Extravascular Implantable Cardioverter-Defibrillator (ICD) Systems in Children
by Mariusz Birbach1*, Monika Brzezińska2, Wojciech Lipiński1, Maria Miszczak-Knecht2, Agnieszka Kaszuba2, Katarzyna Pręgowska2, Maria Posadowska2, Katarzyna Bieganowska2
1Department of Pediatric Cardiothoracic Surgery, the Children’s Memorial Health Institute, Aleja Dzieci Polskich 20, 04-730 Warszawa, Poland
2Department of Cardiology, the Children’s Memorial Health Institute, Aleja Dzieci Polskich 20, 04-730 Warszawa, Poland
*Corresponding author: Mariusz Birbach, Department of Pediatric Cardiothoracic Surgery, Aleja Dzieci Polskich 20, 04-730 Warszawa, Poland
Received Date: 11 November 2024
Accepted Date: 16 November 2024
Published Date: 20 November 2024
Citation: van Vollenstee FA, Tintinger GR, van der Merwe MT (2024) Extravascular Implantable Cardioverter-Defibrillator (ICD) Systems in Children. J Surg 9: 11183 https://doi.org/10.29011/2575-9760.11183
Abstract
Aims: In the pediatric population, implantable cardioverter-defibrillator (ICD) therapy is indicated for primary and secondary prevention of sudden cardiac death (SCD). We present our experience with non-transvenous ICD system implantation in 28 out of 98 pediatric patients treated with an ICD in the Children’s Memorial Health Institute in Warsaw, Poland from August 2004 to March 2021.
Methods and results: We analyzed a group of 28 children at a mean age of 6.6 years with a mean weight of 22.7 kg at the time of implantation. The surgical technique included a small left anterolateral thoracotomy with the placement of sub-pleural ICD leads in 22 children (78%) and a sternotomy with pericardial ICD leads in 6 children (22%). In 5 of the 6 sternotomy patients, an ICD lead was placed through the epicardial transverse sinus; in 1 patient, an epicardial ICD patch was implanted.
Conclusion: There is no uniform methodology for the implantation of non-transvenous ICD systems in children. The method of implantation should be individually selected based on the patient’s weight and whether there are any congenital heart defects or an ICD system is implanted when repairing a heart defect. Most of our patients had a sub-pleural high-energy lead implanted with an ICD generator placed under the left costal arch. In our experience, such a lead and ICD generator placement is optimal in small children. Placing the defibrillator in the pocket under the left thoracic arch enables relatively safe and uncomplicated replacement when the battery is depleted.
Keywords: Children; Extra-cardiac; ICD; Implantation; Lead placement; Sub-pleural
Introduction
In the pediatric population, Implantable Cardioverter-Defibrillator (ICD) therapy is indicated for primary and secondary prevention of Sudden Cardiac Death (SCD), with an annual implantation rate of less than 1 per million. Children represent less than 1% of all patients with ICDs. The pediatric patients at risk of potentially life-threatening ventricular arrhythmias associated with cardiac arrest and death are those with channelopathies, surgically repaired Congenital Heart Disease (CHD), and cardiomyopathies. The transvenous ICD lead implant may be limited because of patient size or venous or cardiac anatomy.
Implanting an ICD in children may reduce the risk of SCD in patients suffering from life-threatening arrhythmias [1]. However, there is no uniform methodology for the implantation of ICDs in children, which may be a challenge, particularly in young children [2-4]. Congenital heart defects with intracardiac shunts, previous surgeries, and small or obstructed/blocked vessels [5] may have an impact on the implantation technique. Moreover, the rapid growth of the child, their physical activity, and the risk of damage to the lead may influence the choice of ICD implantation method. The technique of implantation should allow surgical-suitable ICD replacement in the future with relatively low risk. For the child, it should allow them to take part in normal activity, even certain sports [6]. No uniform consensus has been published in ICD system implantation methodology according to the bodyweight of the child, as has been done for pacemaker implantation [7]. The technique for implanting ICDs in our group of patients varied depending on their clinical status, but in most of the patients, we used the technique proposed by Swiss authors [8-11] with some modifications.
Methods
The implantation technique included a small anterolateral thoracotomy, usually via the 5th intercostal space with sub-pleural ICD lead placement in 22 children and sternotomy with pericardial ICD lead placement in 6 children (Figure 1A-D).
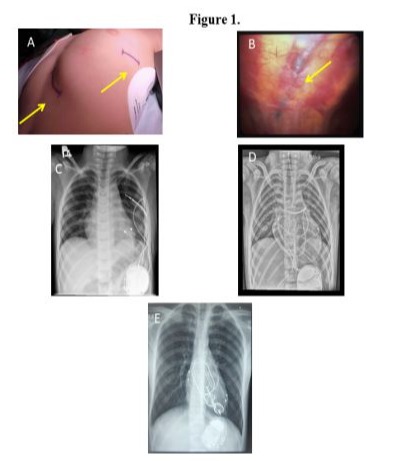
The operations were performed between 2004 and 2021 in our institution. Usually, the ICD lead was placed through the 4th intercostal space in a tunnel created under the parietal pleural wall (Figure 1B). We used ICD leads from St. Jude Medical in 22 patients and from Medtronic in the remaining 6 patients (Table 1).
|
Patient |
Age at
operation (years) |
Body weight
(kg) |
Diagnosis |
CPR |
Implantation
technique |
ICD lead
placement |
Pacing |
ICD lead |
Pacing lead |
ICD |
APpropriate
shocks |
Inappropriate
shocks |
|
1 |
10.01 |
32.00 |
LQTs |
yes |
thoracotomy |
subpleural |
DDD |
SJM 7122 |
Medtronic 4968 |
SJM Epic +DR |
no |
no |
|
2 |
5.07 |
19.00 |
TA, PA, DORV |
yes |
thoracotomy |
subpleural |
VVI |
SJM 7122 |
Medtronic 4968 |
SJM Atlas II+DR |
no |
no |
|
3 |
0.93 |
8.00 |
Brugada syndrome |
yes |
sternotomy |
transverse sinus |
DDD |
SJM 7122 |
Medtronic 4968 |
Medtronic Protecta DR |
yes |
no |
|
4 |
8.08 |
27.00 |
LQTS |
yes |
thoracotomy |
subpleural |
VVI |
SJM 7122 |
Medtronic 4968 |
Medtronic Protecta VR |
yes |
no |
|
5 |
11.55 |
31.10 |
TGA,VSD |
yes |
thoracotomy |
subpleural |
VVI |
SJM 7122 |
Medtronic 4968 |
Biotronik Iforia 3 VR |
no |
no |
|
6 |
4.79 |
18.30 |
LQTs |
yes |
thoracotomy |
subpleural |
VVI |
SJM 7042 |
Medtronic 4968 |
SJM Epic +VR |
yes |
no |
|
7 |
18.10 |
68.00 |
LQTs |
yes |
sternotomy
CPB |
transverse sinus |
DDD |
SJM 7120 |
Medtronic 4968 |
SJM Current +DR |
no |
no |
|
8 |
6.62 |
24.60 |
Brugada syndrome |
yes |
thoracotomy |
subpleural |
VVI |
SJM 7122 |
Medtronic 4968 |
Medtronic Evera S VR |
no |
no |
|
9 |
1.01 |
7.10 |
HCM, WPW syndrome |
yes |
sternotomy |
transverse sinus |
DDD |
Medtronic 6932 |
Medtronic 4968 |
Medtronic Marquis VR |
no |
yes |
|
10 |
7.24 |
25.00 |
RCM |
no |
thoracotomy |
subpleural |
VVI |
SJM 7122 |
Medtronic 4968 |
Medtronic Protecta VR |
yes |
no |
|
11 |
4.72 |
16.20 |
LQTs |
yes |
thoracotomy |
subpleural |
VVI |
SJM 7042 |
Medtronic 4968 |
SJM Epic +VR |
yes |
no |
|
12 |
9.22 |
25.50 |
LQTs |
yes |
thoracotomy |
subpleural |
DDD |
SJM 7122 |
Medtronic 4968 |
Medronic Evera S DR |
no |
no |
|
13 |
15.76 |
31.00 |
PA, VSD, RVOTO |
yes |
sternotomy
CPB |
transverse sinus |
DDD |
SJM 7122 |
Medtronic 4968 |
SJM Atlas II+DR |
yes |
no |
|
14 |
3.36 |
11.00 |
LQTs |
yes |
thoracotomy |
subpleural |
DDD |
SJM 7122 |
Medtronic 4968 |
SJM Atlas II VR, Medtronic Evera S
DR |
no |
yes |
|
15 |
8.03 |
23.50 |
LQTs |
yes |
sternotomy |
transverse sinus |
DDD |
Medtronic 6935 |
Medtronic 4968 |
Medtronic DDMC3D1 Evera MRI |
no |
no |
|
16 |
8.24 |
27.00 |
LQTs |
no |
thoracotomy |
subpleural |
VVI |
SJM 7122 |
Medtronic 4968 |
I-SJM Epic+VR, II-Protecta VR |
no |
no |
|
17 |
4.14 |
18.50 |
LQTs |
yes |
thoracotomy |
subpleural |
DDD |
SJM 7122 |
Medtronic 4968 |
SJM Current +DR |
yes |
yes |
|
18 |
7.97 |
32.10 |
LQTs |
no |
thoracotomy |
subpleural |
DDD |
Medtronic 6935 |
Medtronic 4968 |
Medtronic Evera S DR |
yes |
no |
|
19 |
5.50 |
20.00 |
LQTs |
yes |
thoracotomy |
subpleural |
DDD |
SJM 7122 |
Medtronic 4968 |
SJM Current +DR |
no |
no |
|
20 |
2.21 |
13.00 |
RCM |
yes |
thoracotomy |
subpleural |
VVI |
SJM 7122 |
Medtronic 4968 |
Medtronic Evera S VR |
yes |
no |
|
21 |
11.05 |
35.00 |
CPVT |
yes |
thoracotomy |
subpleural |
DDD |
SJM 7122 |
Medtronic 4968 |
Medtronic Evera S DR |
no |
no |
|
22 |
2.88 |
14.00 |
CPVT |
yes |
thoracotomy |
subpleural |
DDD |
SJM 7122 |
Medtronic 4968 |
Medtronic Protecta DR |
no |
no |
|
23 |
12.24 |
45.00 |
LQTs |
yes |
sternotomy
CPB |
epicardial patch |
DDD |
Medtronic 6721S |
Medtronic 4968 |
Biotronik Lexos DR-T |
yes |
no |
|
24 |
2.34 |
12.10 |
LQTs |
yes |
thoracotomy |
subpleural |
VVI |
SJM 1572 |
Medtronic 4968 |
SJM Epic +VR |
yes |
no |
|
25 |
5.18 |
17.90 |
LQTs |
yes |
thoracotomy |
subpleural |
VVI |
SJM 7122 |
Medtronic 4968 |
Medtronic Evera S VR |
no |
no |
|
26 |
0.55 |
7.20 |
LQTs |
yes |
thoracotomy |
subpleural |
VVI |
Medtronic 6937 |
Medtronic 4968 |
SJM Fortify VR |
no |
no |
|
27 |
0.10 |
4.80 |
SQTs |
yes |
thoracotomy |
subpleural |
VVI |
Medtronic 6937 |
Medtronic 4968 |
SJM Fortify Assura VR |
no |
no |
|
28 |
7.55 |
23 |
LQTs |
yes |
thoracotomy |
subpleural |
DDD |
SJM 7122 |
Medtronic 4968 |
Medtronic Evera S DR |
No |
no |
LQTs: long QT syndrome; TA: tricuspid atresia; PA: pulmonary atresia; DORV: double outlet right ventricle; TGA: transposition of great arteries; VSD: ventricular septal defect; HCM: hypertrophic cardiomyopathy; WPW: Wolff-Parkinson-White syndrome; RCM: restrictive cardiomyopathy; RVOTO: right ventricular outflow tract obstruction; CPVT: catecholaminergic polymorphic ventricular tachycardia, SQTs: short QT syndrome.
Table 1: Baseline Preoperative and Operative Characteristics.
In 3 of the 6 sternotomy patients, the ICD was implanted during cardiopulmonary bypass to repair heart defects, while the remaining 3 patients had an ICD implanted without a cardiopulmonary bypass. In 5 of the 6 sternotomy patients, the ICD lead was placed through the epicardial transverse sinus; an epicardial ICD patch was implanted in 1 patient (Figure 1D,E). All of our patients required pacing leads. We used dual-chamber pacing in 15 children and single-chamber pacing in the remaining 13 children (Figure 2).
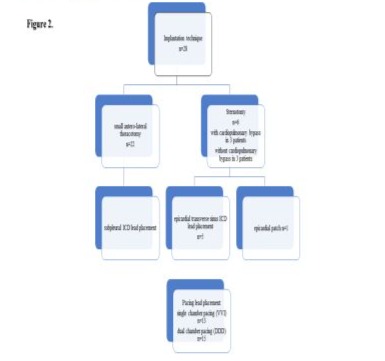
To place the pacing lead, the pericardium was opened carefully and according to the anatomy of the phrenic nerve, parallel to its course. The apex and wall of the left ventricle were exposed, as was the appendage of the left atrium, where necessary. The pacing lead was fixed on the epicardial surface of the left ventricle according to the anatomy of the left coronary artery and its branches, especially in small children. If a patient required dual-chamber pacing, the atrial pacing lead was fixed to the surface of the left atrial appendage. We used pacing leads from Medtronic in all 28 patients (Table 1). Ethicon Prolene 6/0 and 5/0 monofilament sutures were used to affix the pacing wires. The ventricular and atrial leads were tested for sensing and pacing thresholds; the impedance of the pacing and ICD lead coil were measured. An ICD generator was placed in a pocket created through a subcostal incision under the left thoracic rib arch, below the diaphragm and above the peritoneum. The ends of the pacing wires, as well as the ICD lead, were translocated from the pleural cavity to the ICD pocket. When connected to ICD, the device was affixed and sutured to the left thoracic rib arch using nonabsorbable Ethicon Ethibond polyester sutures to avoid the device migrating (Figure 1C,D,E). At the end of the procedure, a test of the defibrillation thresholds was performed. We used ICDs from St. Jude Medical, Medtronic, and Biotronic (Table 1).
A chest tube sized according to the patient’s weight was placed in the left pleura and the wound was closed. The chest tube was usually removed on the next postoperative day. In the case of ICD lead implantation through the sternotomy, the ICD lead was placed in the transverse sinus with three fixation points to the surrounding tissues: in the area of the superior vena cava, in the pericardium at the pulmonary artery trunk, and in the pericardium at the apex of the heart. Pacing leads were implanted either on the right atrial surface and on the anterior surface of the right ventricle or on the left ventricular and left atrial surfaces. As with the sub-pleural technique, an ICD pocket was created under the left costal arch (Figure 1C,D,E).
Results
Out of the 98 pediatric ICD implantations in our institution, we analyzed the 28 pediatric patients who had undergone extravascular implantation of an ICD with the placement of a sub-pleural or epicardial ICD lead between 2004 and 2021. There were 17 male and 11 female patients with a mean age of 6.6 years and a median age of 6.0 years. The oldest patient was 18.1 years, while the youngest was 0.1 years old (37 days). The mean body weight of the patients was 22.7 kg with a median of 21.5 kg (4.8-68). The mean follow-up for our group was 7.6 years. Channelopathies were diagnosed in 22 children and cardiomyopathies in 3. In 3 patients, an ICD system was implanted during a surgery for congenital heart disease. The patients’ characteristics and diagnoses are presented in Table 2.
|
Patients’
characteristics and diagnosis n=28 |
|
|
male
(n) |
17 |
|
female
(n) |
11 |
|
age
at implantation (years) |
6.6
(0.1 - 18.1) |
|
median
(years) |
6 |
|
body
weight (kg) |
22.7
(4.8 - 68) |
|
median
(kg) |
21.5 |
|
Diagnosis
n=28 |
|
|
Long
QT syndrome (LQTs) |
17 |
|
Short
QT syndrome |
1 |
|
Congenital
heart defect* |
3 |
|
Brugada
syndrome |
2 |
|
Catecholaminergic
polymorphic ventricular tachycardia (CPVT) |
2 |
|
Restrictive
cardiomyopathy |
2 |
|
Hypertrophic
cardiomyopathy |
1 |
|
*Congenital
heart defect patients n=3 |
|
|
*tricuspid
atresia, pulmonary atresia, double outlet right ventricle (TA, PA, DORV) after
the Fontan procedure |
|
|
*transposition
of great arteries, ventricular septal defect (TGA, VSD) |
|
|
*pulmonary
atresia, ventricular septal defect, right ventricular outflow tract
obstruction (PA, VSD, RVOTO) |
|
The ICD system was secondary prevention in 25 patients (89%) and primary prevention in 3 (11%). Cardiopulmonary resuscitation (CPR) was required in all 25 patients from the secondary prevention group who underwent cardiac arrest. The patients’ follow-up is presented in Table 3.
|
Patient |
Age at
operation (years) |
Bodyweight (kg) |
Diagnosis |
Follow up (years) |
APpropriate
shocks |
InaPpropriate
shocks |
ICD device
replacement (years post-implantation) |
ICD lead
replacement (years post-implantation) |
|
1 |
10.01 |
32.00 |
LQTs |
12.63 |
no |
no |
5,19 |
no |
|
2 |
5.07 |
19.00 |
TA, PA, DORV |
11.85 |
no |
no |
4.54 |
no |
|
3 |
0.93 |
8.00 |
Brugada syndrome |
4.87 |
yes |
no |
no |
no |
|
4 |
8.08 |
27.00 |
LQTS |
4.99 |
yes |
no |
no |
no |
|
5 |
11.55 |
31.10 |
TGA,VSD |
6.20 |
no |
no |
no |
no |
|
6 |
4.79 |
18.30 |
LQTs |
13.44 |
yes |
no |
3.69 |
no |
|
7 |
18.10 |
68.00 |
LQTs |
10.64 |
no |
no |
no |
no |
|
8 |
6.62 |
24.60 |
Brugada syndrome |
3.13 |
no |
no |
no |
no |
|
9 |
1.01 |
7.10 |
HCM, WPW syndrome |
17.40 |
no |
yes |
no |
no |
|
10 |
7.24 |
25.00 |
RCM |
4.97 |
yes |
no |
no |
no |
|
11 |
4.72 |
16.20 |
LQTs |
9.18 |
yes |
no |
1.02/4/4.19 (3x) |
1.02 |
|
12 |
9.22 |
25.50 |
LQTs |
4.09 |
no |
no |
no |
no |
|
13 |
15.76 |
31.00 |
PA, VSD, RVOTO |
9.31 |
yes |
no |
no |
no |
|
14 |
3.36 |
11.00 |
LQTs |
11.62 |
no |
yes |
3.69/3.41/0.98 (3x) |
8.08 |
|
15 |
8.03 |
23.50 |
LQTs |
0.98 |
No |
noi |
no |
no |
|
16 |
8.24 |
27.00 |
LQTs |
12.44 |
no |
no |
7.58 |
no |
|
17 |
4.14 |
18.50 |
LQTs |
9.17 |
yes |
yes |
2.69 |
2.69 |
|
18 |
7.97 |
32.10 |
LQTs |
4.8 |
yes |
no |
no |
no |
|
19 |
5.50 |
20.00 |
LQTs |
8.09 |
no |
no |
no |
no |
|
20 |
2.21 |
13.00 |
RCM |
4.47 |
yes |
no |
no |
no |
|
21 |
11.05 |
35.00 |
CPVT |
4.65 |
no |
no |
no |
no |
|
22 |
2.88 |
14.00 |
CPVT |
5.35 |
no |
no |
no |
no |
|
23 |
12.24 |
45.00 |
LQTs |
14.98 |
yes |
no |
3.71 |
no |
|
24 |
2.34 |
12.10 |
LQTs |
3.25 |
yes |
no |
0.07/3.18 (2x) |
no |
|
25 |
5.18 |
17.90 |
LQTs |
4.57 |
no |
no |
no |
no |
|
26 |
0.55 |
7.20 |
LQTs |
8.17 |
no |
no |
no |
no |
|
27 |
0.10 |
4.80 |
SQTs |
7.63 |
no |
no |
no |
no |
|
28 |
7.55 |
23 |
LQTs |
0.76 |
no |
no |
no |
no |
LQTs: long QT syndrome; TA: tricuspid atresia; PA: pulmonary atresia; DORV: double outlet right ventricle; TGA: transposition of great arteries; VSD: ventricular septal defect; HCM: hypertrophic cardiomyopathy; WPW: Wolff-Parkinson-White syndrome; RCM: restrictive cardiomyopathy; RVOTO: right ventricular outflow tract obstruction; CPVT: catecholaminergic polymorphic ventricular tachycardia, SQTs: short QT syndrome
Table 3: Patients’ Follow Up.
There were no major complications during the procedure or the postoperative period. In 1 patient, operated on at the age of 3 years, we observed displacement of the ICD lead from the sub-pleural channel that was created. The electrode measurements remained unchanged during the follow-up. The patient was operated on 8 years after the first surgery due to damage to the ventricular pacing lead. The surgery was performed via a left anterolateral rethoracotomy. The ventricular pacing lead and the ICD lead were replaced with new ones, a left atrial pacing lead was additionally implanted, and the ICD was changed due to battery depletion. The postoperative course was uneventful (Figure 3 A,B). The youngest patient, who suffered from short QT syndrome, underwent surgery on their 37th day of life. An ICD was placed in the pleural cavity because of the patient’s size (Figure 3C).
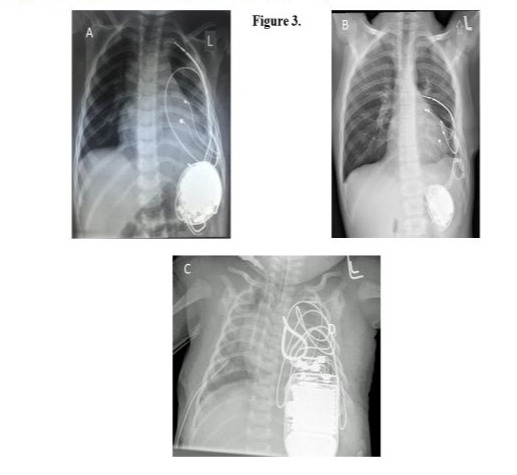
Perioperative antibiotic prophylaxis with cloxacillin, and recently with cefazolin, was administered in every patient. There was 1 infection complication that required removal of the ICD system 5 weeks post-implantation due to a systemic Cryptococcus neoformansfungal infection. This infection was not related to the ICD implantation. A new ICD system was implanted intravascularly 1 year after the fungal infection was diagnosed and the treatment initiated. This child was diagnosed with transposition of the great arteries and had undergone an arterial switch operation 11 years earlier. After being discharged from the hospital, all patients remained under the care of the outpatient cardiology clinic. The first appointment was scheduled 4 to 8 weeks postoperative, and then every 5 to 6 months depending on the clinical condition or whenever appropriate or inappropriate shock of the ICD was detected.
In the follow-up, 11 patients had appropriate shocks and 3 patients had inappropriate shocks (Table 3). Of the 11 patients with appropriate shocks, 9 had undergone implantation as primary prevention and 2 as secondary prevention. Two patients with inappropriate shocks were implanted with an ICD system as primary prevention; both of them had lead damage during follow-up that required a lead exchange. Nine patients (32%) out of 28 required reoperation and ICD replacement due to battery depletion a mean of 5.19 years after the first implantation. Of these 9 patients, 3 patients underwent 2 replacement procedures and 1 patient had 3 replacement procedures in the postoperative follow-up. Three patients required concomitant ICD lead replacement due to lead damage between 1 and 8 years after the first surgery. Three patients required removal of the ICD system, the first patient only 5 weeks after implantation due to a systemic fungal infection. The second patient required removal of the ICD 13 years post-implantation, after a successful ablation procedure and no appropriate shocks detected in the follow-up period. A third patient had their ICD removed during a cardiac transplant which was needed because of restrictive cardiomyopathy 1 year after ICD implantation. There were 2 deaths in our study group due to electric storms 8 years and 3 years after ICD implantation. One of these patients had undergone 3 ICD replacements, while the other had 2 ICD replacements - all due to appropriate shocks. Both patients suffered from LQT syndrome and both had undergone sympathectomy, 1 patient left-sided and the other bilateral.
Discussion
There is no uniform methodology for the implantation of ICD systems in children. Transvenous lead implantation is not always possible and is not the best option in very young children. The majority of pediatric patients with LQTs or Brugada syndrome are under risk of SCD due to ventricular fibrillation and require an ICD system, as in our patients. In our patients implanted with extravascular ICD leads, the technique included anterolateral mini-thoracotomy via the 5th intercostal space, with sub-pleural ICD lead placement in 22 children and sternotomy in 6 children. Small anterolateral thoracotomy was a reasonably safe procedure for ICD and pacing lead implantation in the children with no major complications from our cohort. The pocket created under the left thoracic rib arch for the ICD generator gives reasonably good surgical access for eventual replacement of the ICD when the battery becomes depleted. Moreover, it is hidden under the ribs and it does not bulge out from under the skin, as may occur when it is placed in the rectus abdominis muscle sheath or subcutaneously. This yields promising cosmetic results, as the ICD is covered by the costal arch and in this way also protects the device and the leads from damage during physical activity. Electrode damage is more common in children than in adults, and amounts to up to 21% [12]. Moreover, such a position of the ICD in relation to the position of the lead gives an optimal electrical field for the implanted system.
Other techniques of ICD placement have been proposed, such as between the pericardium and the diaphragm in a horizontal position underneath the heart. Such placement provides a good electrical field, but may result in a more invasive procedure when the ICD generator must be replaced due to battery depletion [9]. Alternative methods of ICD lead implantation in subcutaneous tissue have been proposed in children with some caution [13, 14]. This method may be advisable for a select group of patients without the need for pacing electrode implantation [3]. There were no such patients in our group. An alternative to such procedures would be to make available ICD and pacing leads and the ICD itself in sizes intended for pediatric patients, though these are not currently available.
Conclusion
Our study presents a single-center experience in extravascular sub-pleural and pericardial ICD lead implantation in pediatric patients. Such access seems to be optimal in children requiring ICD implantation when they are too small for the intravascular implantation of multiple electrodes. Based on our cohort of 28 patients, we can conclude that sub-pleural or epicardial lead placement, together with the pericardial placement of both ventricular and atrial pacing leads, may be the optimal approach for children. Placing the ICD generator in a pocket created under the left costal arch allows for relatively safe and uncomplicated replacement and offers a good cosmetic effect that avoids bulging from the ICD in the subcutaneous tissue. The method of extravascular implantation of ICD systems in children that we used showed full effectiveness of defibrillation, relatively few damaged electrodes and a small number of inappropriate shocks. Unfortunately, there is not much literature data on ICD implantation in children. This makes it impossible to compare the results of implantation methods in different centers and limits the choice of the most appropriate method of implantation in pediatric patients
References
- Silka MJ, Kron J, Dunnigan A, Dick M 2nd (1993) Sudden cardiac death and the use of implantable cardioverter-defibrillators in pediatric patients. The Pediatric Electrophysiology Society 87: 800-807.
- Watanabe H, Hayashi J, Haga M, Saito M, et al. (2001) Successful implantation of a cardioverter defibrillator in an infant. Ann Thorac Surg 72: 2125-2127.
- Kriebel T, Ruschewski W, Gonzalez y Gonzalez M, Walter K, Kroll J, et al. (2006) ICD Implantation in Infants and Small Children: The Extracardiac Technique, Pacing Clin Electrophysiol 29: 1319-1325.
- Stephenson EA, Batra AS, Knilans TK, M Gow R, Gradaus R, et al. (2006) A multicenter experience with novel implantable cardioverter defibrillator configurations in the pediatric and congenital heart disease population. J Cardiovasc Electrophysiol 17: 41-46.
- Cannon BC, Friedman RA, Fenrich AL, Fraser C, McKenzie ED, et al. (2006) Innovative techniques for placement of implantable cardioverter-defibrillator leads in patients with limited venous access to the heart. Pacing Clin Electrophysiol 29: 181-187.
- Saarel EV, Law I, Berul CI, Ackerman M J, Kanter R J, et al. (2018) Safety of Sports for Young Patients With Implantable Cardioverter-Defibrillators. Circ Arrhythm Electrophysiol 11.
- Brugada J, Blom N, Sarquella-Brugada G, Blomstrom-Lundqvist C, Deanfield J, et al. (2013) Pharmacological and non-pharmacological therapy for arrhythmias in the pediatric population: EHRA and AEPC-Arrhythmia Working Group joint consensus statement. European Heart Rhythm Association; Association for European Paediatric and Congenital Cardiology. Europace 15: 1337-1382.
- Dodge-Khatami A, Kadner A, Dave H, Mariette R, Prêtre R, et al. (2005) Left heart atrial and ventricular epicardial pacing through a left lateral thoracotomy in children: a safe approach with excellent functional and cosmetic results. Eur J Cardiothorac Surg 28: 541-545.
- Bauersfeld U, Tomaske M, Dodge-Khatami A, Rahn M, Kellenberger CJ, et al. (2007) Initial experience with implantable cardioverter defibrillator systems using epicardial and pleural electrodes in pediatric patients. Ann Thorac Surg 84: 303-305.
- Tomaske M, Prêtre R, Rahn M, Bauersfeld U (2008) Epicardial and pleural lead ICD systems in children and adolescents maintain functionality over 5 years. Europace 10: 1152-1156.
- Winkler F, Dave H, Weber R, Gaas M, Balmer C (2018) Long-term outcome of epicardial implantable cardioverter-defibrillator systems in children: results justify its preference in paediatric patients. Europace 20: 1484-1490.
- Alexander ME, Cecchin F, Walsh EP, Triedman JK, Bevilacqua LM, et al. (2004) Implications of implantable cardioverter defibrillator therapy in congenital heart disease and pediatrics. J Cardiovasc Electrophysiol 15: 72-76.
- Jarman JW, Lascelles K, Wong T, Markides V, Claque JR, et al. (2012) Clinical experience of entirely subcutaneous implantable cardioverter-defibrillators in children and adults: cause for caution. Eur Heart J 33: 1351-1359.
- Jarman JW, Todd DM (2013) United Kingdom national experience of entirely subcutaneous implantable cardioverter-defibrillator technology: important lessons to learn. Europace 15: 1158-1165
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.