Expert Opinion on the Role of Dapagliflozin in Cardiometabolic Diseases: An Indian Perspective
by Ganapathi Bantwal1, Debmalya Sanyal2, Soumik Goswami3, Vijay Viswanathan4, Jayanta Kumar Panda5, Shreerang Godbole6, Pramod Gandhi7, Anirban Sinha8, Sambit Das9, A K Pancholia10, Suhas Erande11, Beena Bansal12, Thushanth Thomas13, Biplab Bandopadhyay14, Nilakshi Deka15, Rohan Sequeira16, Rakesh Goyal17, Rajnish Dhediya18*, Rajan Mittal18, Bhavesh Kotak18
1Professor and Head of Endocrinology, St. Johns Medical College, Bangalore, India.
2Professor, Department of Endocrinology, KPC Medical College, Kolkata, West Bengal, India.
3Department of Endocrinology, NRS Medical College, Kolkata, West Bengal, India.
4MV Hospital for Diabetes and Prof M Viswanathan Diabetes Research Centre, Royapuram, Chennai, India.
5Professor and Head, PG Department of Internal Medicine, SCB Medical College, Cuttack, Odisha, India.
6Department of Endocrinology, INSTRIDE, 6, Poonam Arcade, J. M. Road, 1170/71 Revenue Colony, Shivaji Nagar, Pune, Maharashtra - 411005, India.
7Gandhi Hospital Shrivardhan, Nagpur, Maharashtra, India.
8Associate Professor, Endocrinology, Medical College Kolkata, India.
9Professor and Head, Department of Endocrinology, Kalinga Institute of Medical Sciences (KIMS), Bhubaneswar, India.
10Head of Department, Medicine and Preventive Cardiology, Arihant Hospital and RC, Indore, Madhya Pradesh, India.
11Department of Endocrinology, Akshay Hospital, Pune, India.
12Door To Care Endocrine and Diabetes Clinic, Gurugram, India.
13KIMS Hospital, Trivandrum, India.
14Thyroid and Harmon care, Raipur, India.
15Apollo Hospital, Guwahati, India.
16Senior consultant and cardio-metabolic physician – Jaslok Hospital, Founder and Managing Director – Queira Technologies (Clinicjet.Com), Mumbai, India.
17The Endocrine Clinic, Ludhiana, India.
18Department of Medical Affairs, Dr Reddy's Laboratories Ltd, Hyderabad, Telangana, India.
*Corresponding author: Rajnish Dhediya, Dr Reddy's Laboratories Ltd, Hyderabad, Telangana, India
Received Date: 18 November, 2024
Accepted Date: 26 November, 2024
Published Date: 03 April, 2025
Citation: Bantwal G, Sanyal D, Goswami S, Viswanathan V, Panda JK, et al. (2025) Expert Opinion on the Role of Dapagliflozin in Cardiometabolic Diseases: An Indian Perspective. Int J Cerebrovasc Dis Stroke 8: 191. https://doi.org/10.29011/2688-8734.100191.
Abstract
Cardiometabolic Disease (CMD) is a growing health concern worldwide, including in India, characterized by a constellation of factors such as metabolic syndrome, obesity, dyslipidemia, diabetes, prediabetes, Non-Alcoholic Fatty Liver Disease (NAFLD), and Chronic Kidney Disease (CKD). Sodium-Glucose Cotransporter-2 Inhibitors (SGLT2is) such as dapagliflozin, have demonstrated promising pleiotropic effects beyond the glycemic efficacy, including improving cardiovascular and renal outcomes in patients with CMD. This article aims to explore the potential of dapagliflozin in managing the full spectrum of CMD based on the expert opinions of diabetologists and endocrinologists. The document discusses the role of SGLT2is across the cardiometabolic spectrum such as prediabetes and diabetes, obesity, hypertension, NAFLD, CKD, Cardiovascular Disease (CVD), and heart failure, and the evidence supporting their use in CMD.
Keywords: Cardiometabolic disease; Metabolic syndrome; Type 2 diabetes mellitus; SGLT2is; Dapagliflozin; Pleiotropic effects; Heart failure; Chronic kidney disease.
Introduction
Cardiometabolic Syndrome (CMS) is a collection of various metabolic abnormalities that are substantial risk factors for Cardiovascular Disease (CVD) [1]. CMS has now emerged as a complex clinical syndrome involving factors like insulin resistance, obesity, dyslipidemia, and increased Blood Pressure (BP) [1,2]. It is also known as insulin resistance syndrome since it is hypothesized that insulin resistance is the primary mechanism responsible for the metabolic abnormalities of the syndrome[1]. It is essential to accurately diagnose CMS considering its increasing incidence to ensure that patients at risk receive the proper screening and care. Several guidelines have defined CMS, which are summarized in Table 1 [3].
|
Guideline |
Definition of CMS |
|
WHO (1998) |
- ↑ insulin levels, impaired glucose tolerance, or impaired fasting glucose |
|
- Any two of the following: - BP ≥140/90 mm Hg - TG ≥150 mg/dl |
|
|
- HDL-C <35 mg/dL or abdominal obesity waist-hip ratio >0.9 along with BMI ≥30 kg/m² and WC >37 inches |
|
|
AHA and NHLBI (2005) |
Any 3 of the following: - FPG ≥ 100 mg/dL - HDL-C <40 mg/dL in men and <50 mg/dL in women, or on treatment - TG ≥ 150 mg/dL or on treatment - WC ≥ 102 cm in men, ≥ 88 cm in women - BP ≥ 130/85 mm Hg, or on antihypertensive medication |
|
IDF, NIH, AHA (2009) |
Any 3 of the following: - FPG ≥ 100 mg/dL or on drug treatment for elevated glucose -HDL-C < 40 mg/dL in males, < 50 mg/dL in females - TG ≥ 150 mg/dL or on drug treatment for elevated TG - WC>102 cm in males, >88 cm in females, specific thresholds for Asians -BP ≥ 130/85 mm Hg or on drug treatment for hypertension |
Table 1: Definition of CMS in various guidelines.
AHA, American Heart Association; CMS, cardiometabolic syndrome; BMI, body mass index; BP, blood pressure; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; IDF, International Diabetes Federation, NIH, National Institutes of Health, NHLBI, National Heart, Lung, and Blood Institute; TG, triglycerides; WC, water column; WHO, World Health Organization.
Insulin resistance and CMS are widespread conditions in India [4-13]. CMS was found in around 42.7% of patients with Type 2 Diabetes Mellitus (T2DM) [14]. A study by Bhalwar et al. estimated that approximately 13–15% of India’s adult population have CMS, with females being more affected (approximately 18– 19% among adult females and 8%–9% among adult males) [4]. Genetic predisposition, sedentary lifestyle, environmental factors, and diet might be responsible for establishing the syndrome [15]. The pathophysiology of CMS includes a) alterations in fatty acid metabolism, b) excess visceral fat, c) ectopic accumulation of fat in the liver and muscles, d) inflammatory cytokines produced by the adipose tissue, e) increased blood pressure due to insulin resistance and f) increased renal sodium reabsorption due to hyperinsulinemia [1].
Cardiometabolic Disease (CMD) includes CMS in addition to dyslipidemia, obesity, diabetes, prediabetes, obesity, NonAlcoholic Fatty Liver Disease (NAFLD), and chronic Kidney Disease (CKD) [16]. Sodium-Glucose Cotransporter-2 Inhibitors (SGLT2is) have been shown to enhance Cardiovascular (CV) and renal outcomes in patients with CMD. The clinical benefits of SGLT2is have been confirmed in patients with and without T2DM. As a result, SGLT2is play an increasingly important role in managing Heart Failure (HF) and CKD, extending beyond T2DM treatment. They have diuretic and natriuretic effects, which lead to decreased preload, Left Ventricular (LV) filling pressure, and improvements in other surrogates of afterload. They also decrease sympathetic tone and uric acid levels, increase hemoglobin levels, and are believed to have anti-inflammatory properties [17].
Need for expert opinion
SGLT2is, like dapagliflozin, have been shown to target various conditions of CMD. The availability of emerging evidence for the beneficial effects of dapagliflozin across the spectrum of CMD is likely to affect the trend in the usage of dapagliflozin in such patients. Considering the evolving landscape of management and the promising role of dapagliflozin, expert opinion is essential to inform clinical practice and advance our understanding of the therapeutic options available for this challenging condition. This review outlines the authors’ perspective on dapagliflozin’s position across the CMD spectrum.
Methodology
A panel of 550 expert diabetologists and endocrinologists from India convened digitally for 55 advisory board meetings to discuss the role of dapagliflozin in CMD. The discussions spanned several categories, including T2DM, prediabetes, obesity, hypertension, NAFLD, CVD, HF, and CKD. The collected evidence on these specific topics was presented during the meetings, followed by discussions highlighting dapagliflozin’s role and positioning across CMD. The writing committee prepared the manuscript, which all expert panel members reviewed to ensure a comprehensive and well-structured document. The recommendations in this article are based on the evidence presented and the discussions summarized from the minutes of the meetings.
Literature Review and Expert Opinion Type 2 Diabetes Mellitus
Current Evidence: SGLT2is reduce the HbA1c by 0.5-1%, favoring body weight and BP [18]. The results of a subgroup analysis performed in a meta-analysis by Xu et al. based on a combination of hypoglycemic agents concluded that dapagliflozin is more effective as add-on therapy with insulin, metformin, or exenatide, among other hypoglycaemic agents in reducing HbA1c levels (P<0.00001 for all) [19].
Pan, et al. conducted a meta-analysis of 18 studies on the effects of SGLT2is in T2DM patients. Results showed significant reductions in body weight, waist circumference, Body Mass Index (BMI), skeletal muscle mass, body fat percentage, subcutaneous fat, fat mass, lean mass, and visceral fat compared to control drugs (sulfonylureas, insulin, metformin, thiazolidinediones, DPP-4i, and GLP-1 Receptor Agonists [GLP-1RA]) [20]. Dapagliflozin has been categorized as an antidiabetic drug associated with moderate weight reduction (between 3.2% and 5%) [21].
A systematic review and meta-analysis of Cardiovascular Outcome Trials (CVOT) by Zelniker et al. showed that both SGLT2is and GLP-1RA have robustly reduced the risk of atherosclerotic Major Adverse Cardiovascular Event (MACE), while only SGLT2is have a profound beneficial effect for reducing the risk of HF and progression of CKD [22]. A network meta-analysis of 13 Randomized Controlled Trials (RCTs) by Ghosal et al. showed that SGLT2is reduced CV death risk in patients with T2DM by 12% (Relative Risk [RR]: 0.88, 95% confidence interval [CI]
0.80–0.96) in comparison with standard treatment. Among all SGLT2is, dapagliflozin brought the highest benefit in the T2DM with HF patient group (11% RR reduction), while empagliflozin has shown a more significant decrease in CV death in patients of Atherosclerotic Cardiovascular Disease (ASCVD) and multiple CV risk factors [23]. Meta-analysis of 23 CVOTs has shown significantly reduced risk (CV) death (RR: 0.88), overall death (RR: 0.87), and MACE with SGLT2is compared to DPP-4is. SGLT2is scored over GLP-1RAs for reducing the risk of HF and renal outcome by 24% and 22%, respectively, while both the classes of drugs were comparable for MACE, CV, and total death. In a systematic review and meta-analysis by Yamada T et al., SGLT2is reduced the risk of renal events by 21% compared to GLP-1RAs in patients with T2DM and CKD[24]. A meta-analysis of 25 RCTs by Chen et al. revealed an increased risk of genital infection (GI), urinary tract infection (UTI), and nasopharyngitis compared to other treatments or placebo [25]. Increased risk of GI and UTI was found in female patients, those with BMI ≥ 30 kg/m2 and receiving SGLT2is treatment for ≥ 6 months [26].
A network meta-analysis of 13 RCTs by Ghosal et al. found that SGLT2is reduced CV death risk by 12% (RR: 0.88, 95% CI: 0.80–0.96) in T2DM patients compared to standard treatment. Dapagliflozin showed the most significant benefit in T2DM with HF (11% RR reduction), while empagliflozin significantly reduced CV death in patients with ASCVD and multiple CV risk factors.
Position: Guideline recommendation for SGLT2is in T2DM
American Diabetes Association (ADA) 2024 guidelines recommend SGLT2is as a preferred choice of therapy in T2DM patients with HF (with either reduced or preserved Ejection Fraction (EF)), CKD (with confirmed eGFR of 20–60 mL/min per 1.73 m2 and/or albuminuria), ASCVD, indicators of high CV risk (including obesity, hypertension, dyslipidemia or albuminuria) and high BMI [27].
Expert Opinion
Pharmacotherapy for hyperglycemia in T2DM should be individualized based on comorbidities, hypoglycemia risk, glycemic goals, and medication availability. While metformin remains the standard first-line treatment, SGLT2is can be used as initial therapy (with or without metformin) in T2DM patients with HF, CKD, CVD, or high BMI. SGLT2is show comparable glycemic efficacy and safety. Empagliflozin may be preferred for T2DM with ASCVD and dapagliflozin for T2DM with HF. Counseling on SGLT2i side effects is advised before initiation. Combining SGLT2is and GLP-1RAs may offer additional cardiovascular benefits, with sequential combination therapy as an option.
Role of SGTL2i in Prediabetes Current Evidence
Prediabetes is defined as an intermediate state of hyperglycemia with glucose levels above the normal state but below the diagnostic levels of diabetes [28]. According to the Indian Council of Medical Research (ICMR)–India DIABetes study, 5.8-14.7% of the population in rural and 7.2-16.2% in urban India have prediabetes. The prevalence of prediabetes exceeds that of diabetes in most Indian states, indicating a large population that may soon develop T2DM. Conversion rate to diabetes is 58.9% among Indians with prediabetes [29].
Intensive lifestyle intervention and antidiabetic drugs such as metformin, thiazolidinediones, and α-Glucosidase Inhibitors (AGI) have shown significant reduction in the incidence of diabetes [30,31]. Recent 2024 ADA guidelines recommend intensive lifestyle modification such as moderate-intensity physical activity for ≥ 150 minutes/week to achieve a weight loss of 7% and metformin in the age group of 25-59 years and BMI of ≥ 35 kg/m2. Additionally, Research Society for Study of Diabetes in India (RSSDI) guideline recommends AGI such as acarbose or voglibose in case of non-tolerance to metformin [32,33]. Several emerging data show the benefits of antidiabetic drugs such as GLP-1RA and SGLT2is in preventing T2DM.
Insulin-resistant conditions such as HF and CKD are linked to a high prevalence of diabetes [34]. A systematic review and metaanalysis conducted by Singh et al. included 5 RCTs to assess the effect of SGLT2is on New-Onset Diabetes (NOD) in adult patients with prediabetes having CKD and HF. The results showed that SGLT2is could significantly reduce the occurrence of NOD in these patients (HR 0.81, P=0.005). However, there was no effect of SGLT2is on HbA1c, indicating that the decrease in the occurrence of NOD observed is unrelated to these drugs’ blood glucose masking effect [35]. A pooled analysis of two phase 3 trials, DAPA-CKD and DAPA-HF demonstrated a reduction in the incidence of NOD in patients with T2DM and CKD with dapagliflozin treatment (Hazard Ratio [HR] 0.67 [95% CI 0.51– 0.88]). Subgroup analysis also showed no difference in the effect of dapagliflozin in the subgroups of age, sex, BMI, glycemic status, baseline CV medication use, SBP, and glomerular filtration rate [34]. A randomized, double-blind crossover trial by Veelen et al. showed that two weeks of dapagliflozin treatment in prediabetic patients lowered 24-hour glucose levels and improved 24-hour and nocturnal fat oxidation, as well as ex vivo mitochondrial oxidative capacity without significant changes in hepatic glycogen stores [36]. The PRE-D trial found a greater reduction in glycemic variability measured by Mean Amplitude Of Glycemic Variability (MAGE) by 17% with dapagliflozin compared to metformin in overweight or obese individuals with prediabetes [37].
Possible mechanisms for a beneficial effect of dapagliflozin in prediabetes include protecting pancreatic beta-cells from glucotoxicity, weight loss, and improvement in hepatic insulin sensitivity. Improving CKD and HF may have contributed to insulin sensitivity [34].
Expert Opinion
Prediabetes is an independent risk factor for CVD, and given its high prevalence in India, clinic-based screening of high-risk individuals (overweight, hypertension, sedentary) is crucial for early detection. Management should be individualized, starting with lifestyle changes, followed by metformin for those with high BMI or poor lifestyle compliance. Other classes of drugs, such as AGIs, may be considered for patients intolerant to metformin. While SGLT2is reduce T2DM incidence in HF and CKD, their long-term benefit in individuals without these conditions needs to be confirmed from clinical trials.
Obesity Current Evidence
Obesity is the primary risk factor for the onset of diabetes. Obesity and diabetes rates have been rising simultaneously, posing a threat to patient mortality and driving up community healthcare costs. A 5% weight loss or more of total body weight improves quality of life, lowers the requirement for diabetes medication, and improves glycemic control. Treatment of diabetes and obesity necessitates a multimodal medical strategy that includes medication, potentially surgical management, and intense lifestyle modification [38].
A meta-analysis by Lazzaroni, et al. has categorized the antidiabetic drugs into the following three groups based on their efficacy for weight loss:
- Mild efficacy for weight loss (less than 3.2% of initial weight): metformin, acarbose, empagliflozin, and exenatide.
- Moderate efficacy for weight loss (between 3.2% and 5%): canagliflozin, ertugliflozin, dapagliflozin, dulaglutide.
- Strong efficacy for weight loss (greater than 5%): liraglutide, semaglutide, tirzepatide.
ADA 2024 guideline recommends high weight loss efficacy antidiabetic drugs such as GLP-1RAs, dual GIP and GLP-1RA, and SGLT2is in patients of T2DM associated with obesity or high BMI [39].
Role of SGLT2is in weight reduction
SGLT2is promote weight loss through several mechanisms. They induce weight loss by excreting glucose via the kidneys and shift energy metabolism toward fat and glucose utilization, increasing oxygen consumption and carbon dioxide exhalation. Additionally, SGLT2is regulate adiponectin and leptin in white adipose tissue, boost fatty acid oxidation, and promote lipolysis, leading to more significant energy expenditure and weight reduction (Figure 1).
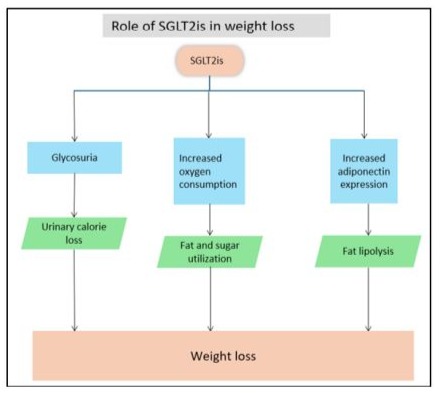
Figure 1: Effect of SGLT2is in reduction of weight.
Several studies have confirmed the effect of SGLT2is in reducing body fat (Table 2).
|
Trial |
Study design |
Study duration |
Inclusion criteria |
Intervention |
Results |
|
Wang et al. (2023) [40] |
Systematic review and meta-analysis-18 RCTs |
- |
T2DM |
SGLT2is (N=1063) |
|
|
Ma et al. (2023) [41] |
Systematic review and meta-analysis -61 RCTs |
- |
-Overweight (BMI ≥ 25 kg/m2) -Obesity (BMI ≥ 30 kg/m2) adults with or without T2DM |
GLP-1RAs SGLT2is (N=17281) |
|
|
Wilding, et al. (2019) [42] |
Non-interventional retrospective cohort study |
195 weeks |
-Adult with T2DM -Prior oral T2DM therapy |
Early versus later Dapagliflozin users (n=3774) |
Early dapagliflozin users experienced baseline-adjusted mean (95% CI) reductions of 3.31% (−4.37, −2.25) in weight versus 4.06% (−5.05, −3.07) in later users, respectively. |
|
Iacobellis, et al. (2020) [43] |
Randomized, double-blind, placebo-controlled trial |
24 weeks |
-Patients with T2DM with BMI ≥ 27 kg/m2 -HbA1c level ≤ 8% on metformin monotherapy. |
Dapagliflozin+ metformin Metformin+ placebo (N=100) |
Dapagliflozin reduced EAT by 20% after 24 weeks (P=0.001) compared to 7% in the metformin group (P=0.009). |
|
Shrikrishnapalasuriyar et al. (2019) [44] |
Real-world evidence study |
- |
Adults with clinical diagnosis of T2DM |
Dapagliflozin (n=101) |
Dapagliflozin was associated with significant reductions in HbA1c (-12.7 mmol/mol, P<0.0001), weight (-8.2 kg, P<0.0001) and BMI (-3.4 kg/m2, P<0.0001) compared to baseline. |
|
FOREFRONT Vishwanathan et al. (2019) [45] |
Real-world evidence study |
52 weeks |
-Adult T2DM patients inadequately controlled (HbA1c>7%) with existing antidiabetic therapy. -Started dapagliflozin at least 3 months before enrolment. |
Dapagliflozin (N=1978) |
-Dapagliflozin significantly reduced body weight (- 1.86 kg, P<0.001). -The maximum decrease in body weight was observed in patients with a BMI of >30 kg/m2. |
|
Shi, et al. (2021) [46] |
Meta-analysis-51 RCTs |
- |
-Adult patients with T2DM -SGLT2is with monotherapy or as an add-on to other hypoglycemic therapy |
SGLT2is (N=23989) |
SGLT2is reduces body weight significantly when given in higher doses. (‒0.346 kg, 95% CI ‒0.437 to ‒0.254). |
|
BMI, body mass index; EAT: Ectopic adipose tissue; GLP-1RA, Glucagon-like peptide-1 receptor agonist; NAFLD, non-alcoholic fatty liver disease; SAT, subcutaneous adipose tissue; SGLT2is, sodium-glucose cotransporter 2 inhibitors; SMD, standardized mean difference; T2DM, type-2 diabetes mellitus; VAT: visceral adipose tissue. |
|||||
Table 2: Clinical studies for weight reduction associated with SGLT2is.
Expert Opinion
Weight management is crucial alongside glycemic control in T2DM patients with overweight or obesity. Aiming for at least 5% weight loss is recommended. Lifestyle modification remains vital, and antidiabetic drugs with weight-lowering effects, such as SGLT2is and GLP-1RAs, should be preferred. While GLP-1RAs offer greater weight loss, SGLT2is are favored in practice due to lower cost and oral administration. Drug choice depends on desired weight loss, patient preferences, and conditions like HF/CKD (favoring SGLT2is) or ASCVD (favoring GLP-1RAs). Dapagliflozin typically reduces weight by 3-5 kg, with greater loss in higher baseline BMI, peaking within 4-6 weeks.
Hypertension
SGLT2is effectively reduces BP by the mechanisms mentioned in Figure 2.
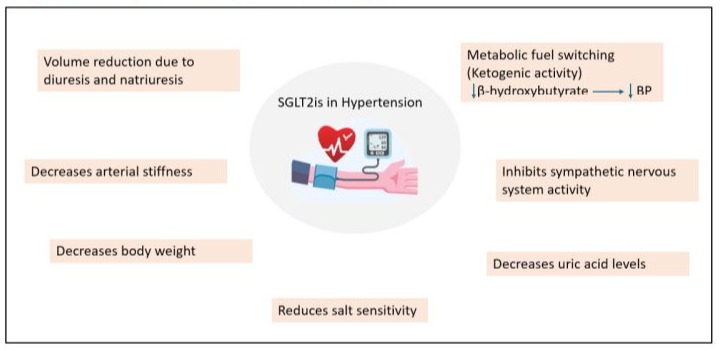
Figure 2: Role of SGLT2is in the reduction of hypertension
Current Evidence
The coexistence of T2DM and hypertension is common. T2DM increases the sodium-glucose transporters’ activity, leading to volume load due to sodium retention. Therefore, controlling BP is crucial to prevent CVD in patients with T2DM[47]. Among antidiabetic drugs, GLP-1RA (semaglutide, liraglutide, exenatide) and SGLT2is (dapagliflozin, empagliflozin, canagliflozin, and ertugliflozin) have shown BP lowering effect over 1 year of treatment in the meta-analysis by Tsapas A, et al. [48].
A summary of clinical studies assessing the antihypertensive effect of dapagliflozin has been enlisted in the table 3.
|
Trial |
Study design |
Study duration |
Inclusion criteria |
Intervention |
Results |
|
Li et al. (2022) [50] |
Systematic review and metaanalysis-16 RCTs |
- |
Adults with chronic HF treated with SGLT2is |
SGLT2is |
• A significant reduction was observed in SBP (1.68 mmHg (95% CI -2.7, -0.66; P=0.001) but not DBP (-1.06 mmHg; 95% CI -3.20, 1.08; P=0.33) by SGLT2is in patients with HF. |
|
Georgianos et al. (2019) [51] |
Meta-analysis |
- |
- |
SGLT2is |
|
|
DECLARE- TIMI 58 Wiviott et al. (2019) [52] |
Randomized, double-blind, multinational, placebo- controlled, phase 3 trial |
4.2 years |
-T2DM with HbA1c 6.5%-12.0% -CrCl of 60 ml or more per minute. |
Dapagliflozin 10 mg (N=8582) Placebo (N=8587) |
A significant BP difference of 2.7mm Hg was observed in the dapagliflozin group vs. 0.7 mm Hg in the placebo group. |
|
McMurray et al. (2019) [53] (DAPA-HF) |
Phase 3, placebocontrolled trial |
18.2 months |
-HFrEF with or without T2DM |
Dapagliflozin 10 mg once daily (N=2373) Placebo (N=2371) |
• A difference of 1.27mm Hg in systolic BP was observed in dapagliflozin group and placebo group (P=0.002). |
|
Sjöström et al. (2015) [54] |
Pooled analysis of 13 placebocontrolled studies |
24 weeks |
-T2DM patients with SBP≥ 140 mmHg |
Dapagliflozin 10mg/day (N=2360) Placebo (N=2295) |
• Dapagliflozin significantly reduced SBP/DBP at week 24 in hypertensive and non-hypertensive patients compared to placebo (by 3.6/1.2 and 2.6/1.2 mmHg, respectively). |
|
Weber et al. 2016 [55] |
Global, randomized, double-blind placebocontrolled trial |
12 weeks |
-Adult T2DM with HbA1c ≥7.0% and ≤ 10.5% -SBP ≥ 140 and ≤ 165 mmHg; DBP ≥85 and ≤ 105 mmHg |
Dapagliflozin 10 mg + ACEi/ARB (N= 302) Placebo + ACEi/ARB (N=311) |
• Dapagliflozin group showed a reduction in SBP by 10.4 mm Hg compared to 7.3 mm Hg in the placebo group (P=0.0010). |
|
Weber et al. 2016 [56] |
Randomised, double-blind, placebo- controlled, phase 3 study |
12 weeks |
-Uncontrolled T2DM With HbA1c 7·0%– 10·5% -Hypertension (systolic 140–165 mm Hg and diastolic 85–105 mm Hg) |
Dapagliflozin 10 mg (N=225) Placebo (N=224) |
• In the dapagliflozin group, seated SBP was greater than placebo by 4.3 mm Hg. |
|
Hao et al. (2020) [57] |
Prospective cohort study |
52 weeks |
-Adult T2DM and primary hypertension -Baseline eGFR ≥60 mL/ min/1.73 m2 |
Dapagliflozin (N=182) Control (N=304) |
• Dapagliflozin showed a significant reduction in 24-hr SBP (P=0.02) and daytime SBP (P=0.009). |
|
Ostrominski et al. (2023) [58] (DELIVER trial) |
Global, multicentre, randomized, double-blind clinical trial |
12 weeks |
-HFpEF or HFmrEF Patients with BP ≥140/90 mm Hg (≥130/80 mm Hg if diabetes) despite treatment with 3 antihypertensive drugs, including a diuretic. |
Dapagliflozin Placebo (N=718) |
• Dapagliflozin reduced SBP by 1 to 3 mm Hg without increasing the risk of hypotension, hypovolemia, or other serious adverse events. |
Abbreviations: ACEi: angiotensin-converting enzyme inhibitors; ARB: angiotensin receptor blockers; CrCl, Creatinine clearance; DBP, diastolic blood pressure; EF, Ejection fraction; HF, Heart Failure; HFmrEF, Heart Failure with mildly reduced ejection fraction; HFpEF, Heart Failure with preserved ejection fraction; HFrEF, Heart failure with reduced ejection fraction; HbA1c, haemoglobin A1C; SBP: systolic blood pressure; T2DM, type 2 diabetes mellitus.
Table 3: Clinical studies evaluating the effect of SGLT2is on blood pressure
Non-alcoholic fatty liver disease (NAFLD)/, Non-alcoholic steatohepatitis (NASH) Effect of antidiabetic drugs on NAFLD has been summarized in table 4.
|
Drug |
Beneficial effects in NAFLD |
|
GLP-1RA |
↓weight, ↑insulin sensitivity, ↓steatosis, ballooning, lobular infiltrates of NASH, ↓ lipotoxicity, and liver fat deposition. |
|
DPP-4i |
↓ lipogenesis, ↓ fibrosis, and NAFLD activity score. |
|
Pioglitazone |
↓ TG levels, ↑ NASH resolution, ↓ ALT and AST levels, and ↓ HOMA-IR. |
|
SGLT2i |
↑hepatic beta-oxidation, ↓hepatic de novo lipogenesis, apoptosis, hepatic inflammation, oxidative stress. |
|
ALT, alanine aminotransferase; AST, aspartate transaminase; DPP-4i, Dipeptidyl peptidase 4 inhibitors; GLP-1RA, Glucagon-like peptide-1 receptor agonists; HOMA-IR: Homeostatic Model Assessment for Insulin Resistance; NASH, Non-alcoholic steatohepatitis; NAFLD, Non-alcoholic fatty liver disease; TG, triglycerides. |
|
Table 4: Effects of antidiabetic agents on NAFLD [60,61].
Studies show that SGLT2is Lower Aspartate Aminotransferase (ALT), Gamma-Glutamyl Transferase (GGT), and Liver Fat Content (LFC) in overweight or obese NAFLD patients. SGLT2is also reduce body weight in patients with or without T2DM, lower plasma triglycerides, and raise HDL, which helps improve dyslipidemia and alleviate hepatic steatosis in NAFLD [62].
|
Trial |
Study design |
Study duration |
Inclusion criteria |
Intervention |
Results |
|
Mantovani et al. (2020) [63] |
Meta-analysis-12 RCTs |
- |
Overweight or obese patients with NAFLD |
SGLT2is |
• SGLT2is effectively reduced ALT, GGT, and absolute percentage of LFC on MRI. |
|
Eriksson et al.(2018) [64] |
Randomized, double-blind, prospective study (EFFECT- II) |
12 weeks |
- T2DM -Patients on a stable dose of metformin, sulfonylurea, or both for at least 3 months. -NAFLD with PDFF >5.5% (as measured by MRI); BMI of 25–40 kg/ m2 |
Dapagliflozin 10 mg (N=21) OM-3CA 4g (N=20) Dapagliflozin 10 mg+ OM-3CA 4g (N=22) Placebo (N=21) |
|
|
Shimizu et al. (2019) [65] |
Randomized, open-label, prospective study |
24 weeks |
-T2DM with HbA1c 6.0–12.0%. -On stable therapy with one to three oral antidiabetic agents with or without insulin for at least 3 months. -NAFLD |
Dapagliflozin 5 mg daily (N=33) Control (N=24) |
|
|
Kinoshita et al. (2020) [66] |
Randomized, open‐label, three‐arm, active control study |
28 weeks |
-T2DM with HbA1c ≥6.5%, BMI ≥22 kg/m2 - NAFLD with ALT ≥25 units/L (men) or ≥17 units/L (women) at screening -Stable dose of diabetes medicine for ≥1.5 months. |
Dapagliflozin 5 mg daily (N=32), Pioglitazone 7.5–15 mg/day (N = 33) or Glimepiride 0.5–1 mg/day (N = 33) |
|
|
Arai et al. (2021) [67] |
Open-label, prospective study |
48 weeks |
-NAFLD (Presence of steatosis in ⩾5% of hepatocytes as per histological findings or fat deposit determined via USG) -T2DM with HbA1c ⩾6.2% despite dietary/ exercise therapies and/or other OHAs for at least 8 weeks. |
SGLT2is (N=44) Non-SGLT2is (N=44) |
(P<0.001), ALT(P=0.02), uric acid (P<0.001), and FIB-4 index (P=0.01) at Week 48.
|
|
ALT, alanine aminotransferase; BMI, body mass index; AST, aspartate aminotransferase; CK, cytokeratin; FIB-4 index, Fibrosis-4 index; FGF21, fibroblast growth factor 21; GGT, γ-glutamyl transferase; LFC, liver fat content; LSM, liver stiffness measurement; OHA, oral hypoglycemic agents; OM-3CA: omega-3-carboxylic acids; PDFF: proton density fat fraction; SGLT2is; sodium-glucose cotransporter 2 inhibitors. *defined as a ⩾10% decline from baseline at Week 48. |
|||||
Table 5: Summary of clinical evidence of SGLT2is in NAFLD/NASH.
Expert Opinion
NAFLD is associated with nearly half of the patients with T2DM. Screening of high-risk populations should be performed for early diagnosis of NAFLD. Lifestyle modification, which promotes weight loss, is the mainstay of therapy for NAFLD. The weight loss and improvement in insulin resistance associated with SGLT2is may be useful to improve the outcome of NAFLD. SGLT2is are a promising therapy for T2DM patients unsuitable for pioglitazone (a commonly used antidiabetic drug for NAFLD associated with T2DM), such as those overweight/obese, HF/at risk of HF.
Cardiovascular disease and Heart Failure
Various mechanisms of SGLT2is are thought to be involved in HF (Figure 3).
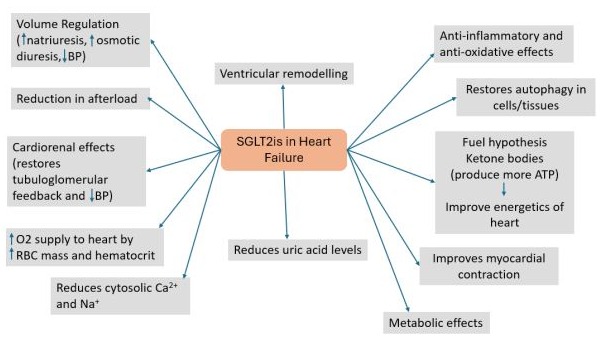
Figure 3: Mechanism of SGLT2is in heart failure.
BP, blood pressure; RBC: Red blood cells.
Current Evidence
Several studies have investigated the effect of SGLT2is on HF and CV outcomes, which are listed below:
|
Trial |
Study design |
Intervention |
Results |
|
Zannad et al (2020) [68] |
Meta-analysis of DAPA-HF (Dapagliflozin) and EMPEROR-Reduced trial (Empagliflozin) |
SGLT2is (Dapagliflozin, Empagliflozin) Placebo (N=8474) |
|
|
Vaduganathan et al. (2022) [69] |
Meta-analysis of DELIVER (Dapagliflozin) and EMPEROR-Preserved (Empagliflozin) trial |
SGLT2is (dapagliflozin, empagliflozin) Placebo (N=12251) |
SGLT2is reduced composite of CVD or first hospitalization for HF (HR 0.80 [95% CI 0.73–0.87]) among the patients of HFmrEF and HFpEF. |
|
Zou et al. (2022) [70] |
Systematic review and meta-analysis |
SGLT2is Control |
|
|
Jhund, et al. (2022) [71] |
Pre-specified meta-analysis of pooled, individual patient-level data from the DELIVER and DAPA-HF trials |
Dapagliflozin (N=11007) |
|
|
Bhatt, et al. (2023) [72] |
Participant-level pooled analysis from the DAPA-HF and DELIVER trials |
Dapagliflozin Placebo (N=11007) |
|
|
Carvalho, et al. (2023) [73] |
Meta-analysis of 9 RCTs |
SGLT2is in AHF (N=2884) |
|
|
Ostromonski, et al. (2023) [74] |
Post-hoc analysis of DELIVER trial |
Dapagliflozin (N=6263) |
Dapagliflozin showed the greatest benefits among those patients with the highest CRM multimorbidity. |
|
Berg, et al. (2021) [75] |
Secondary analysis of DAPA-HF trial |
Dapagliflozin (N=4744) |
|
|
Mizobuchi, et al. (2023) [76] |
Post-hoc analysis of prospective, observational registry |
Early versus late dapagliflozin initiation in AHF (N=118) |
The time to the initiation of dapagliflozin and length of hospital stay showed a significant positive correlation (P<0.001, r=0.46). |
|
AHF, Acute Heart Failure; CRM, cardio-renal-metabolic overlap; CVD, Cardiovascular death; GLP1RA, glucagon-like peptide 1 receptor agonists; KCCQ-TSS, Kansas City Cardiomyopathy Questionnaire-Total Symptom Score; HF, Heart Failure; MACE, major adverse cardiovascular events; SGLT2is, sodium-glucose cotransporter-2 inhibitors; HFmrEF, Heart Failure with mildly reduced ejection fraction; HFpEF, Heart Failure with preserved ejection fraction |
|||
Table 6: Clinical evidence for SGLT2is on HF and CV outcomes.
In the 2023 update of the European Society of Cardiology (ESC) guidelines for HF, the recommendation for SGLT2is has been updated to class IA from class IIa (2021, ESC guideline for HF). Thus, SGLT2is have class IA recommendation across the spectrum of EF in HF [77].
Expert Opinion
Rapid initiation of four Guidelines-Directed Medical Therapy (GDMT) classes for HF within 4 weeks enhances outcomes, with maximum GDMT benefits seen within 30 days. SGLT2is should be initiated once patients are stable, before discharge, without needing dose titration. Dapagliflozin and empagliflozin are suitable for HF patients across the EF spectrum, regardless of diabetes status. Volume status and renal function should be evaluated before starting SGLT2is, which are not recommended if eGFR is <25 ml/min/m².
Renoprotective benefits of SGLT2i in CKD
Current Evidence
Several hypotheses have been proposed to explain the nephroprotective effects of SGLT2is, including reduced renal hyperfiltration via tubuloglomerular feedback, decreased proximal tubule sodium reabsorption, and decreased energy consumption by proximal tubular cells. Additionally, SGLT2is have been shown to protect proximal tubular cells from glucotoxicity, enhance erythropoiesis, improve mitochondrial function, reduce oxidative stress, and decrease autophagy, podocyte injury, and renal inflammation (Figure 4) [78].
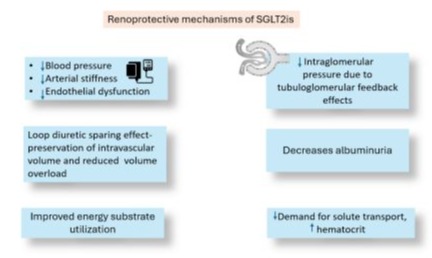
Figure 4: Renoprotective mechanisms of SGLT2is.
The clinical studies evaluating the effects of SGLT2is in CKD are summarised in the table below:
|
Trial |
Study design |
Intervention |
Results |
|
Lin et al. (2022) [79] |
Network meta-analysis |
Dapagliflozin 10 mg |
Compared with controls, dapagliflozin 10 mg (MD = - 3.08, 95% CI - 5.09 to - 1.06) reduced eGFR. Dapagliflozin 10 mg (MD = - 94.15, 95% CI - 111.72 to - 76.59) reduced UACR compared with controls. |
|
Ma et al. (2023) [80] |
Systematic review and metaanalysis |
SGLT2is |
In patients with non-diabetic CKD, treatment with SGLT-2is was associated with:
1.86, P<0.0001).
|
|
Chen et al. (2022) [81] |
Systematic review and metaanalysis |
SGLT2is Placebo |
27.5%.
|
|
Chertow et al. (2021) [82] (DAPA- CKD trial) |
Randomized, doubleblind, placebo-controlled, multicentre trial |
Dapagliflozin 10 mg Placebo |
Patients in the dapagliflozin group experienced a 27% ([95% CI]: −2 to 47%) reduction in the primary composite endpoint* and 29% (−2 to 51%), 17% (−53 to 55%), and 32% (−21 to 61%) reductions in the kidney, cardiovascular and mortality endpoints, respectively, relative to placebo. |
|
Zhang et al. (2024) [83] |
Systematic review and network meta-analysis |
SGLT2is Placebo |
• Results showed that SGLT2is significantly reduced serum uric acid levels in patients with CKD compared with the placebo group (SMD −0.22; 95% CI −0.42 to –0.03; GRADE: low) |
|
Fletcher et al. (2023) [84] |
Meta-analysis of the CREDENCE (Canagliflozin) and DAPA-CKD trials (Dapagliflozin) |
SGLT2is |
15% lower relative risk for discontinuation of RAS blockade in patients receiving SGLT2is (HR, 0.85; 95% CI, 0.74 to 0.99) compared to placebo. |
|
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; GLP1RA, glucagon-like peptide 1 receptor agonists; MD, mean difference; RAS, Renin-angiotensin system; SGLT2is, sodium-glucose cotransporter-2 inhibitors; T2DM, type 2 diabetes mellitus. * Primary composite end points: composite of sustained ≥50% eGFR decline, ESKD, and renal or CV death. |
|||
Table 7: Summary of clinical evidence of SGLT2is in CKD.
Although ACEis and ARBs are the foundational therapy for CKD, they are underused due to adverse effects like hyperkalemia, hospitalization, or Acute Kidney Injury (AKI). Fletcher, et al. assessed the impact of SGLT2is on the discontinuation of these drugs by conducting a joint analysis of CREDENCE and DAPA-CKD trials. Results showed that only 8.7% of patients discontinued ACEi/ARB use. It can be concluded that SGLT2is facilitates the use of ACEis/ARB in patients with albuminuric CKD with and without T2DM [84].
A cost analysis study was conducted by McEwan, et al. to assess the potential medical care cost offsets associated with reduced rates of cardio-renal outcomes. Patients treated with dapagliflozin and standard therapy experienced fewer incidents of End-Stage Kidney Disease (ESKD), and reductions in clinical events were associated with a 33% reduction in total costs [85].
The KDIGO 2024 guideline suggests initiating an SGLT2is as first-line drug therapy for patients with CKD who have an eGFR ≥ 20 ml/min per 1.73 m2 or UACR ≥ 200 mg/g or HF irrespective of the level of albuminuria (1A) [86].
Expert Opinion
Dapagliflozin and other SGLT2is represent the most impactful first-line therapy for individuals with CKD, irrespective of diabetes status. SGLT2is should be initiated as early as possible regardless of dose of Renin-Angiotensin System (RAS) blockers in patients with CKD. They can be continued till the advanced stage of CKD, even in patients on dialysis. The initial drop in eGFR with SGLT2is initiation necessitates monitoring for renal function. Careful monitoring for renal function and adverse effects of SGLT2is should be done with more frequent assessment in patients with advanced CKD.
Positioning of dapagliflozin across CMD
Based on the available clinical evidence and experts’ opinions, we propose the following positioning of dapagliflozin across the spectrum of CMD.
|
Recommendation |
Patient profile |
|
|
Strong clinical evidence for beneficial effect |
1. 2. 3. |
Type 2 Diabetes Mellitus (T2DM) T2DM associated with ASCVD or indicators of high cardiovascular risk Heart Failure (HF) |
|
4. |
Chronic Kidney Disease (CKD) |
|
|
Clinical evidence for a modest benefit |
1. 2. |
High body mass index (BMI) or obesity Hypertension |
|
3. |
Non-alcoholic fatty liver disease (NAFLD) |
|
|
Deficient data to assess the clinical benefit |
1. |
Prediabetes |
Table 8: Positioning of dapagliflozin across cardiometabolic disease.
Conclusion
SGLT2is have demonstrated consistent benefits across three chronic conditions: T2DM, HF, and CKD. These drugs improve glycemic control and confer significant CV and renal protection, making them a valuable therapeutic option for patients with these interrelated conditions. In addition to their primary effects, SGLT2is offer several pleiotropic benefits, including reductions in BP and weight, favorable impacts on lipid profiles, and improvements in insulin resistance and other parameters associated with NAFLD. These additional benefits underscore the potential of SGLT2is to provide comprehensive management across the spectrum of CMD, thereby enhancing overall patient outcomes.
References
- Kirk EP, Klein S (2009) Pathogenesis and Pathophysiology of the Cardiometabolic Syndrome. J Clin Hypertens 11: 761–765.
- Govindarajan G, Whaley-Connell A, Mugo M, Stump K, Sowers JR (2005) The Cardiometabolic Syndrome as a Cardiovascular Risk Factor - The American Journal of the Medical Sciences. Am J Med Sci 330: 311–318.
- Clinical Feature: Cardiometabolic Syndrome: A Package Deal (National Lipid Association) n.d. https://www.lipid.org/lipid-spin/ fall-2022/clinical-feature-cardiometabolic-syndrome-packagedeal#:~:text=Cardiometabolic%20syndrome%2C%20also%20 known%20as,retain%20key%20features%20.
- Bhalwar R (2020) Metabolic syndrome: The Indian public health perspective. Med J Armed Forces India 76: 8–16.
- Bansal S, Paliwal A, Verma V, Chauhan J (2017) Bansal: A study on prevalence of metabolic syndrome. Int J Res Med Sci 5:2641–2643.
- Barik A, Das K, Chowdhury A, Rai RK (2018) Barik: Metabolic syndrome among rural Indian adults. Clinical Nutrition ESPEN 23: 129–135.
- Prasad DS, Kabir Z, Dash AK, Das BC (2012) Prevalence and risk factors for metabolic syndrome in Asian Indians: A community study from urban Eastern India. J Cardiovasc Dis Res 3: 204–211.
- Khan Y, Lalchandani A, Gupta AC, Khadanga S, Kumar S (2018) Prevalence of metabolic syndrome crossing 40% in Northern India: Time to act fast before it runs out of proportions. J Family Med Prim Care 7: 118–123.
- Harikrishnan S, Sarma S, G S, Jeemon P, Krishnan MN, Venugopal K, et al. (2018) Prevalence of metabolic syndrome and its risk factors in Kerala, South India: Analysis of a community based cross-sectional study. PLOS ONE 13: e0192372.
- Bhat RA, Parray Irshad, Ahmad Z (2014) Prevalence of the Metabolic Syndrome among North Indian Adolescents Using Adult Treatment Panel III and Pediatric International Diabetic Federation Definitions. J Diabetes Metab 5.
- Venugopal V, Dongre AR, Saravanan S (2019) Prevalence and Determinants of Metabolic Syndrome among the Rural Adult Population of Puducherry. Indian J Community Med 44: 21–25.
- Madan J, Narsaria A (2016) Prevalence of metabolic syndrome in Mumbai City, India. Journal of Obesity and Metabolic Research 3: 16.
- Kapil U, Khandelwal R, Ramakrishnan L, Khenduja P, Gupta A, et al. (2018) Prevalence of metabolic syndrome and associated risk factors among geriatric population living in a high altitude region of rural Uttarakhand, India. J Family Med Prim Care 7: 709–716.
- Anto EO, Frimpong J, Boadu WIO, Tamakloe VCKT, Hughes C, et al. (2022) Prevalence of Cardiometabolic Syndrome and its Association With Body Shape Index and A Body Roundness Index Among Type 2 Diabetes Mellitus Patients: A Hospital-Based Cross-Sectional Study in a Ghanaian Population. Front Clin Diabetes Healthc 2: 807201.
- Bovolini A, Garcia J, Andrade MA, Duarte JA (2021) Metabolic Syndrome Pathophysiology and Predisposing Factors. Int J Sports Med 42: 199–214.
- Shi S, Huang H, Huang Y, Zhong VW, Feng N (2023) Lifestyle Behaviors and Cardiometabolic Diseases by Race and Ethnicity and Social Risk Factors Among US Young Adults, 2011 to 2018. Journal of the American Heart Association 12: e028926.
- Rastogi A, Januzzi JL (2023) Pleiotropic Effects of Sodium-Glucose Cotransporter-2 Inhibitors in Cardiovascular Disease and Chronic Kidney Disease. Journal of Clinical Medicine 12: 2824.
- Tentolouris A, Vlachakis P, Tzeravini E, Eleftheriadou I, Tentolouris N (2019) SGLT2 Inhibitors: A Review of Their Antidiabetic and Cardioprotective Effects. Int J Environ Res Public Health 16: 2965.
- Xu X, Xu W, Zhuo Q, Yan Y (2022) The efficacy and safety of dapagliflozin combined with oral hypoglycemic agents in patients with type 2 diabetes: a systematic review and meta-analysis. Annals of Palliative Medicine 11.
- Pan R, Zhang Y, Wang R, Xu Y, Ji H, et al. (2022) Effect of SGLT2 inhibitors on body composition in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. PLOS ONE 17: e0279889.
- Lazzaroni E, Ben Nasr M, Loretelli C, Pastore I, Plebani L, et al. (2021) Antidiabetic drugs and weight loss in patients with type 2 diabetes. Pharmacol Res 171: 105782.
- Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, et al. (2019) Comparison of the Effects of Glucagon-Like Peptide Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors for Prevention of Major Adverse Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus. Circulation 139: 2022–2031.
- Ghosal S, Sinha B (2023) Exploring the comparative cardiovascular death benefits of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a frequentist and Bayesian network meta-analysis-based scoring. Front Endocrinol (Lausanne) 14: 1168755.
- Yamada T, Wakabayashi M, Bhalla A, Chopra N, Miyashita H, et al. (2021) Cardiovascular and renal outcomes with SGLT-2 inhibitors versus GLP-1 receptor agonists in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and network meta-analysis. Cardiovasc Diabetol 20: 14.
- Chen L, Xue Q, Yan C, Tang B, Wang L, et al. (2023) Comparative safety of different recommended doses of sodium–glucose cotransporter 2 inhibitors in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis of randomized clinical trials. Front Endocrinol (Lausanne) 14: 1256548.
- Sridharan K, Sivaramakrishnan G (2024) Genito-urinary infectious adverse events related to sodium glucose cotransporter-2 inhibitors: a network meta-analysis and meta-regression. Expert Rev Clin Pharmacol 17: 515–524.
- ElSayed NA, Aleppo G, Bannuru RR, Bruemmer D, Collins BS, et al. (2024) 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2024. Diabetes Care 47.
- Hostalek U (2019) Global epidemiology of prediabetes - present and future perspectives. Clinical Diabetes and Endocrinology 5: 5.
- Pradeepa R, Mohan V (2021) Epidemiology of type 2 diabetes in India. Indian J Ophthalmol 69: 2932–2938.
- Jonas DE, Crotty K, Yun JDY, Middleton JC, Feltner C, et al. (2021) Screening for Prediabetes and Type 2 Diabetes Mellitus: An Evidence Review for the US Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality (US); 2021.
- Hostalek U, Campbell I (2021) Metformin for diabetes prevention: update of the evidence base. Curr Med Res Opin 37: 1705–1717.
- 3. Prevention or delay of diabetes and associated comorbidities: standards of care in diabetes—2024. Diabetes Care 2024; 47: S43– 51.
- RSSDI Clinical Practice Recommendations 2022. n.d.
- Rossing P, Inzucchi SE, Vart P, Jongs N, Docherty KF, et al. (2022) Dapagliflozin and new-onset type 2 diabetes in patients with chronic kidney disease or heart failure: pooled analysis of the DAPA-CKD and DAPA-HF trials. Lancet Diabetes Endocrinol 10: 24–34.
- Singh AK, Singh A, Singh R (2023) New-onset diabetes with sodiumglucose cotransporter-2 inhibitors in prediabetes: An updated metaanalysis and possible mechanisms. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 17: 102789.
- Veelen A, Andriessen C, Op den Kamp Y, Erazo-Tapia E, de Ligt M, Mevenkamp J, et al. (2023) Effects of the sodium-glucose cotransporter 2 inhibitor dapagliflozin on substrate metabolism in prediabetic insulin resistant individuals: A randomized, double-blind crossover trial. Metabolism 140: 155396.
- Færch K, Blond MB, Bruhn L, Amadid H, Vistisen D, et al. (2021) The effects of dapagliflozin, metformin or exercise on glycaemic variability in overweight or obese individuals with prediabetes (the PRE-D Trial):a multi-arm, randomised, controlled trial. Diabetologia 64: 42–55.
- Aras M, Tchang BG (2021) Obesity and Diabetes. Nursing Clinics of North America 56: 527–41.
- 8. Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Care in Diabetes–2024 n.d.
- Wang X, Wu N, Sun C, Jin D, Lu H (2023) Effects of SGLT-2 inhibitors on adipose tissue distribution in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Diabetol Metab Syndr 15: 113.
- Ma H, Lin Y-H, Dai L-Z, Lin C-S, Huang Y, et al. (2023) Efficacy and safety of GLP-1 receptor agonists versus SGLT-2 inhibitors in overweight/ obese patients with or without diabetes mellitus: a systematic review and network meta-analysis. BMJ Open 13: e061807.
- Wilding JPH, Rigney U, Blak BT, Nolan ST, Fenici P, et al. (2019) Glycaemic, weight, and blood pressure changes associated with early versus later treatment intensification with dapagliflozin in United Kingdom primary care patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 155: 107791.
- Iacobellis G, Gra-Menendez S (2020) Effects of Dapagliflozin on Epicardial Fat Thickness in Patients with Type 2 Diabetes and Obesity. Obesity (Silver Spring) 28: 1068–1074.
- Shrikrishnapalasuriyar N, Shaikh A, Ruslan AM, Sharaf G, Udiawar M, et al. (2020) Dapagliflozin is associated with improved glycaemic control and weight reduction at 44 months of follow-up in a secondary care diabetes clinic in the UK. Diabetes Metab Syndr 14: 237–239.
- Viswanathan V, Singh KP (2019) Use of Dapagliflozin in the Management of Type 2 Diabetes Mellitus: A Real-World Evidence Study in Indian Patients (FOREFRONT). Diabetes Technology & Therapeutics 21: 415–422.
- Shi F-H, Li H, Shen L, Fu J-J, Ma J, et al. (2021) High-dose sodiumglucose co-transporter-2 inhibitors are superior in type 2 diabetes: A meta-analysis of randomized clinical trials. Diabetes Obes Metab 3: 2125–2136.
- K S, A T, K N (2023) Recent understandings about hypertension management in type 2 diabetes: What are the roles of SGLT2 inhibitor, GLP-1 receptor agonist, and finerenone? Hypertension Research : Official Journal of the Japanese Society of Hypertension 46.
- Tsapas A, Karagiannis T, Kakotrichi P, Avgerinos I, Mantsiou C, et al. (2021) Comparative efficacy of glucose-lowering medications on body weight and blood pressure in patients with type 2 diabetes: A systematic review and network meta-analysis. Diabetes Obes Metab 23: 2116–2124.
- Li M, Yi T, Fan F, Qiu L, Wang Z, et al. (2022) Effect of sodium-glucose cotransporter-2 inhibitors on blood pressure in patients with heart failure: a systematic review and meta-analysis. Cardiovasc Diabetol 21: 139.
- Georgianos PI, Agarwal R (2019) Ambulatory Blood Pressure Reduction With SGLT-2 Inhibitors: Dose-Response Meta-analysis and Comparative Evaluation With Low-Dose Hydrochlorothiazide. Diabetes Care 42: 693–700.
- Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, et al. (2019) Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 380: 347–357.
- McMurray JJV, DeMets DL, Inzucchi SE, Køber L, Kosiborod MN, et al. (2019) A trial to evaluate the effect of the sodium–glucose cotransporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA-HF). European Journal of Heart Failure 21: 665–675.
- Sjöström CD, Johansson P, Ptaszynska A, List J, Johnsson E (2015) Dapagliflozin lowers blood pressure in hypertensive and nonhypertensive patients with type 2 diabetes. Diabetes and Vascular Disease Research 12: 352–358.
- Weber MA, Mansfield TA, Alessi F, Iqbal N, Parikh S, et al. (2016) Effects of dapagliflozin on blood pressure in hypertensive diabetic patients on renin–angiotensin system blockade. Blood Pressure 25: 93–103.
- Weber MA, Mansfield TA, Cain VA, Iqbal N, Parikh S, et al. (2016) Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double-blind, placebo-controlled, phase 3 study. The Lancet Diabetes & Endocrinology 4: 211–220.
- Hao Z, Sun Y, Wen Y, Cui L, Li G, et al. (2020) Effects and Mechanisms of Dapagliflozin Treatment on Ambulatory Blood Pressure in Diabetic Patients with Hypertension. Med Sci Monit 26: e925987-1-e925987-9.
- Ostrominski JW, Vaduganathan M, Selvaraj S, Claggett BL, Miao ZM, et al. (2023) Dapagliflozin and Apparent Treatment-Resistant Hypertension in Heart Failure With Mildly Reduced or Preserved Ejection Fraction: The DELIVER Trial. Circulation 148: 1945–1957.
- Xia M-F, Bian H, Gao X (2019) NAFLD and Diabetes: Two Sides of the Same Coin? Rationale for Gene-Based Personalized NAFLD Treatment. Front Pharmacol 10.
- Shalimar null, Elhence A, Bansal B, Gupta H, Anand A, et al. (2022) Prevalence of Non-alcoholic Fatty Liver Disease in India: A Systematic Review and Meta-analysis. J Clin Exp Hepatol 12: 818–829.
- American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Non-alcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings - Endocrine Practice n.d.
- Androutsakos T, Nasiri-Ansari N, Bakasis A-D, Kyrou I, Efstathopoulos E, et al. (2022) SGLT-2 Inhibitors in NAFLD: Expanding Their Role beyond Diabetes and Cardioprotection. International Journal of Molecular Sciences 23: 3107.
- Xu R, Lian D, Xie Y, Chen Z, Wang Y, et al. (2023) SGLT-2 Inhibitors for Non-Alcoholic Fatty Liver Disease: A Review. FBL 28: 134.
- Mantovani A, Petracca G, Csermely A, Beatrice G, Targher G (2020) Sodium-glucose cotransporter-2 inhibitors for treatment of nonalcoholic fatty liver disease: A meta-analysis of randomized controlled trials. Metabolites 11: 22.
- Eriksson JW, Lundkvist P, Jansson P-A, Johansson L, Kvarnström M, et al. (2018) Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia 61: 1923–1934.
- Shimizu M, K S, K K, T J, T I, et al. (2019) Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab 21.
- Kinoshita T, Shimoda M, Nakashima K, Fushimi Y, Hirata Y, et al. (2020) Comparison of the effects of three kinds of glucose‐lowering drugs on non‐alcoholic fatty liver disease in patients with type 2 diabetes: A randomized, open‐label, three‐arm, active control study. J Diabetes Investig 11: 1612.
- Arai T, Atsukawa M, Tsubota A, Mikami S, Ono H, et al. (2021) Effect of sodium-glucose cotransporter 2 inhibitor in patients with non-alcoholic fatty liver disease and type 2 diabetes mellitus: a propensity scorematched analysis of real-world data. Ther Adv Endocrinol Metab 12.
- Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, et al. (2020) SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 396: 819–29.
- Vaduganathan M, Docherty KF, Claggett BL, Jhund PS, Boer RA de, et al. (2022) SGLT2 inhibitors in patients with heart failure: A comprehensive meta-analysis of five randomised controlled trials. Lancet 400: 757–767.
- Zou X, Q S, Po V, G G, Cc L, et al. (2022) Sodium-Glucose Cotransporter-2 Inhibitors in Patients With Heart Failure : A Systematic Review and Meta-analysis. Annals of Internal Medicine 175.
- Jhund PS, Kondo T, Butt JH, Docherty KF, Claggett BL, et al. (2022) Dapagliflozin across the range of ejection fraction in patients with heart failure: a patient-level, pooled meta-analysis of DAPA-HF and DELIVER. Nat Med 28: 1956–1964.
- Bhatt AS, Kosiborod MN, Vaduganathan M, Claggett BL, Miao ZM, et al. (2023) Effect of dapagliflozin on health status and quality of life across the spectrum of ejection fraction: Participant-level pooled analysis from the DAPA-HF and DELIVER trials. Eur J Heart Fail 25: 981–988.
- Carvalho PEP, Veiga TMA, Simões e Silva AC, Gewehr DM, Dagostin CS, et al. (2023) Cardiovascular and renal effects of SGLT2 inhibitor initiation in acute heart failure: a meta-analysis of randomized controlled trials. Clin Res Cardiol 1–12.
- Ostrominski JW, Thierer J, Claggett BL, Miao ZM, Desai AS, et al. (2023) Cardio-Renal-Metabolic Overlap, Outcomes, and Dapagliflozin in Heart Failure With Mildly Reduced or Preserved Ejection Fraction. JACC Heart Fail 11: 1491–503.
- Berg DD, Jhund PS, Docherty KF, Murphy SA, Verma S, et al. (2021) Time to Clinical Benefit of Dapagliflozin and Significance of Prior Heart Failure Hospitalization in Patients With Heart Failure With Reduced Ejection Fraction. JAMA Cardiol 6: 499–507.
- Mizobuchi S, Saito Y, Miyagawa M, Koyama Y, Fujito H, et al. (2023) Early Initiation of Dapagliflozin during Hospitalization for Acute Heart Failure Is Associated with a Shorter Hospital Stay. Intern Med 62: 3107–3117.
- Bauersachs J, Soltani S (2024) [Heart failure: Update of the ESC 2023 guidelines]. Herz 49: 19–21.
- Kalay Z, Sahin OE, Copur S, Danacı S, Ortiz A, et al. (2022) SGLT-2 inhibitors in nephrotic-range proteinuria: emerging clinical evidence. Clin Kidney J 16: 52–60.
- Lin J, Wang S, Wen T, Zhang X (2022) Renal protective effect and safety of sodium-glucose cotransporter-2 inhibitors in patients with chronic kidney disease and type 2 diabetes mellitus: a network metaanalysis and systematic review. Int Urol Nephrol 54: 2305–2316.
- Ma C, Li X, Li W, Li Y, Shui F, et al. (2023) The efficacy and safety of SGLT2 inhibitors in patients with non-diabetic chronic kidney disease: A systematic review and meta-analysis. Int Urol Nephrol 55: 3167– 174.
- Chen H-B, Yang Y-L, Yu T-H, Li Y-H (2022) SGLT2 inhibitors for the composite of cardiorenal outcome in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Eur J Pharmacol 936: 175354.
- Chertow GM, Vart P, Jongs N, Toto RD, Gorriz JL, et al. (2021) Effects of Dapagliflozin in Stage 4 Chronic Kidney Disease. J Am Soc Nephrol 32: 2352–2361.
- Zhang L, Zhang F, Bai Y, Huang L, Zhong Y, et al. (2024) Effects of sodium-glucose cotransporter-2 (SGLT-2) inhibitors on serum uric acid levels in patients with chronic kidney disease: A systematic review and network meta-analysis. BMJ Open Diabetes Res Care 12: e003836.
- Fletcher RA, Jongs N, Chertow GM, McMurray JJV, Arnott C, et al. (2023) Effect of SGLT2 Inhibitors on Discontinuation of Renin– angiotensin System Blockade: A Joint Analysis of the CREDENCE and DAPA-CKD Trials. J Am Soc Nephrol 34: 1965.
- McEwan P, Hafner M, Jha V, Correa-Rotter R, Chernin G, et al. (2023) Translating the efficacy of dapagliflozin in chronic kidney disease to lower healthcare resource utilization and costs: a medical care cost offset analysis. J Med Econ 26: 1407–1416.
- Rossing P, Caramori ML, Chan JCN, Heerspink HJL, Hurst C, et al. (2022) Executive summary of the KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease: an update based on rapidly emerging new evidence. Kidney International 102: 990–999.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.