union Council Directive 93/42/EEC amended by 2007/47/EC, MEDDEV 2. 12-1 revision 6 and amendments, and local legislation on clinical investigations involving medical devices. Patients provided their informed consent before study participation.
References
- Huang Q, Xu LL, Wu T, Mu YZ. (2022). New Progress in Therapeutic Modalities of Striae Distensae. Clin Cosmet Investig Dermatol. 15: 2101-15.
- D’Agostino A, La Gatta A, Stellavato A, Cimini D, Corsuto L, et al. (2022). Potential of Biofermentative Unsulfated Chondroitin and Hyaluronic Acid in Dermal Repair. Int J Mol Sci. 23:1-10.
- Oakley AM, Patel BC. (2024). Stretch Marks. StatPearls. Treasure Island (FL): 1-5.
- Adatto M. (2023). Clinical Evaluation of the Efficacy of Fractional Radiofrequency for the Treatment and Reduction of Stretch Marks: A Prospective Study. J Cosmet Dermatol. 22: 214-21.
- Elsaie ML, Baumann LS, Elsaaiee LT. (2009). Striae Distensae (Stretch Marks) and Different Modalities of Therapy: An Update. Dermatol Surg. 35: 563-73.
- Alsharif SH, Alghamdi AS, Alhumaidi WA, AlRobaish OA, Al Hamoud MH, et al. (2023). Treatment of Striae Distensae with Filler Injection: A Systematic Review. Clin Cosmet Investig Dermatol. 16: 837-45.
- Ud-Din S, McGeorge D, Bayat A. (2016). Topical Management of Striae Distensae (Stretch Marks): Prevention and Therapy of Striae Rubrae and Albae. J Eur Acad Dermatol Venereol. 30: 211-22.
- Mendes N, Alves PJ, Barros M, Rodrigues JM, Machado J. (2022). A Narrative Review of Current Striae Treatments. Healthcare (Basel). 10: 1-10.
- Hague A, Bayat A. (2017). Therapeutic Targets in the Management of Striae Distensae: A Systematic Review. J Am Acad Dermatol. 77: 559-68.
- Ciardo S, Pezzini C, Guida S, Del Duca E, Ungar J, et al. (2021). A Plea for Standardization of Confocal Microscopy and Optical Coherence Tomography Parameters to Evaluate Physiological and Para-Physiological Skin Conditions in Cosmetic Science. Exp Dermatol. 30:911-22.
- Draelos ZD, Gold MH, Kaur M, Olayinka B, Grundy SL et al. (2010). Evaluation of an Onion Extract, Centella Asiatica, and Hyaluronic Acid Cream in the Appearance of Striae Rubra. Skinmed. 8: 80-6.
- Morganti P, Palombo P, Fabrizi G, Palombo M, Persechino S. (2001). Biweekly In-Office Treatment of Striae Distensae: A Long-Term Daily Use of Topical Vitamin C. J Appl Cosmetol. 19: 107-12.
- Gupta A, Kroumpouzos G. (2021). A Novel Treatment of Striae Distensae with Pneumatic Injection of Hyaluronic Acid. J Appl Cosmetol. 39: 17-24.
- MacGillis D, Vinshtok Y. (2021). High-Velocity Pneumatic Injection of Non-Crosslinked Hyaluronic Acid for Skin Regeneration and Scar Remodeling: A Retrospective Analysis of 115 Patients. J Cosmet Dermatol. 20: 1098-103.
- Guida S, Longo C, Amato S, Rossi AM, Manfredini M, Ciardo S, et al. (2023). Laser Treatment Monitoring with Reflectance Confocal Microscopy. Medicina (Kaunas). 59: 1-10.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.