Expanding the Reconstructive Toolbox: External Oblique Myocutaneous Flap for Large Chest Wall Defects
by Silvy C. Akrawe, M.D.; Rijul S. Maini*, D.O.; Dunya Atisha, M.D., FACS.
Department of Plastic and Reconstructive Surgery, Henry Ford Health, Detroit, MI, USA 2799 W Grand Blvd, Detroit, MI 48202, USA
*Corresponding Author: Rijul S. Maini D.O., Department of Plastic and Reconstructive Surgery, Henry Ford Health, Detroit, MI, USA 2799 W Grand Blvd, Detroit, MI 48202, USA
Received Date: 03 January 2026
Accepted Date: 08 January 2026
Published Date: 12 January 2026
Citation: Akrawe S.C.; Maini R.S.; Atisha D (2026) Expanding the Reconstructive Toolbox: External Oblique Myocutaneous Flap for Large Chest Wall Defects J Surg 11:11536 https://doi.org/10.29011/2575-9760.011536
bstract
Background: Breast cancer resection can result in large chest wall defects. An underutilized but promising alternative to traditional workhorse and free flap reconstructive methods is the External Oblique Myocutaneous Flap (EOMCF). In this case series, we describe our institutional experience using the EOMCF for single-stage closure of large cancer-related chest wall defects.
Methods: A retrospective review of patients between 2014-2024 at Henry Ford Health (HFH) with advanced or recurrent breast cancer who underwent extensive mastectomy and EOMCF chest wall reconstruction. Extracted data included patient demographics, cancer history, resection margins, recurrence rates, post-operative complications, and overall survival. The senior author performed all reconstructive surgeries.
Results: Six women with mean age 67.2 years underwent EOMCF reconstruction following breast/chest wall cancer excision. Three patients had Radiation-Induced Angiosarcoma (RIAS), two invasive lobular carcinoma (ILC), and one Invasive Ductal Carcinoma (IDC). Average chest wall defect measured 30.83x18.33 cm. Mean defect area coverage with EOMCF was 575 cm². Average operative time was 2.6 hours. No complete flap loss, postoperative hematoma, or abdominal wall hernia occurred. Two patients experienced partial flap necrosis. Three patients had disease recurrence within five years. Three mortalities occurred: two from metastasis, and one from chemotherapy-associated leukemia. Three patients are living without disease recurrence.
Conclusion: The EOMCF is a reliable reconstructive option for large chest wall defects. In elderly or medically complex patients, it allows durable wound closure and minimal donor-site morbidity. Although oncologic outcomes depend on tumor biology and resection margins, the EOMCF is a valuable tool in chest wall reconstruction.
Keywords: Breast Cancer Reconstruction; Chest Wall Defects; Chest Wall Reconstruction; External Oblique Myocutaneous Flap; Reconstructive Surgery; Single-Stage Closure
Introduction
Extensive resections of chest wall soft tissue for cancer extirpation, particularly following breast cancer or radiationinduced angiosarcoma, can result in large, complex soft tissue defects that are challenging to reconstruct. Achieving reliable and timely coverage is essential not only for wound healing, but also to avoid delays in post-resection adjuvant therapy, which has been shown to reduce recurrence and mortality [1]. In many cases, reconstruction must be staged, especially when tumor margins are uncertain. However, when oncologic control can be confirmed pre-operatively, a single-stage approach is ideal, allowing patients to recover and proceed with adjuvant treatment more expeditiously. Workhorse pedicled flaps commonly used for chest wall reconstruction include the pectoralis major, latissimus dorsi, and rectus abdominis flaps. While effective in many cases, their size and vascular reliability may be insufficient to provide coverage for larger defects extending towards the clavicle or past the midline [2]. Additionally, free flap reconstruction may not be feasible and has a risk of complete flap failure. An underutilized yet promising alternative is the External Oblique Myocutaneous Flap (EOMCF). With dual blood supply from the deep circumflex iliac artery and segmental intercostal perforators, this Mathes and Nahai type V flap provides a generous surface area coverage (averaging 400 cm2 and reaching up to 800 cm2 in an obese individual), preserves some cutaneous innervation via the 6th - 12th intercostal nerves, allows for harvest without patient repositioning, and typically permits primary donor site closure [3-5]. Despite its anatomical advantages, the use of EOMCF in oncologic chest wall reconstruction is seldom reported in literature. There remains a need to better define its clinical utility, outcomes, and place within the reconstructive algorithm. In this case series, we describe our institutional experience using the external oblique myocutaneous flap for single-stage closure of large chest wall defects following cancer resection. We highlight surgical technique, patient outcomes, and flap feasibility as a reconstructive option for managing extensive chest wall defects.
Materials and Methods
Patients and Study Design
A retrospective review was conducted of patients with advanced or recurrent breast cancer who underwent extensive mastectomy and chest wall reconstruction using an External Oblique Myocutaneous Flap (EOMCF) at Henry Ford Health between September 2014 and September 2024. Extracted data included patient demographics, cancer history, tumor pathology, resection margins, recurrence rates, overall survival, and post-operative complications.
Histopathological Evaluation
Patients with Ductal Carcinoma In Situ (DCIS), Invasive Ductal Carcinoma (IDC), or Invasive Lobular Carcinoma (ILC) underwent preoperative biopsies, which were histologically confirmed by the pathology department prior to surgery and were planned for wide local excision or mastectomy. In cases of Radiation-Induced Angiosarcoma (RIAS), pre-operative punch biopsies were taken 5 cm circumferentially from the visible tumor margins and sent for pathology. Once pathology confirmed absence of malignancy in the punch biopsy samples, surgical oncology proceeded with wide local excision and a single stage reconstruction was performed by plastic surgery using EOMCF
Surgical Technique
All reconstructive surgeries were performed by the senior author. Under general anesthesia, patients were placed in a supine position with both arms abducted at 90 degrees. All patients received prophylactic preoperative antibiotics. Preoperative skin incision lines were marked to delineate the extent of the mastectomy, including the tumor and/or overlying skin, as well as the design for the ipsilateral EOMCF (Figure 1, 2A). Following wide local excision, the ipsilateral EOMCF was harvested. A midline incision was made through the skin and subcutaneous tissue, extending down to the anterior rectus fascia. The caudal border of the flap was designed in a “V” shape to facilitate V-Y primary closure and allow for a greater arc of cephalad rotation (Figure 1, 2A, 2B). The skin flap was elevated from medial to lateral in the subcutaneous plane, sparing the anterior rectus fascia until the semilunar line was identified. At this point, the external oblique aponeurosis was incised longitudinally, and the external oblique muscle was identified and separated from the underlying internal oblique. Dissection continued laterally between the external and internal oblique muscles to the midaxillary line. The external oblique muscle was then divided from its attachments to the anterior iliac crest and costal margin. The deep circumflex iliac artery was ligated and divided. SPY angiography was used to confirm adequate perfusion to the flap. Once sufficient vascular supply was verified, drains were placed beneath the flap, which was then rotated cephalad and inset into the defect. Closure was performed in three layers: Scarpa’s fascia was approximated first, followed by placement of deep dermal sutures, and completed with a subcuticular suture for skin closure (Figure 2C).
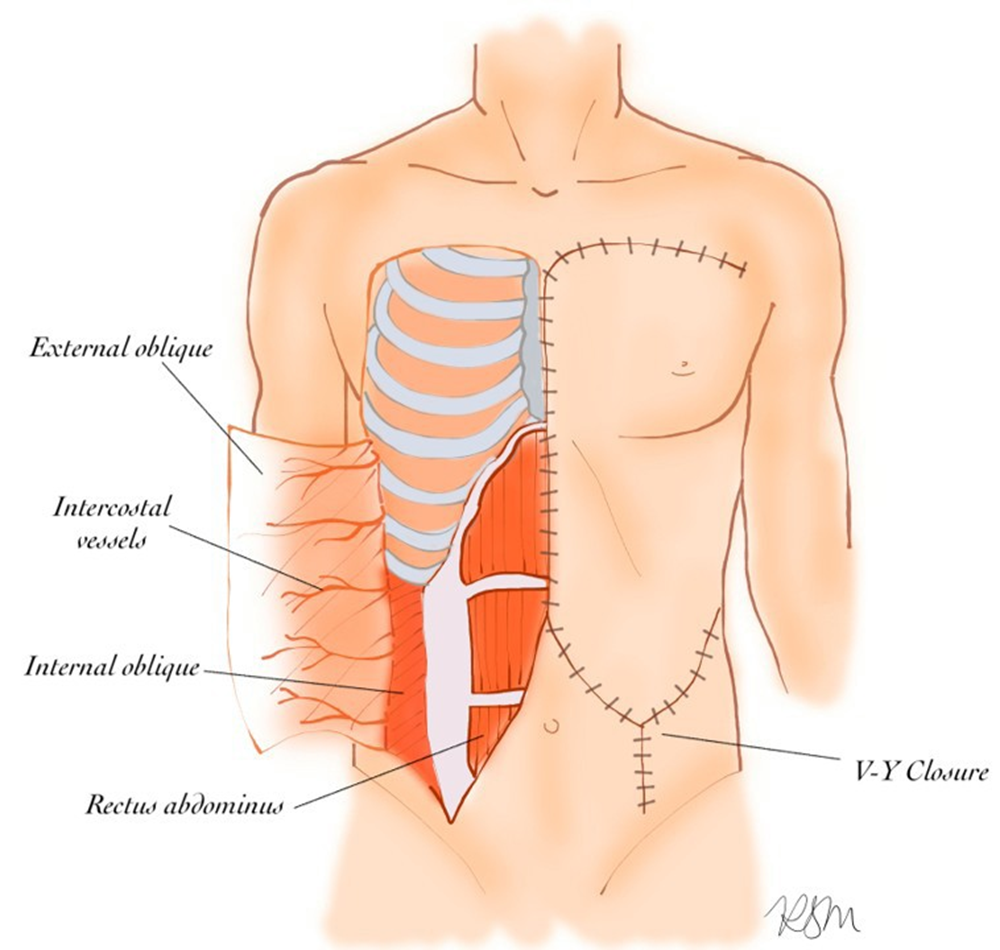
Figure 1: Elevation of external oblique to be used as rotational advancement flap (left). Closure of donor site in V-Y fashion to allow further advancement and greatest coverage (right). Illustration by author R.S.M.
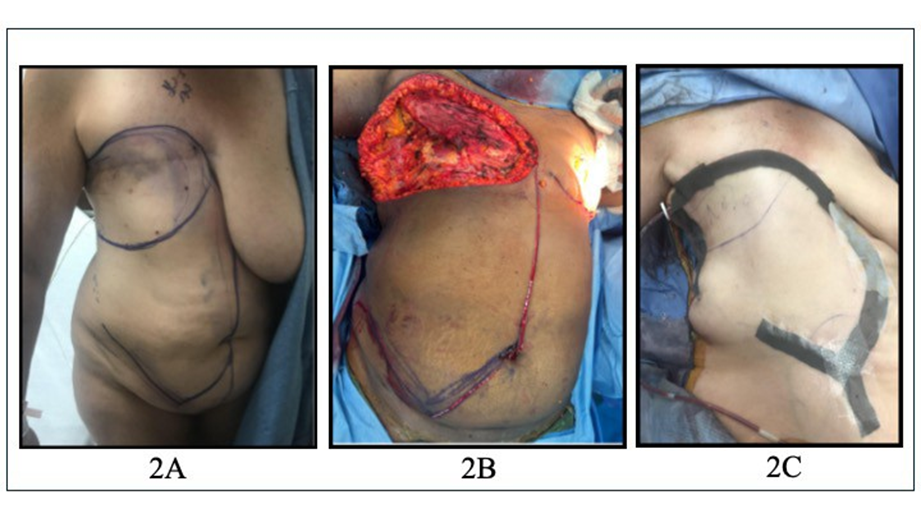
Figure 2: [A] External Oblique Myocutaneous Flap (EOMCF) preoperative markings; [B] Chest wall defect (top), intraoperative incision pattern (bottom); [C] V-Y closure.
Results
Over a ten-year period, six women were identified who underwent External Oblique Myocutaneous Flap (EOMCF) reconstruction following breast/chest wall cancer excision. The mean age was 67.2 years (range: 42-89 years), and the mean Body Mass Index (BMI) was 29.0 kg/m² (range: 22.7-34.7 kg/m²). Two patients (33%) were former smokers, while four (66%) had never smoked. Three patients (50%) had hypertension, and two (33%) had comorbidities such as hypercholesterolemia, diabetes, and/or coronary artery disease (Table 1). At the time of reconstruction, three patients carried a diagnosis of radiation-induced angiosarcoma (RIAS) following prior external beam radiation to the chest wall. All patients underwent immediate reconstruction at the time of wide local excision. Individual outcomes are detailed (Table 2). The mean tumor span was 86.75 mm (range: 42-150 mm). Following excision, the average chest wall defect measured 30.83 cm in length (range: 25-40 cm) and 18.33 cm in width (range: 10-25 cm). The mean area of the chest wall skin defect reconstructed with an EOMCF was 575 cm² (range: 250-800 cm²). The average pathologic margin was 16.7 mm (range: 2-35 mm). Mean operative time for flap harvest and inset was 2.6 hours (range 1.6-3.9). Patients required an average of 2.5 drains (range 2-4), which were on average removed during the first or second postoperative visit. There were no cases of complete flap loss, postoperative hematoma, or abdominal wall hernia. All patients healed within an appropriate timeframe. Two patients (33%) experienced partial flap necrosis—one managed surgically with a 12 × 4 cm skin graft, and the other treated conservatively with bedside debridement and local wound care. During follow-up, three patients experienced disease recurrence within five years. There were three total mortalities: two due to metastatic progression, occurring at a mean of 30.5 months postoperatively, and one secondary to chemotherapy-associated leukemia. Three patients are living without evidence of disease recurrence (Table 3).
Table 1: Patient smoking history and comorbidities.
|
Patient Smoking History and Comorbidities |
|
|
Smoking Status |
Patients (n=6) |
|
Never |
4/6 (66.7%) |
|
Former |
2/6 (33.3%) |
|
Comorbidities |
|
|
None |
1/6 (16.7%) |
|
Hypertension |
3/6 (50%) |
|
High Cholesterol |
2/6 (33.3%) |
|
Diabetes Mellitus |
2/6 (33.3%) |
|
Coronary Artery Disease |
2/6 (33.3%) |
Table 2: Patient demographics, cancer type, tumor and defect characteristics, pathologic margins, and operative times.
|
Patient Demographics and Cancer Characteristics |
|||||||||
|
Patient |
Age |
BMI |
Cancer |
Tumor Span (cm) |
Defect Length (cm) |
Defect Width (cm) |
Defect Area (cm2) |
Path Margins (mm) |
OR time (min.) |
|
1 |
76 |
31.7 |
RIAS |
8.0 |
40 |
20 |
800 |
15 |
95 |
|
2 |
89 |
30.9 |
RIAS |
4.2 |
30 |
25 |
750 |
25 |
141 |
|
3 |
65 |
28.3 |
RIAS |
7.5 |
30 |
16 |
480 |
10 |
157 |
|
4 |
42 |
34.7 |
ILC |
15.0 |
30 |
19 |
570 |
2 |
232 |
|
5 |
67 |
22.7 |
ILC |
19.6 |
25 |
10 |
250 |
35 |
124 |
|
6 |
64 |
25.7 |
IDC |
4.7 |
30 |
20 |
600 |
10 |
185 |
|
Avg. (range) |
67.2 (42- 89) |
29.0 (22.7- 34.7) |
8.67 (4.2-15) |
30.83 cm (25- 40) |
18.33 cm (10-25) |
575 cm2 (250-800) |
16.7 mm (2-35) |
156 min (95-232) |
|
Table 3: Patient complications, adjuvant chemotherapy, time to recurrence, disease specific survival*, and 5-year survival. *(>months) means the patient is living at the time this study was completed.
|
Patient Demographics and Treatment Characteristics |
|||||||||
|
Patient |
Age |
BMI |
Cancer |
Complications |
Time to Adjuvant therapy (months) |
Type of Adjuvant Therapy |
Time to Recurrence(months) |
DiseaseSpecific Survival After Definitive Resection and Flap Coverage (months)* |
5-year survival |
|
1 |
76 |
31.7 |
RIAS |
none |
N/A |
N/A |
20.6 |
Yes (>76.0) |
Yes |
|
2 |
89 |
30.9 |
RIAS |
Seroma, infection, full thickness necrosis, abdominal bulge |
N/A |
N/A |
23.0 |
No (31.5) |
No (metastasis) |
|
3 |
65 |
28.3 |
RIAS |
Full thickness necrosis |
3.0 |
Chemo + radiation |
14.2 |
Yes (29.0) |
No (chemoinduced leukemia) |
|
4 |
42 |
34.7 |
ILC |
Bilateral PE |
2.0 |
Chemo + radiation |
N/A |
Yes (>14.3) |
Yes |
|
5 |
67 |
22.7 |
ILC |
none |
3.0 |
Chemo |
N/A |
Yes (>28) |
Yes |
|
6 |
64 |
25.7 |
IDC |
none |
1.1 |
Chemo |
N/A |
No (29.5) |
No (metastasis) |
|
Avg. (range) |
67.2 (42- 89) |
29.0 (22.7- 34.7) |
2.3 (1.1-3.0) |
19.3 (14.2-23.0) |
|||||
Discussion
Key Findings and Potential Clinical Advantages of EOMCF
A major strength of this technique is its ability to facilitate singlestage reconstruction following radical tumor resection. In our cohort, negative surgical margins were achieved in all patients, with an average clearance of 16.7 mm, allowing for the timely initiation of adjuvant therapy. This is particularly critical in aggressive malignancies such as Radiation-Induced Angiosarcoma (RIAS), where treatment delays can have a significant impact on prognosis [6]. The use of the EOMCF enabled radical oncologic resection with immediate defect closure—an essential prerequisite for maintaining treatment timelines and optimizing disease control. Compared to free flaps, the External Oblique Myocutaneous Flap (EOMCF) offers several practical advantages, particularly for elderly or medically complex patients. Flap harvest and inset are technically straightforward, do not require microsurgical skill, do not require patient repositioning, and do not require higher level of post-operative care. and were completed in an average operative time of 2.6 hours-significantly shorter than other flap or microsurgical reconstructions. By avoiding prolonged operative time and eliminating the need for microsurgical anastomosis, the EOMCF serves as a valuable reconstructive option for large chest wall defects in a single-stage procedure. While free flaps remain a mainstay in chest wall reconstruction, the EOMCF represents a technically simpler, shorter, and lower-risk alternative in appropriately selected cases. If executed correctly with maintenance of at least two perforators, there is essentially no risk of complete flap loss, which is not the case with free-flap reconstruction. In the setting of RIAS, the mean time to recurrence has been reported to be 10 months (range 3-18) with mean disease specific survival being 3.1 years (range 1-6) [7]. While the prognosis is bleak, our RIAS patients who underwent resection and reconstruction with EOMCF had a mean time to recurrence of 19.3 months, over 9 months more than the reported mean time to recurrence. Two of our RIAS patients had mean disease-specific survival times of 52.5 months (range 29-76), roughly 4.4 years, which is greater than the reported mean disease-specific survival of 3.1 years [7]. In this patient population, the EOMCF may be a viable and less morbid reconstructive option compared to other reconstructive methods.
Complications
Complications were observed in three patients, primarily those with significant comorbidities and concurrent metastatic disease. An 89-year-old patient (patient 2) with coronary artery disease and hypertension developed a small area of flap necrosis, which was ultimately resolved with conservative management. Patient 3 experienced a small area of full-thickness flap necrosis, necessitating coverage with a 12 x 4 cm split-thickness skin graft. Patient 4, who developed bilateral pulmonary embolisms, was in the setting of metastatic disease at the time of the operation. There were no cases of complete flap failure, postoperative hematoma, or abdominal wall hernia. These findings suggest that while the EOMCF is a reliable reconstructive option, postoperative outcomes are closely influenced by patient selection and underlying comorbidities.
Limitations
This study is limited by its small sample size, retrospective design, and heterogeneity of cancer diagnoses. These limitations precluded statistical comparisons with alternative reconstructive techniques. Nonetheless, given the complexity of large chest wall defects addressed by this approach and the inclusion of patients with Radiation-Induced Angiosarcoma (RIAS)—a particularly aggressive and rare malignancy—this series provides valuable insights into the reconstructive utility of the EOMCF. Future multicenter or prospective studies are needed to better define patient selection criteria, complication rates, and long-term outcomes compared to other reconstructive options. Additionally, since most patients in this cohort were overweight or obese with ample donor tissue for flap advancement, caution should be taken when considering this technique for very lean patients with limited available tissue.
Conclusion
The External Oblique Myocutaneous Flap (EOMCF) is a reliable and technically straightforward reconstructive option that provides effective coverage following wide local excision of advanced breast and chest wall cancers. This flap enables durable wound closure with minimal donor-site morbidity, even in elderly or medically complex patients and in the context of large, irradiated defects. While oncologic outcomes depend primarily on tumor biology and resection margins rather than the reconstructive technique, the EOMCF consistently meets its reconstructive goals, reinforcing its value as an essential tool in the chest wall reconstruction toolbox.
Acknowledgements: None
Ethical Approval: All procedures performed in this study were in
accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study (retrospective), formal consent is not required.
Conflict of Interest Statement: The senior author Dunya Atisha M.D. is a key opinion leader for MTF Biologics. The authors have no conflicts of interest to disclose.
References
- EBCTCG (Early Breast Cancer Trialists’ Collaborative Group) (2014) Effect of radiotherapy after mastectomy and axillary surgery on 10year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. The Lancet 383: 2127-2135.
- Matros E, Disa J (2011) Uncommon Flaps for Chest Wall Reconstruction. Seminars in Plastic Surgery 25: 55-59.
- Billington A, Dayicioglu D, Smith P, Kiluk J (2019) Review of Procedures for Reconstruction of Soft Tissue Chest Wall Defects Following Advanced Breast Malignancies. Cancer Control 26: 1073274819827284.
- Schlenz I, Burggasser G, Kuzbari R, Eichberger H, Gruber H, et al. (1999) External oblique abdominal muscle: a new look on its blood supply and innervation. The Anatomical Record 255: 388-395.
- Bogossian N, Chaglassian T, Rosenberg PH, Moore MP (1996) External Oblique Myocutaneous Flap Coverage of Large ChestWall Defects Following Resection of Breast Tumors. Plastic & Reconstructive Surgery 97: 97-103.
- Torres KE, Ravi V, Kin K, Yi M, Guadagnolo BA (2012) Long-Term Outcomes in Patients with Radiation-Associated Angiosarcomas of the Breast Following Surgery and Radiotherapy for Breast Cancer 20: 1267-1274.
- Alves I, Marques JC (2018) Radiation-induced angiosarcoma of the breast: a retrospective analysis of 15 years’ experience at an oncology center. Radiologia Brasileira 51: 281-286.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.