Extensive Peritumoral Retraction Clefts in Post-Neoadjuvant Breast Carcinoma are Associated with Aggressive Biology and Poor Prognosis
by Teresa CF. Gutman1*, Angela S. Rezende2, Karin S. Cunha3, Fabiana R. Rodrigues3, Vania GS. Lopes3
1Department of Pathology, Gaffrée Guinle University Hospital - Federal University of the State of Rio de Janeiro – UNIRIO, Rio de Janeiro, Brazil.
2Division of Pathology, Brazilian National Cancer Institute (INCA), Rio de Janeiro, R.J, Brazil.
3Department of Pathology, Fluminense Federal University (UFF), Niterói, Brazil.
*Corresponding author: Teresa CF. Gutman, Federal University of the State of Rio de Janeiro (UNIRIO), Department of Pathology, Mariz e Barros Street 775, Tijuca, Rio de Janeiro, Brazil.
Received Date: 04 September, 2025
Accepted Date: 10 September, 2025
Published Date: 12 September, 2025
Citation: Gutman TCF, Rezende AS, Cunha KS, Rodrigues FR, Lopes VGS (2025) Extensive Peritumoral Retraction Clefts in Post-Neoadjuvant Breast Carcinoma are Associated with Aggressive Biology and Poor Prognosis. J Oncol Res Ther 10: 10303. https://doi.org/10.29011/2574-710X.10303
Abstract
Peritumoral retraction clefts (RCs) in breast carcinoma are increasingly recognized as indicators of biological aggression rather than mere artifacts. This study investigates the prevalence and prognostic significance of extensive RCs in invasive breast carcinoma following neoadjuvant chemotherapy. Here, a retrospective cohort of 100 neoadjuvant chemotherapy -treated invasive breast carcinoma cases was analyzed. The extent of RCs on H&E-stained sections was quantified and categorized as <70% or ≥70% of tumor involvement. Associations with clinicopathological features, including molecular subtypes, lymphovascular invasion (EL) confirmed by D2-40 immunohistochemistry, and survival outcomes were evaluated using appropriate statistical methods. Extensive RCs (≥70%) were identified in 42% of cases and demonstrated significant correlations with younger age (p=0.027), HER2 positivity (p=0.018), high Ki-67 index (p=0.038), and the presence of EL (p<0.001). Crucially, no cases with extensive RCs were free of axillary lymph node metastases (ypN0). Patients with extensive RCs had a markedly shorter mean overall survival (4.5 years) compared to those with less prominent RCs (8 years). Multivariate analysis confirmed advanced nodal stage (ypN2, ypN3) as the strongest independent predictor of mortality. In conclusion, extensive peritumoral retraction clefts are a robust histological marker of aggressive tumor biology, lymphatic dissemination, and poor survival in invasive breast carcinoma, including in the post- neoadjuvant chemotherapy setting. Their assessment provides valuable prognostic information that could help identify high-risk patients for more intensive management.
Keywords: Retraction clefts; Breast cancer; Neoadjuvant therapy; Prognosis; Lymphovascular invasion; D2-40.
Abbreviations
RCs - Retraction Clefts
H&E - Hematoxylin and Eosin
EL - Lymphovascular Invasion
ER - Estrogen Receptor
PR - Progesterone Receptor
HER2 - Human Epidermal Growth Factor Receptor 2
Ki-67 - Cell Proliferation Index
IHC - Immunohistochemistry
NST - No Special Type
IDC - Infiltrating Ductal Carcinoma
DCIS - Ductal Carcinoma In Situ
FISH - Fluorescence In Situ Hybridization
TMA - Tissue Microarray
AES - 3-Aminopropyltriethoxysilane
ROC - Receiver Operating Characteristic
SD - Standard Deviation
ypT - Post-chemotherapy Pathological Tumor Size
ypN - Post-chemotherapy Pathological Nodal Stage
ypM - Post-chemotherapy Pathological Distant Metastasis
HR - Hazard Ratio
CI - Confidence Interval
pCR - Pathological Complete Response
RCB - Residual Cancer Burden.
Introduction
The presence of artifacts known as peritumoral retraction clefts (RCs), also referred as retraction fissures or retraction artifacts, is frequently observed in carcinomas of various organs and systems, including the breast, prostate, gastrointestinal tract, urinary tract, oral mucosa, skin, and stroma, among others [1-6]. Previously, the RCs observed in invasive carcinomas were interpreted by pathologists as retraction artifacts resulting from tissue fixation and slide preparation stained with hematoxylin and eosin (H&E) [7].
The mechanism underlying the formation of retraction clefts is not fully understood [8]. While some studies have suggested that these clefts may be fixation artifacts, their presence in frozen sections of invasive breast carcinoma indicates that they represent true spaces rather than being solely artifacts of formalin fixation [9]. These artifacts were described as clear spaces that separate tumor cell nests or cords from the adjacent stroma, typically following the rounded or angular contours of these structures and without endothelial lining [10].
Currently, the formation of RCs is understood to be a morphological sign of underlying biological changes rather than a mere artifact. These clefts are a consequence of phenotypic changes in the tumor stroma, where cells often adopt a myofibroblastic appearance, and may reflect stromal remodeling linked to lymphangiogenesis within the tumor tissue [11].
Functionally, RCs are an indicator of aggressive tumor biology and are thought to play a direct role in lymphatic spread. They may represent an early stage of vascular invasion, where the process of transforming mesenchymal cells into fully formed lymphatic endothelial cells is not yet complete [12]. The apparent stromal retraction around carcinoma cells signifies critical alterations in tumor-stroma interactions. These changes facilitate lymphatic dissemination, tumor progression, and the proliferation and endothelialization of lymphatic-like spaces [12].
The origin of RCs is still debated. Some studies associate them with the loss of a basal cell layer in breast and prostate carcinomas, while others propose they are formed by an abnormal, reactive stroma surrounding the tumor nest [3,6]. Despite these findings, the precise biological mechanism and its full clinical significance remain unknown.
Supporting the stromal change hypothesis, immunohistochemical (IHC) studies have demonstrated a strong correlation between RCs and myofibroblastic phenotypic changes in stromal cells. In one study, this was evidenced using anti-CD34 and anti-vimentin antibodies and was observed in 92% of cases of invasive breast carcinoma of no special type [14].
Clinically, the extent of RCs has been graded and shows a significant correlation with adverse pathological features in invasive breast carcinoma. This association suggests that prominent RCs are a marker of more aggressive disease and a worse patient prognosis [15,16].
A study of 199 infiltrating ductal carcinoma (IDC) and 188 ductal carcinoma in situ (DCIS) cases used H&E-stained slides to categorize RCs on a 0 to 4+ scale based on the percentage of tumor involvement (1+: 1-25%; 2+: 26-50%; 3+: 51-75%; 4+: 76-100%). RCs were far more prevalent and extensive in invasive cancer, being observed in 84.4% of IDC cases compared to only 16% of DCIS cases (P < 0.0001). Notably, significant retraction (scored as 2+ or greater) was found in 38.7% of IDC cases but in merely 0.53% of DCIS specimens. The authors proposed that this stark difference suggests RCs represent true involvement of pre-lymphatic spaces rather than a fixation artifact [13].
The clinical significance of extensive RCs is a subject of ongoing research. Several studies have reported that prominent RCs are significantly associated with aggressive features, including larger tumor size, high histological grade, lymphovascular invasion, axillary lymph node metastasis, and worse overall and disease-free survival [17]. Some research indicates that extensive RCs are an independent predictive factor for poor outcomes, regardless of lymph node status [18]. However, this view is not universal; another study found no convincing association between RCs and clinicopathological characteristics or prognosis, suggesting that divergent results in the literature may stem from differences in stratification methods and racial demographics of the study populations [19].
The development of specific lymphatic endothelial markers, such as podoplanin (D2-40), has been crucial for this field. Podoplanin is a transmembrane glycoprotein that is highly expressed in lymphatic capillary endothelium but not in blood vessels, allowing for a clear distinction between lymphatic invasion and retraction artifacts [20,21]. It is important to note that D2-40 is also expressed in other normal and neoplastic tissues, including mesothelial, basal, and myoepithelial cells, which requires careful morphological interpretation to avoid false positives [22]. The confirmed presence of D2-40-positive tumor emboli is significantly associated with a poorer prognosis and reduced disease-free survival [23]. This growing body of evidence underscores the importance of investigating tumor lymphangiogenesis and metastatic pathways to inform the development of novel anti-tumor therapies [24].
In this context, the present study aims to investigate and correlate the extent of RCs with prognosis and clinicopathological characteristics in invasive breast carcinoma following neoadjuvant chemotherapy. We conducted a detailed histopathological and prognostic evaluation of RCs using both H&E staining and immunohistochemical analysis.
Materials and Methods
This retrospective analytical study utilized a convenience sample of 100 patients with invasive breast carcinoma who received neoadjuvant therapy at a Brazilian public hospital between 2009 and 2011.
Inclusion criteria comprised patients of both sexes, aged 18 years or older, with available clinical data from requisitions and/or medical records, and accessible paraffin-embedded tissue blocks of adequate quality and quantity for immunohistochemical analysis. All H&E stained histological slides from each case were reviewed to identify representative tumor areas. Retraction clefts were not assessed in intratumoral regions showing treatment-related response, as these areas often contained artifactual spaces secondary to chemotherapy effects. Cases exhibiting clear spaces surrounding tumor cell clusters with reverse polarity, characteristic of invasive micropapillary carcinoma, were excluded from the analysis.
Immunohistochemical staining for D2-40 was performed on all cases to confirm the presence or absence of true lymphovascular emboli. Manual tissue microarray (TMA) construction was subsequently carried out [25]. This involved extracting three 3-mm cylindrical cores perpendicular to the tissue surface from each donor block, resulting in a total of 300 cores from 100 patients. For orientation and spatial mapping, ten control cores from non-neoplastic tissue were included in each recipient TMA block. Standard technical procedures were followed; histological sections were mounted on slides coated with 2% 3-aminopropyltriethoxysilane (AES). Immunostaining was performed using a monoclonal mouse anti-human podoplanin antibody (DAKO®), with antigen retrieval conducted in citrate buffer (pH 6.0) overnight at 6°C. Positive staining was defined as strong linear immunoreactivity localized to the membrane and cytoplasm of endothelial cells.
The TMA slides were digitally scanned using an Aperio® ScanScope CS system, generating high-resolution svs image files. The surface area of each tissue core was calculated as approximately 7 mm² (A = π × (d/2)² = 3.142 × (1.5)²), yielding a total evaluable area of 21 mm² per case.
Lymphovascular emboli were defined as tumor cell clusters within D2-40-positive endothelial-lined spaces. Retraction clefts were identified as clear, stroma-free spaces surrounding nests or cords of tumor cells, lacking an endothelial lining and showing negative D2-40 staining. These were evaluated across all histological sections by estimating the proportion of tumor cell nests surrounded by clefts in 10 microscopic fields (20× objective magnification). An average score per case was calculated and categorized as follows: high (≥70%), moderate (11–69%), mild (6–10%), or negative (<5%), in accordance with established criteria. [13].All H&E and immunohistochemical slides were independently evaluated by three pathologists to assess the extent of peritumoral retraction. Interobserver agreement was excellent, with a Fleiss' kappa coefficient of 0.82, indicating a high level of concordance in the assessment of RC extent.
Estrogen receptor (ER), progesterone receptor (PR), cell proliferation index (Ki-67), human epidermal growth factor receptor 2 (HER2) status were retrieved from medical records, based on original diagnostic IHC assays. HER2-equivocal cases (score 2+) were reflex-tested using fluorescence in situ hybridization (FISH), adhering to ASCO/CAP guidelines. A Ki-67 index ≥14% was used to define high proliferative activity, as per St. Gallen International Expert Consensus recommendations [26].
Data were compiled in Microsoft Excel® and analyzed using SPSS version 25.0, with a statistical significance threshold of p < 0.05. Receiver operating characteristic (ROC) curve analysis was employed to determine the optimal cutoff value (70%) for RC extent using overall survival as the state variable. Categorical variables were compared using Pearson’s chi-square test. Survival outcomes were analyzed using the Kaplan–Meier method, with comparisons between groups stratified by RC extent (<70% vs. ≥70%) and lymphovascular invasion (positive vs. negative) performed using the log-rank test. Due to heterogeneity in treatment regimens among patients, the potential confounding effects of therapy could not be fully adjusted for in the statistical analyses.
Results
The demographic data revealed a patient age range of 26 to 84 years, with a mean age of 57 years. The cohort was predominantly female (98%). Regarding tumor size, the majority of tumors (62 patients) were classified as ypT2 (2-5 cm), followed by ypT3 (30 patients), with only eight cases being ypT1.
The most frequent histopathological type was invasive breast carcinoma of no special type (NST; ductal, 86%), followed by lobular (7%), mucinous (3%), invasive papillary (2%), mixed (lobular and ductal, 1%), and metaplastic (1%) carcinomas. Histological grade II (moderately differentiated) was the most prevalent (47%), followed by grade III (23%). The histological grade could not be determined in 13% of cases due to treatment-related artifacts that compromised accurate assessment.
The extent of RCs varied widely, from 6% to 84% of the tumor perimeter. An optimal cut-off value of 70% for defining "extensive RCs" was determined using ROC curve analysis against key clinicopathological characteristics. Based on this, cases were stratified into two groups: RC <70% (58% of cases) and RC ≥70% (42% of cases).
Among invasive carcinomas with extensive RCs (≥70%), 41% were of the NST subtype. Conversely, within the group with less prominent RCs (<70%), 43% were also of the NST subtype. Negative RCs were observed in 13% of cases; these were predominantly associated with special-type carcinomas (8%) or with extensive post-chemotherapy fibrosis in NST carcinomas (5%).
The presence of extensive RCs (≥70%) showed statistically significant correlations with several aggressive tumor features. These included younger patient age (56.5%, p = 0.027), HER2-positive status (57%, p = 0.018), the presence of lymphovascular emboli (81%, p < 0.001), and a high cellular proliferation index (Ki67 ≥14%, 73.8%, p = 0.038). Notably, a subset of cases (20%) with less prominent RCs (<70%) also had an unfavorable outcome; these were associated with HER2 positivity despite the absence of visible vascular emboli.
A highly significant finding was that no extensive RCs were observed in the axillary metastasis-free group (ypN0 = 28%); this group was strongly associated with a lower RC extent (p < 0.001). The absence of extensive RCs demonstrated a 100% negative predictive value for axillary lymph node metastasis in this cohort. A histopathological pattern of small neoplastic nests surrounded by extensive RCs (≥70%) was correlated with lymphovascular invasion and a poorer prognosis. In contrast, cases featuring larger tumor nests with less prominent RCs (<70%) were associated with a more favorable prognosis (Figure 1).
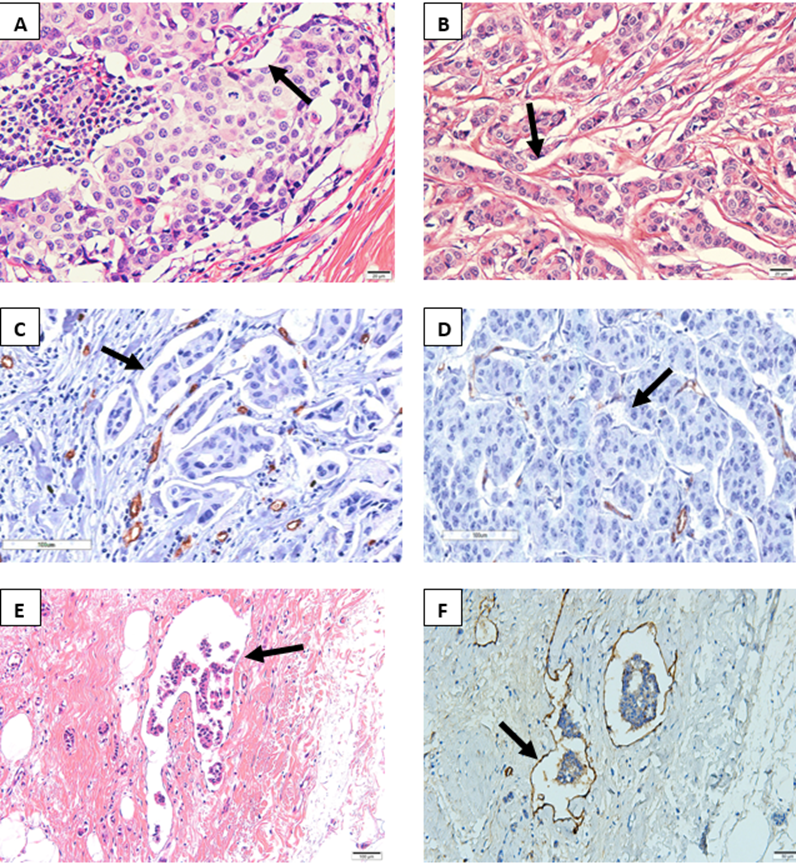
Figure 1: Extent of peritumoral retraction clefts identified by the absence of D2-40 antibody expression in post-neoadjuvant invasive breast carcinoma ductal of no special.
A- Clear space (arrow) affecting <70% of tumor cells (H&E, 40x) B- Clear space (arrow) affecting >70% of tumor cells (H&E, 20x). C- Numerous clear spaces affecting >70% of neoplastic cells without endothelial lining (IHC CD34, 20x). D- Few clear spaces (arrow) affecting <70% of neoplastic cells without endothelial lining (IHC D2-40, 20x). E-Microphotograph of lymphatic vessel with neoplastic embolus (arrow) within the peritumoral stroma. (H&E, 20x). F- D2-40 highlighting a lymphatic vessel surrounding a tumor embolus (arrows). D2-40 (IHC D2-40, 20x).
The most frequent molecular subtypes observed were Luminal A, which was predominantly associated with tumors exhibiting <70% RCs (41.4%), followed by Luminal B HER2-negative, which was most common in tumors with ≥70% RCs (35.7%). However, statistical analysis revealed no significant association between extensive RCs (≥70%) and other parameters, including tumor size, ER/PR status, molecular subtype, or the presence of distant metastasis (Table 1).
|
Parameter |
Category |
Total |
Retraction Clefts |
||
|
<70% |
≥70% |
p* |
|||
|
N |
100 |
58 (58.0%) |
42 (42.0%) |
- |
|
|
Age (years) |
Median (SD) |
57 (12.5) |
60 (12.9) |
56.5 (10.9) |
0.027 |
|
Tumor size |
<2 cm |
8 (8.0%) |
6 (10.3%) |
2 (4.8%) |
0.122 |
|
2-5 cm |
62 (62.0%) |
39 (67.2%) |
23 (54.8%) |
||
|
≥5 cm |
30 (30.0%) |
13 (22.4%) |
17 (40.5%) |
||
|
Molecular subtypes |
Luminal A |
34 (34.0%) |
24 (41.4%) |
10 (23.8%) |
0.071 |
|
Luminal B HER2- |
34 (34.0%) |
19 (32.8%) |
15 (35.7%) |
||
|
Luminal B HER2+ |
15 (15.0%) |
4 (6.9%) |
11 (26.5%) |
||
|
HER2-enriched |
6 (6.0%) |
4 (6.9%) |
2 (4.8%) |
||
|
Triple Negative |
11 (11.0%) |
7 (12.1%) |
4 (9.5%) |
||
|
ER |
Positive |
75 (75.0%) |
45 (77.6%) |
30 (71.4%) |
0.483 |
|
Negative |
25 (25.0%) |
13 (22.4%) |
12 (28.6%) |
||
|
PR |
Positive |
78 (78.0%) |
44 (75.9%) |
34 (81.0%) |
0.544 |
|
Negative |
22 (22.0%) |
14 (24.1%) |
8 (19.0%) |
||
|
HER2 |
Positive |
68 (68.0%) |
34 (58.6%) |
34 (81.0%) |
0.018 |
|
Negative |
32 (32.0%) |
24 (41.4%) |
8 (19.0%) |
||
|
Ki67 |
<14% |
38 (38.0%) |
27 (46.6%) |
11 (26.2%) |
0.038 |
|
≥14% |
62 (62.0%) |
31 (53.4%) |
31 (73.8%) |
||
|
EL D240 |
Positive |
56 (56.0%) |
22 (37.9%) |
34 (81.0%) |
<.001 |
|
Negative |
44 (44.0%) |
36 (62.1%) |
8 (19.0%) |
|
|
|
ypT |
0 |
3 (3.0%) |
3 (5.2%) |
0 (0.0%) |
0.182 |
|
1 |
14 (14.0%) |
9 (15.5%) |
5 (11.9%) |
|
|
|
2 |
43 (43.0%) |
28 (48.3%) |
15 (35.7%) |
|
|
|
3 |
12 (12.0%) |
6 (10.3%) |
6 (14.3%) |
|
|
|
4 |
28 (28.0%) |
12 (20.7%) |
16 (38.1%) |
|
|
|
ypN |
0 |
28 (28.0%) |
28 (48.3%) |
0 (0.0%) |
<.001 |
|
1 |
22 (22.0%) |
11 (19.0%) |
11 (26.2%) |
|
|
|
2 |
21 (21.0%) |
9 (15.5%) |
12 (28.6%) |
|
|
|
3 |
29 (29.0%) |
10 (17.2%) |
19 (45.2%) |
|
|
|
ypM |
0 |
78 (78.0%) |
47 (81.0%) |
31 (73.8%) |
0.389 |
|
1 |
22 (22.0%) |
11 (19.0%) |
11 (26.2%) |
|
|
|
* Pearson χ2 test |
|||||
Table 1: Association between the extent of retraction clefts and standard clinical, pathological, and biological features of invasive breast carcinoma.
SD: standard deviation; ER: estrogen receptor; PR: progesterone receptor; HER2: human epidermal growth factor receptor 2; Ki67: cell proliferation index; EL: lymphatic emboli; yp: post-chemotherapy pathological staging; T: tumor size; N: number of lymph nodes involved; M: distant metastasis.
In the multivariate Cox regression analysis, both the ypN2 (metastases in 4–9 lymph nodes) and ypN3 (>10 lymph nodes) categories were independently associated with a significantly increased risk of mortality, as indicated by elevated hazard ratios (HRs). This finding indicates that patients in these groups had a significantly lower probability of overall survival compared to those with no lymph node metastases (ypN0) (Table 2).
|
Variables |
HR(95% CI) |
p* |
|
RCs |
0.85 (0.448-1.623) |
0.627 |
|
Age |
0.993 (0.969-1.019) |
0.606 |
|
Ki67 |
0.849 (0.213-3.383) |
0.817 |
|
HER2 |
0.725 (0,376-1.398) |
0.337 |
|
PR |
0.561 (0.193-1.635) |
0.29 |
|
ER |
1.375 (0.571-3.310) |
0.478 |
|
EL_D240 |
0.669 (0.311-1.436 |
0.302 |
|
Tumor size (<2 cm) |
- |
- |
|
Tumor size (2-5 cm) |
1.537 (0.458-5.154) |
0.486 |
|
Tumor size (≥5 cm) |
0.691 (0.310-1.542) |
0.367 |
|
Molecular Subtype - Luminal A |
- |
- |
|
Molecular Subtype - Luminal B HER2- |
0.631 (0.202-1.975) |
0.429 |
|
Molecular Subtype - Luminal B HER2+ |
1.449 (0.622-3.379) |
0.39 |
|
Molecular Subtype - HER2+ |
1.063 (0.383-2.951) |
0.907 |
|
Molecular Subtype - Triple negative |
1.017 (0.274-3.775) |
0.98 |
|
ypT(0) |
- |
- |
|
ypT(1) |
0.455 (0.034-6.066) |
0.551 |
|
ypT(2) |
2.000 (0.127-31.627) |
0.623 |
|
ypT(3) |
4.736 (0.273-82.017) |
0.285 |
|
ypT(4) |
6.301 (0.427-93.043) |
0.18 |
|
ypN(0) |
- |
- |
|
ypN(1) |
2.465 (0.913-6.658) |
0.075 |
|
ypN(2) |
3.563 (1.277-9.940) |
0.015 |
|
ypN(3) |
5.150 (1.777-14.929) |
0.003 |
|
ypM |
1.533 (0.827-2.842) |
0.175 |
|
HR: hazard ration; CI: confidence interval; RCs: retraction clefts; ER: estrogen receptor; PR: progesterone receptor; yp: post-chemotherapy pathological staging; T: tumor size; N: number of lymph nodes involved; M: distant metastasis; HER2: human epidermal growth factor receptor 2; Ki67: cell proliferation index; RE: estrogen receptor; PR: progesterone receptor; EL: lymphatic emboli. |
||
Table 2: The correlation between clinicopathological features and overall survival in breast cancer patients.
Patients with a lower extent of retraction clefts (<70%, comprising 42% of the cohort) had a significantly longer average survival time of eight years. In contrast, those with extensive retraction clefts (≥70%, comprising 58% of the cohort) had a markedly shorter average survival of 4.5 years. Furthermore, the presence of lymphatic tumor emboli (found in 56% of cases) was associated with a poor prognosis, with an estimated survival time of 4.1 years. Patients without identifiable neoplastic emboli had a more favorable outcome, with an average survival time of 7.7 years (Kaplan-Meier analysis, 95% CI) (Figure 2).
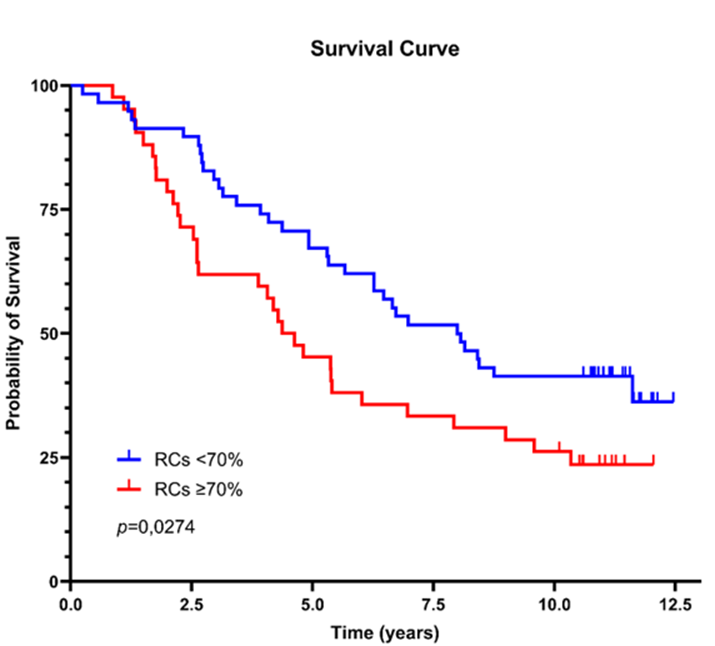
Figure 2: Survival curves based on the extent of retraction clefts and the presence of neoplastic emboli.
A clear correlation was observed between the extent of RCs and the patient's response to neoadjuvant chemotherapy. RCs were completely absent in 18 cases, which included all three patients who achieved a pathological complete response (pCR), one patient with a minimal response, and 14 with a moderate response.
Conversely, extensive retraction clefts, defined as involving ≥70% of the tumor area, were predominantly identified in cases with a high residual tumor burden, being present in 66 such cases. A smaller degree of retraction (<70% involvement) was less common and was associated with a varied response, observed in one case with minimal response, one with extensive burden, and 16 cases with a moderate response.
Discussion
The findings of this study robustly align with and extend the growing body of evidence that peritumoral RCs are not mere fixation artifacts but rather morphologic indicators of aggressive tumor biology. Our observed RC prevalence of 87% falls within the wide range (15.5% to 92%) reported in the literature for invasive breast carcinoma, a variability that may be attributed to differences in histological subtypes, grading systems, and interobserver assessment [4,10]. More critically, the prevalence of extensive RCs (≥70%) in our cohort was 42%, a figure remarkably consistent with the 41% reported by [14] in a large series of invasive breast carcinomas of no special type (NST) [14]. This concordance across diverse populations strengthens the reproducibility of this histological feature.
The most salient finding of our study is the potent association between extensive RCs and established markers of poor prognosis. The strong correlation with lymphatic emboli (D2-40 positive, p<0.001) and the stark absence of extensive RCs in the axillary metastasis-free group (ypN0, p<0.001) provide compelling evidence that RCs are intimately linked to the process of lymphatic invasion and metastasis. This supports the hypothesis proposed by [13] that these spaces may represent "pre-lymphatic" pathways or early stages of vascular co-option, where tumor-stromal interactions facilitate dissemination before the complete formation of canonical lymphatic vessels [13]. Our results thus validate and confirm the work of several groups who have identified RCs as a key correlate of lymphatic emboli; and nodal metastasis [15,17].
Furthermore, the significant associations with younger age, HER2 positivity, and a high Ki-67 index paint a picture of a highly proliferative and aggressive tumor phenotype prone to stromal remodeling. The association with HER2-enriched biology is particularly intriguing. While we did not find a significant link to a specific molecular subtype, the strong individual correlation with HER2 positivity suggests that the signaling pathways driving HER2 amplification (e.g., PI3K/AKT, MAPK) may also promote the stromal reactions and loss of adhesion that manifest as RCs. This aligns with the concept that HER2-positive tumors often exhibit a distinct microenvironment [27].
The survival analysis offers the most clinically significant insight. The dramatic disparity in mean overall survival (4.5 years for RC ≥70% vs. 8 years for RC <70%) underscores the prognostic power of this simple histological assessment. This finding is in line with previous studies that have consistently found extensive RCs to be a marker of reduced disease-free and overall survival [15,14,16]. Importantly, while our multivariate analysis confirmed advanced nodal stage (ypN), not RC extent itself, as the ultimate independent predictor of mortality, this does not diminish the value of RCs. Instead, it positions them as a powerful morphological correlate and an integrated marker of the aggressive biological process that leads to nodal metastasis. The extensive RCs are a visible signature of a tumor-stromal interaction conducive to invasion and dissemination. Therefore, they serve as a highly effective histological marker for identifying those tumors with a high probability of having already achieved that poor prognostic nodal stage at the time of diagnosis. The fact that the RC <70% group included a subset of HER2-positive cases with poor outcomes suggests that RCs are a strong but not exclusive marker; other pathways of aggression undoubtedly exist.
A novel aspect of our study, to the best of our knowledge not previously reported in the literature, is the focus on the post-neoadjuvant chemotherapy setting. We observed that RCs were absent in all patients who achieved a pathological complete response (pCR), suggesting that effective tumor cell eradication also abolishes this stromal reaction. Conversely, extensive RCs were predominantly found in cases with a high residual cancer burden (RCB). This implies that the tumor-stromal ecosystem that generates RCs is also one that confers resistance to chemotherapy. This finding has potential clinical utility; the presence of significant RCs in post-neoadjuvant chemotherapy specimens could help identify a patient subgroup with residual, chemo-resistant disease that remains at high risk for recurrence, potentially warranting escalated or novel adjuvant therapies.
We acknowledge the limitations of our study. The retrospective design and modest sample size, particularly for certain molecular subtypes, limit the statistical power for some subgroup analyses and the ability to fully adjust for heterogeneous treatment regimens. Furthermore, the assessment of RCs, while validated by multiple pathologists, can be challenged by treatment-related fibrosis and artifacts, necessitating careful examination. As noted by [19], discrepancies in the literature regarding the prognostic value of RCs can arise from differences in stratification methods and cohort demographics [19]. Our study, conducted in a Brazilian population, adds valuable data to this global conversation.
Conclusion
In conclusion, our data compellingly argue that extensive peritumoral retraction clefts are a potent histological marker of aggressive tumor behavior in invasive breast carcinoma. They are strongly associated with lymphatic invasion, nodal metastasis, a proliferative phenotype, and significantly reduced survival. Their evaluation in the post-neoadjuvant setting may provide additional prognostic information beyond standard RCB assessment. We advocate for the routine reporting of RC extent in pathological assessments and encourage future prospective, multi-institutional studies with standardized scoring systems to definitively establish its role in risk stratification and clinical decision-making. Future research should investigate the potential of integrating RC assessment into established prognostic systems, such as the Nottingham Index or RCB staging, to enhance their predictive power for disease recurrence in breast cancer patients.
Acknowledgments: This study was supported by the Postgraduate Program in Pathology at Fluminense Federal University, Niteroi, Rio de Janeiro, Brazil. The authors thank the Department of pathology from Antonio Pedro University Hospital, Fluminense Federal University, Niteroi; and all the patients who contributed to this study.
Authors' contributions: TCFG and ASR: Design of the work, acquisition of data, interpretation of data, and drafting the work, KSC, FRR and VGSL: Conception/design and intellectual contributions to the work, and substantive revisions.
Funding: The authors declare that no funds, grants, or other support were received to the development of this work.
Availability of data and materials: The data that support the findings of this study is not publicly available, as they were used under license for research purposes. However, they can be accessed upon reasonable request and with permission of the first author.
Conflict of interest: All contributing authors declare that they have no financial or personal relationships with individuals or organizations that could potentially influence or bias the current work.
Ethics approval and consent to participate: This work was approved by the Research Ethics Committee of Fluminense Federal University (CAAE 43900620.8.0000.5243). This research adheres to the standards outlined in National Health Commission (CNS) Resolution Nº 466 of 2012 and in Operational Standard nº 001 of 2013 of the CNS. All patients provided signed informed consent through the Consent to Participate declaration.
References
- Bujas T, et al. (2008) Peritumoral retraction clefting correlates with advanced stage squamous cell carcinoma of the esophagus. Pathology oncology research: Pathol Oncol Res 14:443–447.
- Džombeta T, Krušlin B (2018) High Grade T1 Papillary Urothelial Bladder Cancer Shows Prominent Peritumoral Retraction Clefting. Pathology oncology research: Pathol Oncol Res 24:567–574.
- Kruslin B, et al. (2003) Periacinar retraction clefting in the prostatic needle core biopsies: an important diagnostic criterion or a simple artifact? Virchows Arch 443:524–527.
- Jain D, Tikku G, Bhadana P, Dravid C, Grover RK (2019) The Impact of Peritumoral Retraction Clefting & Intratumoral Eosinophils on Overall Survival in Oral Squamous Carcinoma Patients. Pathology oncology research 25:183–189.
- Martínez-ortega JI (2024) Peritumoral Clefts in Basal Cell Carcinoma: Matrix Metabolism and Primary Cilium. Cureus 16:e58316.
- Ulamec M, et al. (2012) Periacinar retraction clefting and d2-40 expression in prostatic adenocarcinoma. Pathology oncology research 18:365–370.
- Zargaran M (2019) Clinicians’ role in the occurrence of oral biopsy artifacts as a potential diagnostic dilemma. Dental and Medical Problems 56:299–306.
- Acs G, et al. (2012) The extent of retraction clefts correlates with lymphatic vessel density and VEGF-C expression and predicts nodal metastasis and poor prognosis in early-stage breast carcinoma. Modern Pathology 25:163–177.
- Cserni G (1999) Pitfalls in frozen section interpretation: a retrospective study of palpable breast tumors. Tumori 85:15–18.
- Zaorsky NG, et al. (2012) Differentiating lymphovascular invasion from retraction artifact on histological specimen of breast carcinoma and their implications on prognosis. Journal of Breast Cancer 15:478–480.
- Bieniasz-krzywiec P, Mazzone M (2020) PoEMs edit breast cancer outcome. Aging 12:4045–4047.
- Alpaugh ML, Barsky SH (2001) The molecular basis of inflammatory breast carcinoma. Breast Cancer Research and Treatment 69:239-331.
- Irie J, et al. (2007) Artefact as the pathologist’s friend: peritumoral retraction in in situ and infiltrating duct carcinoma of the breast. International Journal of Surgical Patholog 15:53–59.
- Acs G, et al. (2015) The presence of extensive retraction clefts in invasive breast carcinomas correlates with lymphatic invasion and nodal metastasis and predicts poor outcome: a prospective validation study of 2742 consecutive cases. The American Journal of Surgical Pathology 39:325–337.
- Acs G, et al. (2007) Extensive retraction artifact correlates with lymphatic invasion and nodal metastasis and predicts poor outcome in early stage breast carcinoma. The American Journal of Surgical Pathology 31:129–140.
- Deng HY, et al. (2018) [Extensive peritumoral retraction clefts and prognosis in invasive breast carcinomas of no specific type]. Zhonghua Bing Li Xue Za Zhi 47:196–200.
- Li W, et al. (2022) The presence of retraction clefts correlates with lymphovascular invasion and lymph node metastasis and predicts poor outcome: Analysis of 2497 breast cancer patients. Annals of Diagnostic Pathology 61:152047.
- Leniček T, Kos M (2018) The correlation of peritumoral clefts with myofibroblastic stromal reaction in invasive ductal breast carcinoma. Liječnički vjesnik 140:190-199.
- Huang L, et al. (2021) The Prognostic Value of Retraction Clefts in Chinese Invasive Breast Cancer Patients. Pathology oncology research 27:1609743.
- Chen Jia-Mei, et al. (2021) Lymphatic Endothelial Markers and Tumor Lymphangiogenesis Assessment in Human Breast Cancer. Diagnostics 12:4.
- Kahn HJ, Marks A (2002) A new monoclonal antibody, D2-40, for detection of lymphatic invasion in primary tumors. Laboratory Investigation 82:1255–1257.
- Kalof NA, Cooper K (2009) D2-40 immunohistochemistry--so far! Advances in Anatomic Pathology 16:62–64.
- Abe N, et al. (2016) Clinicopathological significance of lymphangiogenesis detected by immunohistochemistry using D2-40 monoclonal antibody in breast cancer. Fukushima Journal of Medical Science 62:57–63.
- Schoppmann SF (2005) Lymphangiogenesis, inflammation and metastasis. Anticancer Research 25:4503–4511.
- Pires ARC, Andreiuolo FM, De souza SR (2006) TMA for all: a new method for the construction of tissue microarrays without recipient paraffin block using custom-built needles. Diagnostic Pathology 1:14.
- Thürlimann B (2024) St. Gallen International Breast Cancer Conferences & Consensus. Translational Breast Cancer Research 5:22.
- García-martínez A, et al. (2021) Hedgehog gene expression patterns among intrinsic subtypes of breast cancer: Prognostic relevance. Pathology, Research and Practice 223:153478.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.