Fibrous Dysplasia of the Jaws: From Diagnosis to Treatment. A Case Series
by Garibaldi J*, Pozzetti E, Cassinotto E, Merlini A
Department of Odontostomatology, Galliera Hospital, Genoa, Italy.
*Corresponding author: Garibaldi J, Department of Odontostomatology, Galliera Hospital, Genoa, Italy. Email: enrico.pozzetti.96@ icloud.com
Received Date: 29 August, 2023
Accepted Date: 06 September, 2023
Published Date: 08 September, 2023
Citation: Garibaldi J, Pozzetti E, Cassinotto E, Merlini A (2023) Fibrous Dysplasia of the Jaws: From Diagnosis To Treatment. A Case Series. Dent Adv Res 8: 205. https://doi.org/10.29011/2574-7347.100205.
Abstract
Introduction: fibro-osseous lesions of the jaws comprise a diverse group of pathological conditions characterized by the replacement of bone with newly formed mineralized fibrous tissue. Fibrous dysplasia (FD) is a tumor-like developmental anomaly caused by a congenital mutation in the GNAS gene, leading to the replacement of bone with fibrous connective tissue containing irregular bony trabeculae. The application of piezoelectric technology in oral bone surgery is highly advantageous, including precise and selective hard tissue cutting, preservation of vital structures, improved surgical field visibility, and reduced post-operative discomfort for patients.
Material and Methods: Herein, we present a case series involving two patients that referred to Galliera Hospital (Genoa, Italy), with an expansive mass involving the right upper jaw (Case 1) and the left side of the mandible (Case 2). Piezoelectric instrumentation was used for incisional biopsies, after the elevation of a flap to expose the lesion.
Results: histopathological examination confirmed the diagnosis of FD, and conservative management was opted due to the benign nature of the condition.
Conclusion: given the benign nature of the diagnosed condition and the absence of patient discomfort or facial deformities, literature suggests regular annual check-ups as surgical treatment is not requested. The application of piezoelectric instruments proved to be a valid and effective method.
Keywords: Piezosurgery; Fibrous Dysplasia; Biopsy; Oral Pathology; Bone Pathology
Introduction
Fibro-osseous lesions of the jaws encompass a heterogeneous group of pathological conditions characterized by the substitution of normal bone with mineralized fibrous tissue. These lesions include fibrous dysplasia, cemento-osseous dysplasia, and ossifying fibroma [1]. FD results from a congenital mutation in the GNAS gene (20q13.32), leading to the replacement of bone with fibrous connective tissue containing irregular bony trabeculae. The disease's prevalence is often difficult to estimate due to its asymptomatic nature [2-4]. FD can affect a single bone (monostotic fibrous dysplasia) and interests 70-85% of the patient affected by FD; maxilla is interested more frequently than mandible, but usually mandible lesions are truly monostotic, instead maxillary involvement often is associated with adjacent bone lesions (zygoma, sphenoid, ethmoid, frontal bone, temporal bone, occiput) and it is known as craniofacial fibrous dysplasia. A minority of patients exhibits multiple bones involvement, and this condition is named polyostotic fibrous dysplasia, which shows female predilection. Rarely, the polyostotic form can be associated with other clinical signs. In fact, it may also lead to skin pigmentation, known as Jaffe-Lichtenstein syndrome, or it can be linked to skin and multiple endocrine abnormalities, named as McCune-Albright syndrome, or even manifest as intramuscular myxomas, designated as Mazabraud syndrome This pathological condition, in comparison to other fibro-osseous disorders, is caused by a congenital mutation of the GNAS gene, which encodes for the α-subunit of a stimulatory G protein. Specifically, this genetic mutation leads to the constitutive activation of adenylyl cyclase and elevated levels of cyclic AMP. These elevated levels act on downstream signaling pathways, resulting in the replacement of normal bone with fibrous tissue and immature bone [5]. The extent and severity of the lesions associated with FD depend on the timing of the genetic mutation. If it occurs at the embryonic level, affecting a totipotent stem cells, it leads to abnormalities in osteoblasts, melanocytes, and endocrine cells. Conversely, if the mutation arises in a progenitor osteoblastic cell, only osteoblastic cells are affected.
The constitutive activation of this G protein leads to impaired osteoblastic differentiation, excessive melanin production, hyperplasia, and hyperfunction of endocrine cells. Simultaneously, the mutated osteoblastic cells, due to hyperexpression of IL-6, stimulate osteoclastic activity, contributing to the expansion of fibrous masses [6].
FD tends to stabilize upon skeletal maturation, and conservative management is preferred. However, in cases of cosmetic and functional disturbances, a surgical debulking procedure can be beneficial. Surgical therapy is the key treatment in some cases and aims to prevent functional complications and improve aesthetics. It encompasses various approaches, from a more conservative ones (curettage, reshaping of the bone margins), which carry a higher risk of recurrence, to radical excision with immediate reconstruction, which provides the highest success rate. In 1990 Chen and Noodrof ideated a decisional algorithm, dividing the craniofacial region in 4 zones based on the functional and aesthetic consequences of the pathology in relation to the unique anatomical characteristics of each area.
- Zone 1: area above the alveolar maxillary bone, including the frontal, nasal, orbital, ethmoidal, zygomatic, and superior maxillary regions. In this area the reconstruction of the defect is achievable without altering aesthetics and functionality. For this reason, radical resection of the lesion is recommended.
- Zone 2: area of the cranial region covered by the scalp. Aesthetic appearance is not as crucial as in Zone 1. Both different options can be considered, based on the surgeon's evaluation.
- Zone 3: central part of the cranial base, including the petrous, mastoid, and pterygoid regions, where cranial nerves and major blood vessels are localized. This zone presents a particularly risky surgical access, so surgical approach is advised against unless the lesion shows symptoms.
- Zone 4: it’s the area interested by our two cases, includes the mandible and maxilla. Resection of one of these bones requires prosthetic replacement of the lost elements, which cannot fully replicate the functionality of natural dentition. Therefore, a conservative therapy approach is preferred [7].
Yet, it's important to note that in some cases, this approach might be ineffective, as 20% to 50% of patients experience pathological tissue regrowth after surgery [8-9].
Invented in 1988 by Tomaso Vercellotti, piezoelectric surgery is a precise technique for bone cutting. It relies on ultrasonic microvibrations and "pressure electrification". Ultrasonic vibration is generated through the expansion and contraction of quartz or Rochelle salts when subjected to an electric tension. These ultrasonic vibrations, used in dentistry, typically within the frequency range of 25-29 kHz and with an oscillation amplitude of 60-210 µm, provide a maximum power of up to 50 W. This technology enables selective cutting of hard structures while minimizing damage to soft tissues [10-11]. Piezoelectric technology proves highly valuable in oral bone surgery. It facilitates the protection of soft tissues during selective hard tissue cutting, preserves vital structures, induces cavitation effects, improves visibility within the surgical field, and minimizes post-operative discomfort for patients.
The FD is a rare pathological condition that often affects the craniofacial bones. The lesions associated with this disease are frequently asymptomatic, although in some cases they can cause functional issues due to nervous or vascular involvement. Radiographic assessment is essential for identifying the lesions, but a definitive diagnosis is only achieved through histopathological examination. As per the literature, given the benign nature of the diagnosed condition and the absence of patient discomfort or facial deformities, regular annual check-ups are recommended as surgical treatment is not warranted. There is a lack of literature regarding piezoelectric surgery and its application in the treatment and biopsy of fibrous dysplasia. However, within the constraints of the case report, it demonstrates a valid and alternative method that surpasses the limitations of traditional instrumentation.
Materials and Methods
This case series involved the selection of two patients afflicted by FD of the jaws: the first patient is a male with the lesion located in the maxilla (Case 1), and the second patient is female with the lesion affecting the mandible (Case 2).
Case 1: a 12-year-old male patient from Catanzaro, Italy, was referred to the Odontostomatology Department of Galliera Hospital in Genoa, Italy. The patient presented an expansive mass involving the right upper jaw (Fig. 2) and retained right permanent upper canine. Upon clinical examination, the patient exhibited a hard lesion causing altered eruption of the dental element 1.3, a lack of space in the dental arch, subsequent to the exfoliation of the deciduous canine, and a buccal protuberance of the permanent canine, in close proximity to the lateral incisor root. Initial bidimensional radiographic examination (OPT) revealed a ground-glass opacity within the right maxillary sinus and malposition of the permanent canine. To delineate the lesion in three dimensions and to locate the right upper canine accurately, cone-beam computed tomography (CBC, Fig. 1) was necessary. The CBCT depicted the buccal position of the canine and defined the borders of the fibro- osseous lesion, which also involved the buccal aspect of the posterior elements, leading to altered passive eruption.
Case 2: a 52-year-old Brazilian female patient was referred to the Odontostomatology Department of Galliera Hospital in Genoa, Italy, with an asymptomatic deformation of the edentulous left posterior mandible (Fig. 8). The OPT revealed an irregular radiopacity in a poorly defined radiolucent area with a cystic appearance. Subsequent CBCT revealed a 20x15x15 mm osteolytic round lesion adjacent to the inferior alveolar canal, exhibiting bifurcation in correspondence with the radiodense mass, indicative of an impacted tooth. Posterior to this radiopacity, a smaller lesion was observed, involving the mandibular ramus (Fig. 6-7).
The patients were informed about the surgical method and the potential risks associated with this procedure, and they provided informed consent.
In Case 1, under local anesthesia (2% Mepivacaine Hydrochloride with 1:100000 adrenaline), a mucoperiosteal flap was raised (Fig. 3), fully exposing the fibro-osseous tissue. An incisional biopsy was then performed (Fig. 4), obtaining a sample measuring 2 cm x 0.6 cm x 0.3 cm (Fig. 5).
In Case 2, the bone biopsy was larger due to the patient requiring extraction of an impacted tooth from within the fibro-osseous tissue. This also allowed for restoration of the correct volume of the left posterior mandible. A sample of tissue was obtained that measured 2 cm x 1.1 cm x 0.9 cm for, using piezoelectric instrumentation (Piezosurgery [Mectron S.p.A., Carasco, Italy]) with an osteotomic microsaw insert (Mectron, OT7S-3).
The excised samples were both placed in a pre-filled jar with a formalin ratio of 10:1, protected from light sources, and sent to the Department of Pathology, Anatomy, and Histology at Galliera Hospital. The wound was closed with resorbable 4/0 braided polyglactin 910 sutures (Vycril, Ethicon). At the seven-day follow-up, the healing progress was optimal.
Results
In both cases, histopathological examination revealed osseous tissue characterized by immature bony trabeculae with irregular shapes and sizes, thin and disconnected from each other, surrounded by a fibroblastic stroma, without evidence of osteoblastic activity. At the periphery of the biopsy specimen, the fibro-osseous tissue merged with bone without encapsulation or a demarcation line. This histological pattern is consistent with a diagnosis of fibrous dysplasia. Genetic testing for GNAS gene mutations was not performed because this investigation has a low statistical sensitivity and is useful for uncertain histopathological diagnoses.
In Case 1, the patient was informed about the altered passive eruption of dental elements in the first quadrant, but the parents refused the suggested surgical crown lengthening to improve this condition. Instead, for the retention of the right permanent upper canine, it was recommended to initiate fixed orthodontic therapy to create the space required for proper permanent eruption. Subsequently, surgical exposure of the canine and orthodontic traction was planned.
In Case 2, the patient exhibited favorable aesthetic results without neurological consequences. The post-operative CBCT at the 3-year follow-up indicated maintenance of the alveolar structure, with partial ossification of the defect.
Case1:
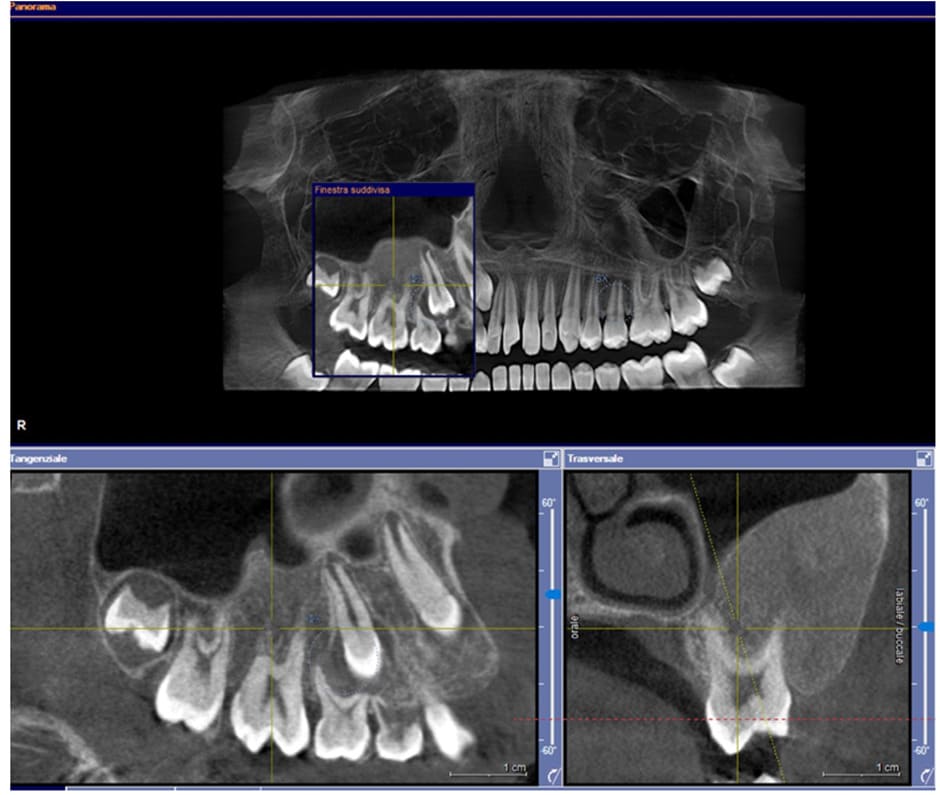
Figure1: Pre-operative CBCT scan.
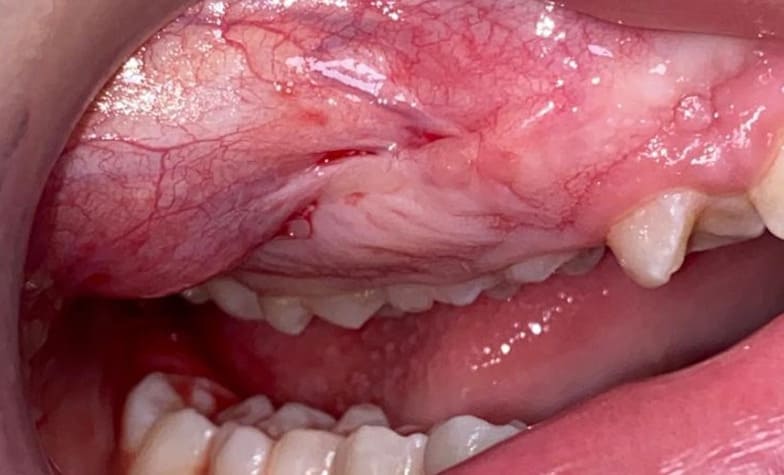
Figure2: Pre-operative situation
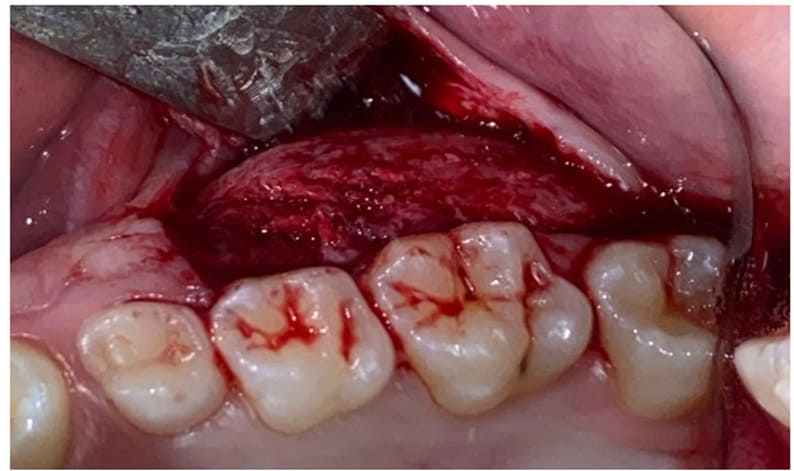
Figure3: Elevation of the flap and exposition of the lesion.
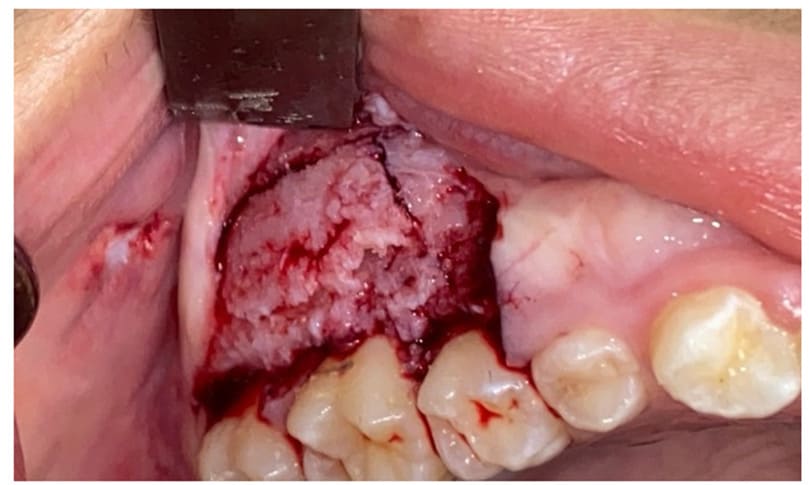
Figure 4: Osteotomy performed with Piezosurgery technique
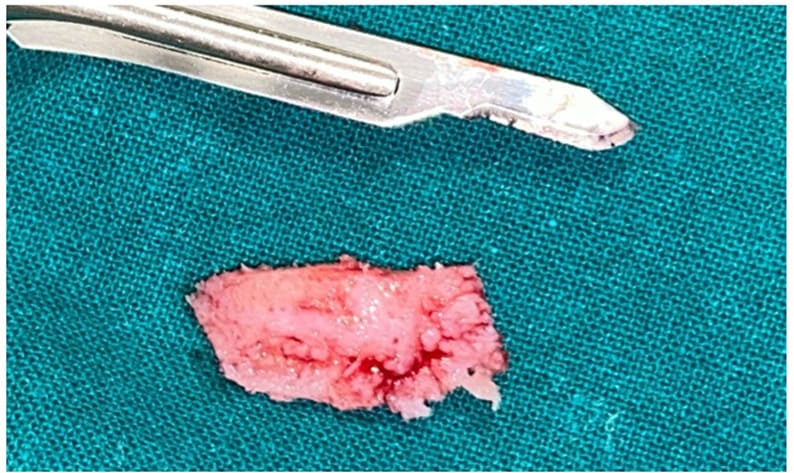
Figure 5: Excised tissue
Case2:
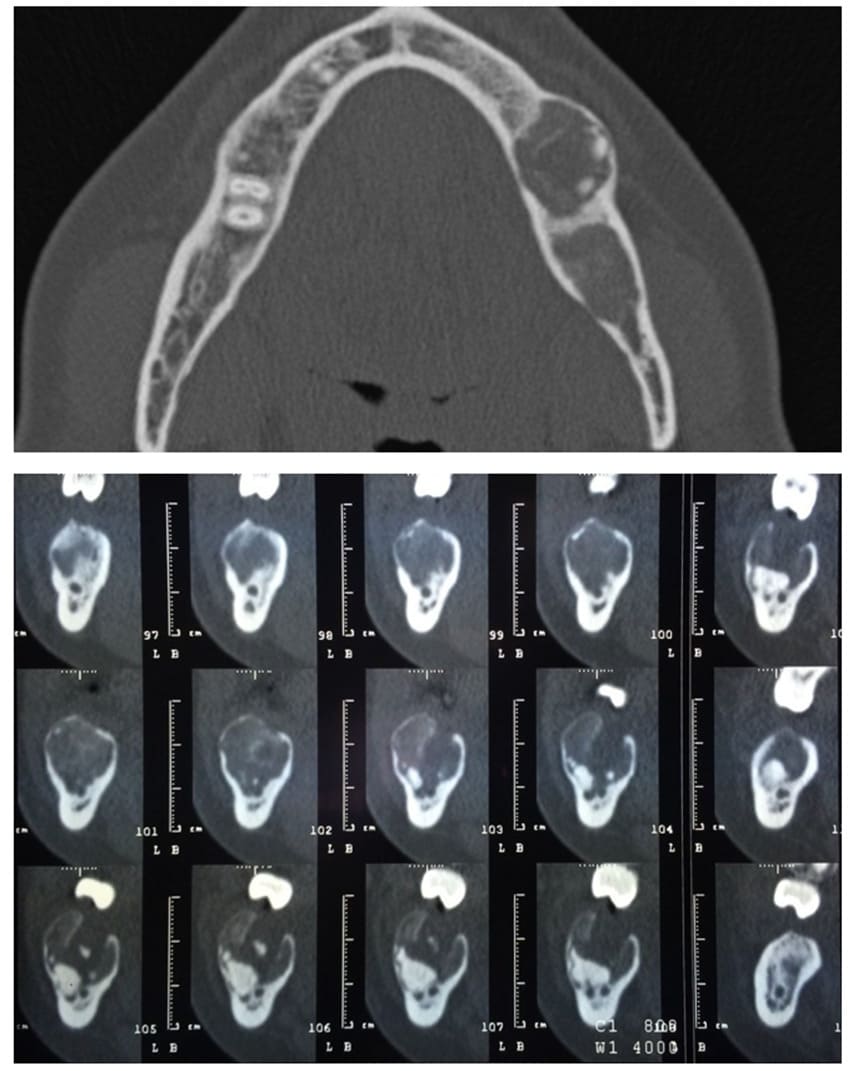
Figure 6-7: pre-operative CBCT scan, the upper transverse section shows the affected mandibular area. In the lower sagittal sections it’s also visible the affected area with the presence of an impacted tooth and the bifurcation of the inferior alveolar nerve.
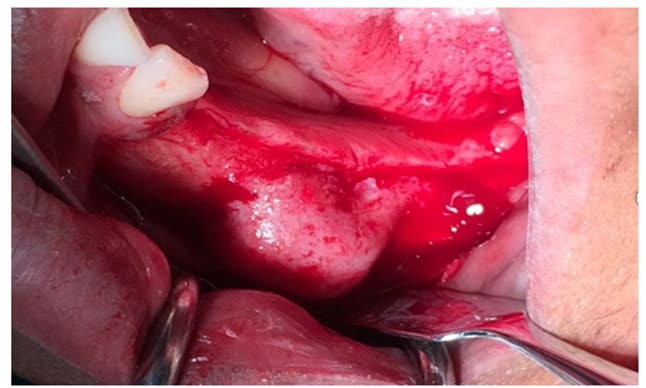
Figure 8: Exposition of the lesion after the flap elevation
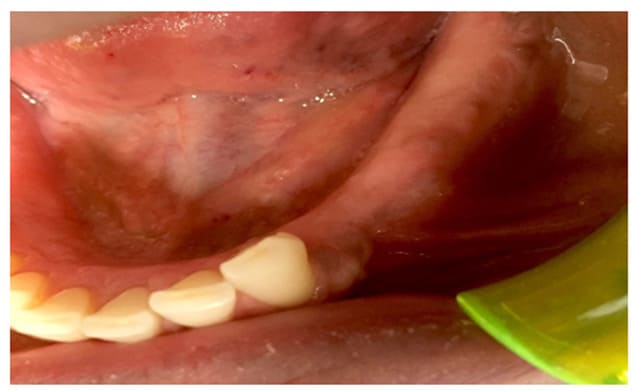
Figure 9: Follow up at 3 months
Histopathological Features:
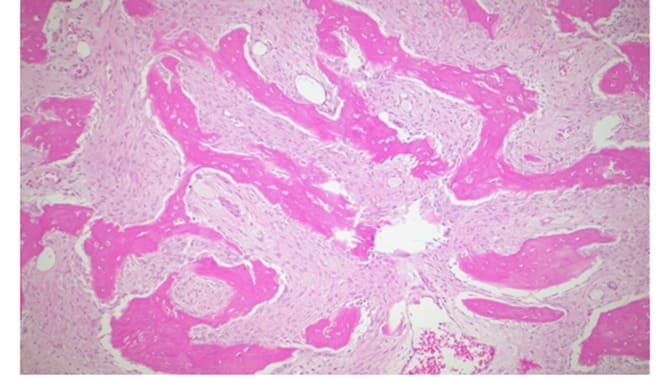
Figure 10: H&E, 25X,: Irregular and curvilinear arrangement of trabeculae.
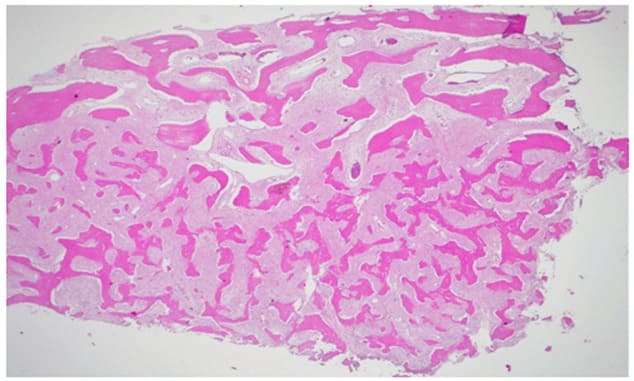
Figure 11: H&E 100X, highlights of the fibrous component.
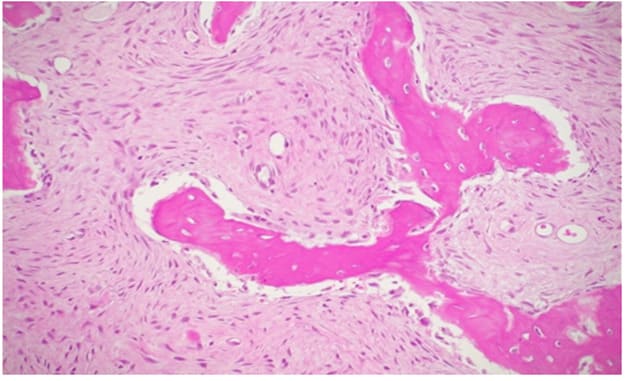
Figure 12: H&E 250X2, areas with predominant fibrous component.
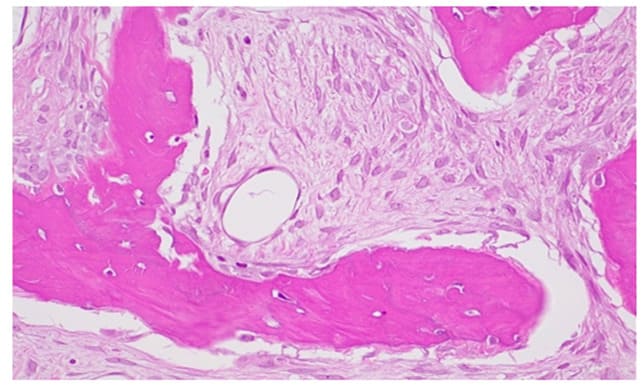
Figure 13: H&E 400X 4, fibroblastic stroma with spindle-shaped elements and the absence of osteoblastic rim.
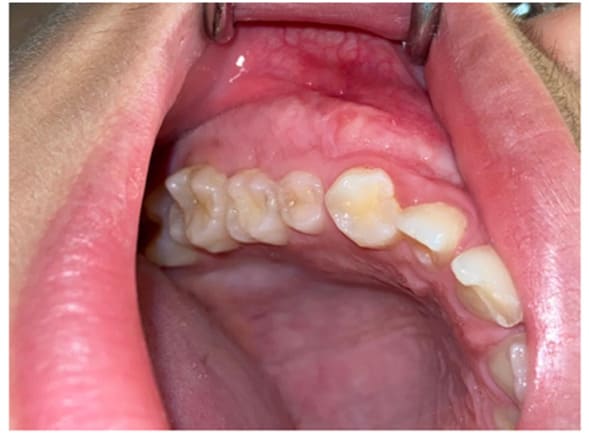
Figure 14: Clinical case post-recovery.
Discussion
FD is a benign congenital progressive disorder characterized by the replacement of normal bone with irregular and immature fibrous-osseous tissue [12]. Early diagnosis can be beneficial for monitoring the progression of this pathological condition. In this context, the role of pediatric dentists is crucial in identifying craniofacial signs of this disorder, while pediatricians are essential for recognizing other deformities, such as malformations of the proximal femur or bowing deformities.
Clinical examination reveals an onset of symptoms in young patients, with a slow growing, potentially painful mass leading to facial asymmetry, pathological fractures, and other distinctive signs. Radiological evaluation is the initial step in assessing the extent of fibrous replacement and bone involvement. This is typically observed as "ground glass" opacification with poorly defined margins. The gold standard for diagnosis remains histological examination, involving a biopsy of the lesion with adequate margins to observe the fusion between healthy bone and fibro-osseous tissue.
Park BY et al demonstrated that serum alkaline phosphatase is a significant marker for detecting the recurrence of fibrous dysplasia after incomplete resection in craniofacial cases [13].
Genetic testing for GNAS mutations can be conducted on lesional tissue or, in a less invasive manner, on peripheral blood samples. This test can be particularly helpful in cases of diagnostic uncertainty, given its relatively low statistical sensitivity1. In this specific case, the histopathological examination exhibited typical signs of fibrous dysplasia, leading the authors to question the utility of genetic testing, in line with existing literature.
Regarding surgical approaches, osteotomies can be performed using traditional rotating instruments, but these require caution as they generate heat and friction, potentially harming anatomical structures in the surgical area. Piezosurgery serves as a valid alternative due to its ultrasonic mechanism, which enables selective cutting of mineralized tissue while preserving soft tissues, including the periosteum and its associated blood supply. This preservation promotes faster recovery and reduces the incidence of post-operative complications, avoiding the need for secondary healing and the remodeling of bone and soft tissue. For these reasons, piezosurgery should be considered the preferred choice for performing osteotomies [14].
In the specific cases presented, piezosurgery allowed for an operation near the maxillary sinus in Case 1, preserving the Schneiderian membrane. It also resulted in less vibration and noise, which were crucial for ensuring compliance in the young patient and enhancing intra- and post-operative comfort. In Case 2, piezoelectric surgery was significant because the fibro-osseous lesion was located above the inferior alveolar canal, enabling the surgeon to work while respecting this important anatomical structure.
Treatment approaches for fibrous dysplasia vary depending on factors such as the extent of fibro- osseous tissue involvement, evident malformations, and clinical manifestations (e.g., pathological fractures, epiphora, diplopia, orbital dystopia, proptosis, strabismus, hearing loss, tinnitus, nasal obstruction), patient age, and the progression of the lesions [15].
Hereafter Valentini et al. proposed a revision of the Chen and Noodrof classification15 suggesting a more radical treatment for lesions arising in Zones 1 and 4. According to the authors, radical resection would be the only resolutive technique to achieve healing, considering the possibilities offered by modern reconstructive techniques that allow appropriate aesthetic and functional outcomes. However, this approach may result in longer recovery times [16].
Furthermore, the authors distinguished the fibro-osseous lesions on the evolution (stable and growing lesions) and on the patient’s age (pediatric and adult). In their decisional tree, they proposed for stable lesions without growth, both in pediatric and adult patients, osteoplasty as the preferred treatment. However, in cases of relapse, radical excision and reconstruction may be necessary.
In pediatric patients with growing lesions, the authors distinguished between large residual lesions, which are managed conservatively to reduce patient discomfort, and small residual lesions that require resection. In adult patients, growing lesions often necessitate demolitive surgery [16].
In 2022, Dalle Carbonare et al conducted a review of the literature on surgical management of fibro- osseous lesions, concluding that in absence of clinical signs and deformities, surgery is unnecessary for asymptomatic patients, who should be managed with expectant observation [17].
In 2019, Ni Y et al investigated the preoperative and postoperative quality of life of 24 patients with fibrous dysplasia of the mandible, comparing conservative and radical surgical treatments using the University of Washington Quality of Life (UW-QoL) questionnaire. Both surgical approaches led to improvements in pain, appearance, and mood. However, activities, recreation, and speech scores worsened in both groups, reflecting reductions in these areas. Notably, the conservative surgical treatment group showed no significant difference in chewing, possibly related to the minimal resection nature of the procedure. Based on their findings, the authors concluded that the treatment of craniofacial fibrous dysplasia should prioritize conservatism as much as possible [17].
Conclusions
The fibrous dysplasia is a rare pathological condition that often affects the craniofacial bones. The lesions associated with this disease are frequently asymptomatic, although in some cases they can cause functional issues due to nervous or vascular involvement. Radiographic assessment is essential for identifying the lesions, but a definitive diagnosis is only achieved through histopathological examination. As per the literature, given the benign nature of the diagnosed condition and the absence of patient discomfort or facial deformities, regular annual check-ups are recommended as surgical treatment is not warranted. There is a lack of literature regarding piezoelectric surgery and its application in the treatment and biopsy of fibrous dysplasia. However, within the constraints of the case report, it demonstrates a valid and alternative method that surpasses the limitations of traditional instrumentation.
Funding:
This research received no external funding.
Institutional Review Board Statement:
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of University of Genoa (protocol code 21084).
Informed Consent Statement:
Informed consent was obtained from all subjects involved in the study. Conflicts of Interest: The authors declare no conflict of interest.
References
- Neville BW, ed. (2002) Oral & Maxillofacial Pathology.
- Metwally T, Burke A, Tsai JY, Collins MT, Boyce AM (2016) Fibrous Dysplasia and Medication- Related Osteonecrosis of the Jaw. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg 74: 1983-1999.
- Davidova LA, Bhattacharyya I, Islam MN, Cohen DM, Fitzpatrick SG (2020) An Analysis of Clinical and Histopathologic Features of Fibrous Dysplasia of the Jaws: A Series of 40 Cases and Review of Literature. Head Neck Pathol 14: 353-361.
- Shaw SE, Chan CH (2023) Non-Odontogenic Tumors of the Jaws. In: StatPearls. StatPearls Publishing.
- Burke AB, Collins MT, Boyce AM (2017) Fibrous dysplasia of bone: craniofacial and dental implications. Oral Dis 23: 697-708.
- Riddle ND, Bui MM (2013) Fibrous dysplasia. Arch Pathol Lab Med 137: 134-138.
- Chen YR, Noordhoff MS (1990) Treatment of craniomaxillofacial fibrous dysplasia: how early and how extensive? Plast Reconstr Surg 86: 835-842; discussion 843-844.
- Pacino GA, Cocuzza S, Tonoli G, Rizzo PB, Tirelli G, et al. (2020) Jawbone fibrous dysplasia: retrospective evaluation in a cases series surgically treated and short review of the literature. Acta Bio-Medica Atenei Parm 92: e2021018.
- Alves N, de Oliveira RJ, Takehana D, Deana NF (2016) Recurrent Monostotic Fibrous Dysplasia in the Mandible. Case Rep Dent 2016:3920850.
- Pereira CCS, Gealh WC, Meorin-Nogueira L, Garcia-Júnior IR, Okamoto R (2014) Piezosurgery applied to implant dentistry: clinical and biological aspects. J Oral Implantol 40: 401-408.
- Pozzetti E, Garibaldi J, Cassinotto E, Figliomeno E, Merlini A (2023) Piezosurgery Removal of Mandibular Tori: A Case Series. Arch Clin Med Case Rep 07.
- Cai M, Ma L, Xu G, Gruen P, Li J, et al. (2012) Clinical and radiological observation in a surgical series of 36 cases of fibrous dysplasia of the skull. Clin Neurol Neurosurg 114: 254-259.
- Park BY, Cheon YW, Kim YO, Pae NS, Lee WJ (2010) Prognosis for craniofacial fibrous dysplasia after incomplete resection: age and serum alkaline phosphatase. Int J Oral Maxillofac Surg 39: 221-226.
- Giampaoli G, Chirumbolo S, Di Ceglie P, Bertossi D, Nocini R (2021) Frontal bone hyperostotic mass associated with fibrous dysplasia in a male patient with systemic lupus erythematosus (SLE). Int J Surg Case Rep 89:106564.
- Lee JS, FitzGibbon EJ, Chen YR, HJ Kim, LR Lustig, et al. (2012) Clinical guidelines for the management of craniofacial fibrous dysplasia. Orphanet J Rare Dis 7 :S2.
- Valentini V, Cassoni A, Terenzi V, Monaca MD, Fadda MT, et al. (2017) Our experience in the surgical management of craniofacial fibrous dysplasia: what has changed in the last 10 years? Acta Otorhinolaryngol Ital Organo Uff Della Soc Ital Otorinolaringol E Chir Cerv-facc 37: 436-443.
- Ni Y, Yang Z, Xu H, Lu P, Wang W, et al. (2019) Assessment of preoperative and postoperative quality of life of 24 patients with fibrous dysplasia of the mandible. Br J Oral Maxillofac Surg 57: 913-917.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.