Evaluating the Appropriateness of Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers: A Scoping Review of Initiation and Ongoing Treatment
by Amr Musa Basheer*, Mohamed Musa Basheer
Medical Student at The Royal College of Surgeons in Ireland – Medical University of Bahrain, P.O. Box 15503, Adliya, Bahrain
*Corresponding author: Amr Musa Basheer, Medical Student at The Royal College of Surgeons in Ireland – Medical University of Bahrain, P.O. Box 15503, Adliya, Bahrain
Received Date: 03 October, 2025
Accepted Date: 16 October, 2025
Published Date: 29 October, 2025
Citation: Basheer AM, Basheer MM (2025) Evaluating the Appropriateness of Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers: A Scoping Review of Initiation and Ongoing Treatment. J Family Med Prim Care Open Acc 9: 291. https://doi.org/10.29011/2688-7460.100291
Abstract
Background: Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are essential in managing numerous cardiovascular and renal disorders. Despite their prevalent application, the suitability of their commencement and continued treatment remains a subject of considerable clinical interest and contention. Objective: To evaluate the suitability of ACIs and ARBs before commencement and throughout therapy, following current guidelines and recent evidence. Data Sources: PubMed, CINAHL, EMBASE, Medline, Wiley, ScienceDirect, Scopus, Google Scholar, and Cochrane Library databases. Guidelines from the American College of Cardiology, American Heart Association, European Society of Hypertension, Journal of Cardiovascular Pharmacology, Clinical Therapeutics, Journal of Clinical Hypertension, and Diabetes Care. Kidney International and Circulation from 2017 to 2024. Data Extraction: The two reviewers (AMB and AMB) independently compiled the data into tabulated formats, encompassing authors and publication year, country, title, methodology, objectives, measures, sampling, sample size, statistical tests, outcomes, themes, key findings, confounding variables, biases, and overall quality. The gathered data were associated with the research questions, aims, and objectives. Results: The preliminary search yielded 187 studies, from which we picked eleven studies using the database. The research identifies five themes that influence the suitability of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before initiation and throughout continuous treatment. The themes include adherence to guidelines and patient outcomes, variations in clinical practice, special populations, and emerging evidence and trends. Major clinical guidelines and governmental agencies highlight a patient-centred approach, focusing on individualized treatment strategies, close monitoring, and dose adjustments to maximize therapeutic benefit while minimizing the risk of adverse outcomes. Conclusion: Current guidelines and evidence support initiating and continuing therapy with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers; however, clinical decisions must be tailored to individual patient profiles and emerging evidence. Additional research is needed to fill knowledge gaps and improve treatment techniques for enhanced patient outcomes.
Keywords: Appropriateness and safety of Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers; Cardiovascular Disease; Hypertension; Heart Failure; Diabetes; Renal Insufficiency; Chronic Kidney Disease; Clinical Guidelines
What is Already Known
ACEIs and ARBs are commonly recommended for cardiovascular and renal disorders, although adherence to evidence-based introduction and monitoring is uneven. What this study adds: This study synthesises global recommendations and evidence, identifies gaps in monitoring for safety and appropriateness, and proposes strategies to enhance the use of ACEIs and ARBs in various patient populations.
Abbreviations: ACC: American College of Cardiology; ACEIs: Angiotensin Converting Enzyme Inhibitors; AHA: American Heart Association; ARBs: Angiotensin Receptor Blockers; BNF: British National Formulary; CVD: Cardiovascular Disease; CKD: Chronic Kidney Disease; CHF: Chronic Heart Failure; eGFR: Estimated Glomerular Filtration Rate; ESC: European Society of Cardiology; HF: Heart Failure; (HFpEF): Heart failure with a preserved Ejection Fraction; RAAS/RAS: Rennin-AngiotensinAldosterone System; RFT: Renal Function Test
Introduction
Angiotensin-Converting Enzyme inhibitors (ACEIs) and Angiotensin Receptor Blockers (ARBs) are among the most frequently prescribed drugs worldwide in healthcare centres and hospitals. These medications have significantly improved the management of hypertension and Chronic Heart Failure (CHF), and have delayed disease progression in individuals with Chronic Kidney Disease (CKD) (European Society of Cardiology [ESC], 2024; British National Formulary [BNF], 2024). Yet, variability exists in their initiation, monitoring, and long-term use across guidelines and clinical practice. The Renin-AngiotensinAldosterone System (RAAS) regulates blood pressure, fluid balance, and electrolyte levels. This system is primarily activated by decreases in blood pressure, blood volume, or sodium levels, leading to hormonal responses. As shown in Figure 1, the RAAS acts mainly through its effectors—such as angiotensin II, aldosterone, and renin—that control vascular tone, sodium reabsorption, and potassium excretion. Angiotensin II, the main effector molecule of the RAAS, causes vasoconstriction by binding to Angiotensin II Type 1 (AT1) receptors, thus increasing blood pressure. It also stimulates aldosterone release from the adrenal glands, promoting salt retention and potassium excretion in the kidneys, which increases blood volume and pressure. Dysregulation of the RAAS is linked to various cardiovascular disorders, including hypertension, heart failure, and CKD (Fyhrquist & Saijonmaa, 2008).
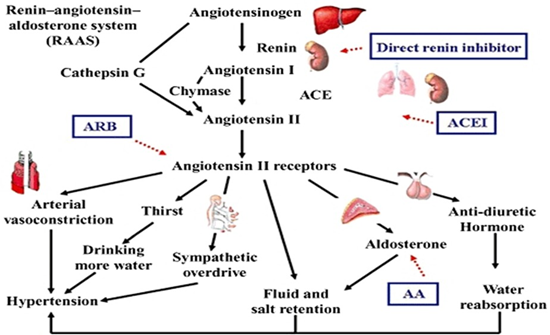
Figure 1: Renin-angiotensin-aldosterone system (RAAS), British Journal of Pharmacology.
Although widely used, ACEIs and ARBs are associated with different side effects and potential medication interactions. Clinicians must carefully evaluate these factors before prescribing these drugs for long-term therapy, with patient safety as a priority. Understanding their suitability before starting treatment and during ongoing management is crucial for optimising patient outcomes, ensuring safety, and reducing potential risks (American College of Cardiology [ACC], 2024; American Heart Association [AHA], 2024). The safety and efficacy of ACEIs and ARBs have been extensively studied; however, clinical practices regarding their use and management continue to evolve. The ACC/AHA (2024) guidelines provide recommendations that influence treatment decisions. Nonetheless, gaps remain in understanding the appropriateness of these medications both before initiation and during continuous therapy. We hypothesise that inconsistencies in guideline recommendations and clinical implementation impact the safety, effectiveness, and outcomes of ACEI and ARB therapy. This review aims to summarise current guidelines, evaluate clinical evidence on management and outcomes, and identify factors influencing the use of ACEI and ARB in real-world practice. The review considers the research question: What are the current guidelines, practices, and evidence guiding the initiation and maintenance of ACEIs and ARBs, and what factors influence their implementation across various clinical settings? It synthesises evidence from clinical guidelines, randomised controlled trials, observational studies, and systematic reviews, highlighting trends, gaps, and barriers to effective deployment. The review consolidates research about ACEIs and ARBs, affirming their efficacy and safety across diverse patient groups and treatment scenarios. Its goal is to summarise, assess, and integrate existing data to inform clinical decisions, while identifying key themes, deficiencies, and future research directions.
Materials and Methods
Study Design
This review analyzes and synthesizes the literature on existing guidelines, practices, and evidence for the suitability of ACE inhibitors and angiotensin receptor blockers before beginning and throughout continuous treatment. This review adhered to the Preferred Reporting Items for Systematic Reviews and MetaAnalyses Protocols (PRISMA) criteria (Page et al., 2021). The principles established by Arksey and O’Malley (2005) were employed, encompassing the identification of straightforward research questions and objectives, formulation of search strategies, selection of pertinent research articles, extraction and organization of data, and ultimately the summarization, analysis, and presentation of findings in the report (Arksey & O’Malley, 2005).
Data Sources and Search
The two reviewers (AMB and MMB) separately performed an extensive literature review on research pertinent to current guidelines, practices, and evidence regarding the appropriateness of ACEIs and ARBs. They also thoroughly searched PubMed, CINAHL, EMBASE, Medline, Wiley, ScienceDirect, Scopus, Google Scholar, and Cochrane Library databases. Guidelines from the American College of Cardiology, American Heart Association, European Society of Hypertension, Journal of Cardiovascular Pharmacology, Clinical Therapeutics, Journal of Clinical Hypertension, and Diabetes Care. Kidney International and Circulation from 2017 to 2024. We utilised the Boolean operators “AND” and “OR” in conjunction with other database descriptions. Keywords, Medical Subject Headings (MeSH) terms, and free-text words in titles, abstracts, and index terms used in the search included “angiotensin-converting enzyme inhibitors,” “angiotensin II type 1 receptor blockers,” “hypertension management,” “heart failure management,” “appropriateness of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor blockers,” “chronic kidney disease management,” “renal insufficiency management,” “renal function monitoring,” “cardiovascular risk assessment,” “angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor blockers monitoring,” and “hyperkalaemia.”
Eligibility Criteria and Selection Relevant Studies
The two reviewers (AMB and MMB) independently assessed the titles, abstracts, keywords, and full texts of articles and guidelines released from 2018 to 2024. This scoping review includes studies involving adult patients (18 years and older) treated with ACEIs or ARBs for disorders such as hypertension, heart failure, chronic kidney disease, and other cardiovascular diseases. The research evaluates ACEIs or ARBs in various therapeutic settings, with a focus on their introduction and ongoing management. Furthermore, research will encompass comparisons of ACEIs and ARBs with other antihypertensive or cardiovascular agents, placebo, or no intervention. Key factors will also be evaluated, including appropriateness, safety, efficacy, and the influence of using ACEIs and ARBs. The review will encompass clinical guidelines, systematic reviews, meta-analyses, randomized controlled trials (RCTs), cohort studies, and observational studies published in peerreviewed journals. Conversely, studies focusing solely on pediatric and non-peer-reviewed articles, such as opinion pieces, editorials, conference abstracts, and non-English-language publications, were excluded. Furthermore, any studies that do not specifically evaluate the initiation or ongoing use of ACEIs and ARBs (for instance, studies that only discuss their mechanisms of action) will also be excluded, as will articles that are not available in full text. In adult patients (≥18 years) with hypertension, heart failure, chronic kidney disease, or other cardiovascular conditions (Population), what is known about the use of ACEIs and ARBs, including their appropriateness, safety, efficacy, and factors influencing use (Concept), across clinical settings involving initiation, ongoing management, or comparison with other cardiovascular therapies (Context)? This review followed the PRISMA flowchart to select studies, as shown in Figure 2, to ensure transparency and rigour in the reporting process.
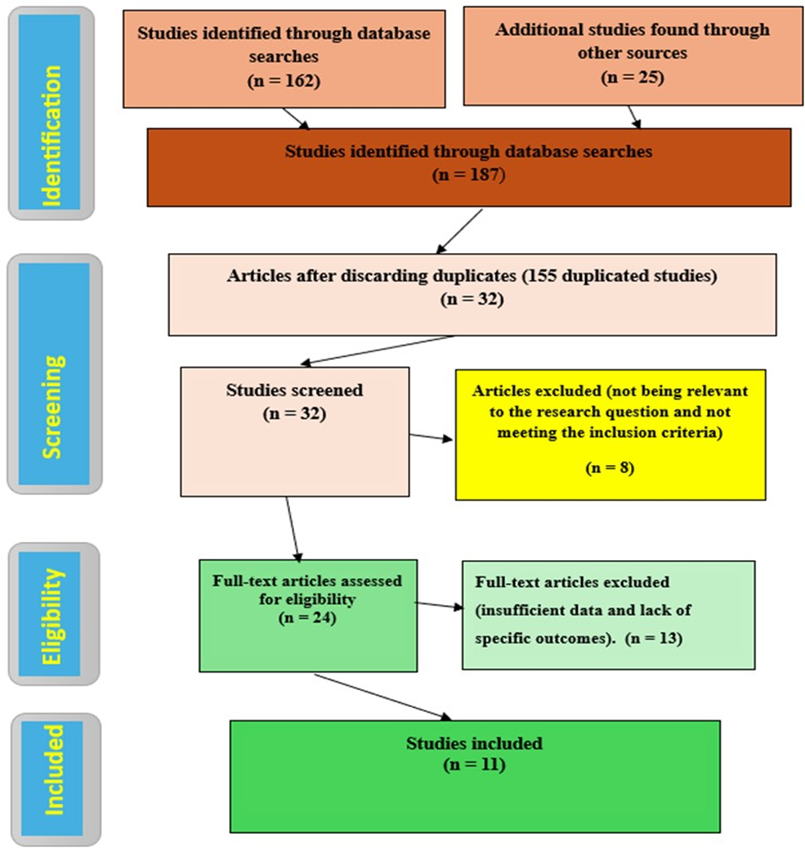
Figure 2: PRISMA Flow Diagram of The Study Selection Process. (n number of articles).
Data Extraction (Collection Process) and Charting
The two reviewers (AMB and SEA) independently summarized the data in tabulated forms, including authors and year of publication, country, title, methodology, aims and goals, measures, sampling, sample size, statistical tests, outcomes, themes, key findings, confounding variables, biases, and overall quality. The extracted data were linked to the research questions, aims, and objectives. The reviewers compared their results, discussed, and resolved disagreements and discrepancies in data extraction. Relevant authors were contacted as needed for additional data, clarification or missing information.
Summarizing and Presenting Findings (Analysis and Synthesis)
We utilized descriptive and thematic analysis to discern patterns, similarities, and discrepancies among studies and guidelines, emphasizing gaps while concentrating on current guidelines, practices, and evidence of the suitability of ACEIs and ARBs, both before initiation and throughout ongoing treatment. The two reviewers (AMB and MMB) independently synthesized, summarized, compared and presented the findings in two main parts: the appropriateness before and during treatment and five themes, including adherence to guidelines, patient outcomes, variations in clinical practice, special populations, and emerging evidence and trends.
Results
The results were organised, emphasizing main topics, discoveries, and implications for therapeutic practice.
Study Selection (Flow of the Studies)
Figure 2 illustrates that 187 studies were identified in the preliminary search. We gathered 162 studies from Science Direct, PubMed, Wiley, Scopus, CINAHL, EMBASE, Google Scholar, and Cochrane. Guidelines from the ACC, AHA), ESC, and relevant journals. Systematic reviews and meta-analyses from journals like the Journal of Cardiovascular Pharmacology, Clinical Therapeutics, and Diabetes Care. Original research articles from Kidney International, Circulation, and Journal of Clinical Hypertension. We generated 25 additional studies from other sources such as university sites, Academia, and ResearchGate. After removing duplicates, 32 studies remained. These were screened, and eight studies that only provided abstracts were excluded. 24
full texts remained, and 13 were excluded. Finally, eleven studies were included in the synthesis, covering a broad spectrum of topics related to ACEIs and ARBs, including guidelines and safety, comparative effectiveness, and emerging research.
Characteristics of Included Studies
Eleven studies fulfilled all inclusion criteria and were summarized in tables, five themes and Key findings. Table 1 outlines the authors’ publishing year, country, title, methodology, objectives, and metrics. Table 2 delineates sampling, sample size, statistic tests, outcomes, and thematic elements. Table 3 illustrates key findings, confounding variables, biases, and overall quality. Theme 1, adherence to guidelines; Theme 2, patient outcomes; Theme 3, variations in clinical practice; Theme 4, special populations; and Theme 5, emerging evidence and trends. We categorized the critical findings into two categories: the appropriateness of ACEIs and ARBs before and during ongoing treatment.
|
No |
Authors & year of publication |
Country |
Title |
Methodology |
Objectives |
Measures |
|
1 |
American
College of Cardiology. (2021) |
USA |
Guidelines
for the use of ACEIs and ARBs 2021 |
Evidence-based
guidelines |
Provide
comprehensive guidelines for ACEI and ARB use in cardiovascular conditions |
Clinical
guidelines |
|
2 |
Li et al. (2021). |
USA |
Appropriateness
of ACEIs and ARBs: A Systematic Review 2020 |
A systematic
review |
Assess the
appropriateness of ACEIs and ARBs in different clinical contexts |
Literature
review, clinical studies |
|
3 |
Singh et al., (2025) |
USA |
ACE
inhibitors |
Clinical
review article. Clinical guidelines, RCTs AND MA. Medical literature. |
To review the
indications, mechanism of action, administration, adverse effects, and
clinical toxicology of ACE inhibitors to improve patient care and outcomes. |
Clinical
practice review |
|
4 |
2017 ACC/AHA hypertension guidelines |
USA |
2017 ACC/AHA
Hypertension Guidelines |
Evidence-based
guidelines |
Outline
guidelines for hypertension management, including ACEIs and ARBs |
Clinical
guidelines |
|
5 |
European
Society of Cardiology (2021) |
Europe |
2021 ESC
Guidelines for the diagnosis and treatment of acute and chronic heart failure |
Evidence-based
guidelines |
Provide
updated guidelines for heart failure management, including ACEIs and ARBs |
Clinical
guidelines |
|
6 |
American
Journal of Obstetrics and Gynecology (2020) |
USA |
Safety of ACE
Inhibitors and Angiotensin Receptor Blockers in Pregnancy: A Systematic
Review 2020 |
Systematic
review |
Assess the
safety of ACEIs and ARBs during pregnancy |
A systematic
review of Clinical data |
|
7 |
Kidney
International (2019) |
Global |
Long-term
Effects of Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor
Blockers on Renal Outcomes 2019 |
Observational
study |
Evaluate
long-term renal outcomes associated with ACEIs and ARBs |
Long-term
observational studies |
|
8 |
Diabetes Care
(2018) |
Global |
ACE
Inhibitors versus Angiotensin Receptor Blockers in Diabetes: A Meta-Analysis
2018 |
Meta-analysis |
Compare the
effectiveness of ACEIs and ARBs in diabetic patients |
Meta-analysis |
|
9 |
Journal of
the American College of Cardiology (2022) |
USA |
Comparative
Effectiveness of ACE Inhibitors and Angiotensin Receptor Blockers in Patients
with Heart Failure with Preserved Ejection Fraction 2022 |
Comparative
effectiveness study |
Compare ACEIs
and ARBs in patients with heart failure with preserved ejection fraction |
Comparative
effectiveness study |
|
10 |
Circulation
(2023) |
USA |
The Evolving
Role of Angiotensin Receptor Blockers in Cardiovascular Disease Management
2023 |
Review
article |
Review
emerging roles and evidence for ARBs in cardiovascular disease management |
Literature
review, recent studies |
|
11 |
Journal of
Clinical Hypertension (2024) |
USA |
ACE
Inhibitors vs. ARBs: Current Evidence and Future Directions 2024 |
Review
article |
Summarize
current evidence on ACEIs and ARBs and discuss future directions in their use |
Current
evidence reviews |
|
ACC: American College of
Cardiology, ACEIs: Angiotensin Converting Enzymes Inhibitors, AHA: American
Health Association, ARBs: Angiotensin Receptor Blockers, USA: United States
of America |
||||||
Table 1: Authors and year of publication, country, titles, methodology, aims and objectives, measures.
|
No |
Authors & year of publication |
Sampling |
Sample size |
Statistical tests |
Outcomes |
Themes |
|
1 |
American
College of Cardiology. (2021) |
Developed by
expert panels and review committees |
N/A
(guideline-based) |
N/A |
Evidence-based
recommendations for therapy |
-Efficacy in
hypertension and heart failure - First-line treatment options - Alternative use in case of
intolerance - Patient-specific recommendations |
|
2 |
Li et al.
(2021) |
Comprehensive
search and review of literature |
30+ studies
reviewed |
Meta-analysis |
Summarizes
effectiveness, safety, and indications for ACEIs and ARBs |
- General
effectiveness of ACEIs and ARBs - Tolerability and patient preference - Guidelines for use - Comparative effectiveness |
|
3 |
Singh et al., (2025) |
The
StatPearls entry is a clinical review article, not a single
primary research study |
N/A (review
of practices) |
N/A |
ACE
inhibitors are first-line for hypertension, heart failure with reduced
ejection fraction (HFrEF), chronic kidney disease (CKD), and post-myocardial
infarction (MI). They provide mortality benefits in these conditions. |
- Safety profile - Clinical Pear |
|
4 |
2017 ACC/AHA hypertension guidelines |
Developed by
expert panels and review committees |
N/A
(guideline-based) |
N/A |
Detailed
recommendations for hypertension treatment, including ACEIs and ARBs |
- Blood
pressure targets - Use of ACEIs and ARBs in
hypertension - Management strategies - Special considerations for
different populations |
|
5 |
European
Society of Cardiology (2021) |
Developed by
expert panels and review committees |
N/A(guideline-based) |
N/A |
Comprehensive
treatment guidelines for heart failure with recommendations for ACEIs and
ARBs |
- Use of
ACEIs and ARBs in heart failure - Acute vs. chronic heart failure
management - Treatment protocols - Patient-specific considerations |
|
6 |
American Journal
of Obstetrics and Gynecology (2020) |
Comprehensive
search and selection of relevant studies |
15+ studies
reviewed |
Metanalysis |
Highlights
risks associated with ACEIs and ARBs in pregnancy |
- Risks
associated with ACEIs and ARBs during pregnancy - Recommendations for use in pregnant
patients - Safety profiles - Alternatives for pregnant patients |
|
7 |
Kidney
International (2019) |
Review of
long-term studies and clinical data |
Not specified |
Longitudinal
analysis |
Shows
long-term benefits of ACEIs and ARBs on renal outcomes, with some risks noted |
- Renal
protection - Long-term efficacy - Adverse effects on renal function - Monitoring and adjustment
strategies |
|
8 |
Diabetes Care
(2018) |
Systematic
search and selection from multiple databases |
20+ studies included |
Meta-analysis |
Compares the
efficacy and safety of ACEIs vs. ARBs in diabetes management |
- Comparative
efficacy in diabetes management - Side effect profiles - Long-term outcomes - Patient subgroups and preferences |
|
9 |
Journal of
the American College of Cardiology (2022) |
Review of
clinical studies and trials |
Not specified |
Comparative
analysis |
Both ACEIs
and ARBs are effective, with nuances in patient response and tolerance |
- ACEIs vs.
ARBs in HFpEF - Clinical outcomes - Treatment response based on patient
characteristics - Recommendations for therapy |
|
10 |
Circulation
(2023) |
Literature
review and synthesis of recent studies |
N/A (review
of multiple studies) |
Review and
synthesis |
ARBs have
expanding indications and evolving evidence in cardiovascular disease
management |
- Expanding
indications for ARBs - Comparative effectiveness - Innovations in ARB therapy - Future research directions |
|
11 |
Journal of
Clinical Hypertension (2024) |
Literature
review and synthesis of recent studies |
N/A (review
of current evidence) |
Review and
synthesis |
Provides an
update on the current state of knowledge regarding ACEIs and ARBs |
- Current
evidence on ACEIs vs. ARBs - Gaps in research - Future research needs - Evolving treatment paradigms |
|
ACEIs:
Angiotensin Converting Enzymes Inhibitors, ARBs: Angiotensin Receptor
Blockers, N/A: Not applicable |
||||||
Table 2: Authors and year of publication, sampling, sample size, statistical tests, outcomes and themes.
|
No |
Authors & year of publication |
Key findings |
Confounders variables |
Biases |
Overall quality |
|
|
1 |
American
College of Cardiology. (2021) |
Emphasizes
appropriate use of ACEIs and ARBs in various cardiovascular conditions, with
specific guidance on dosing and monitoring. |
Variability
in clinical practice, differences in patient populations, regional guidelines |
N/A |
High:
Comprehensive guidelines based on the latest evidence, developed by a leading
cardiology organization, and regularly updated. |
|
|
2 |
Li et al.
(2021) |
ACEIs and
ARBs are effective for hypertension and heart failure; ARBs are preferred in
patients intolerant to ACEIs. |
Differences
in study quality, variations in inclusion criteria, reporting biases |
Selection
bias, publication bias |
Good:
Systematic review focusing on appropriateness, though quality depends on
included studies and potential publication bias. |
|
|
3 |
Singh et al., (2025) |
Common
adverse effects include dry cough (10-20%), dizziness, hypotension, and
elevated creatinine. Serious risks include angioedema and hyperkalemia. Contraindicated
in pregnancy due to fetopathy risk. |
The
StatPearls entry is a clinical review article, not a single
primary research study. Therefore, it does not have its own methodology,
sample size, statistical tests, or measured confounders, as an original
investigation would. |
selection and interpretation of the cited literature, rather than in a specific study design |
Good: Continuing medical education. A practical
review of clinical practice provides insights that are consistent with
guidelines. |
|
|
4 |
2017 ACC/AHA hypertension guidelines |
Recommends
ACEIs as first-line therapy for hypertension, with ARBs as alternatives. |
Variations in
practice standards, patient demographics, evolving evidence |
N/A |
High:
Authoritative guidelines based on extensive evidence and expert consensus
relevant to current clinical practice. |
|
|
5 |
European
Society of Cardiology (2021) |
Emphasizes
the role of ACEIs and ARBs in heart failure management, with specific
recommendations based on patient type. |
Variability
in clinical settings, patient demographics, evolving evidence |
N/A |
High: Comprehensive
guidelines from a leading European cardiology organization, widely respected
and evidence-based. |
|
|
6 |
American
Journal of Obstetrics and Gynecology (2020) |
ACEIs and
ARBs should generally be avoided in pregnancy due to potential risks to the
fetus. |
Variability
in study designs, pregnancy outcomes, confounding factors |
Selection
bias, study design |
High:
Systematic review focusing on a critical aspect of drug safety in pregnancy,
which is important for clinical decision-making. |
|
|
7 |
Kidney International
(2019) |
ACEIs and
ARBs benefit long-term renal protection, though careful monitoring is needed. |
Patient
baseline renal function, concurrent medications, comorbid conditions |
Selection
bias, confounding |
High:
Detailed study focusing on long-term renal outcomes, high relevance to
nephrology and cardiology. |
|
|
8 |
Diabetes Care
(2018) |
ACEIs and
ARBs are effective, with ARBs sometimes preferred due to better tolerance. |
Variability
in study designs, patient characteristics, definitions of outcomes |
Publication
bias |
High:
Meta-analysis provides a rigorous comparison based on multiple studies, high
relevance to diabetic patients |
|
|
9 |
Journal of
the American College of Cardiology (2022) |
There is no
significant difference in effectiveness, and the choice depends on individual
patient factors. |
Patient
disease severity, concurrent treatments, adherence rates |
Confounding
selection bias |
High: A
recent and relevant study published in a leading cardiology journal provides
specific insights into heart failure management. |
|
|
10 |
Circulation
(2023) |
ARBs are
gaining new indications and are playing an increasingly important role in the
management of cardiovascular diseases. |
Variations in
ARB use, differences in patient populations, evolving evidence |
N/A |
High: A
recent review in a prestigious journal offers up-to-date insights into ARB’s
role in cardiovascular management. |
|
|
11 |
Journal of
Clinical Hypertension (2024) |
ACEIs and
ARBs remain critical in treatment; ongoing research is needed to refine their
use and indications. |
Variation in
study designs, patient populations, outcome measures |
Selection
bias, publication bias |
Good: Recent
review that addresses current evidence and future directions, but quality
depends on the breadth of included evidence. |
|
|
ACEIs:
Angiotensin Converting Enzymes Inhibitors, ARBs: Angiotensin Receptor
Blockers, N/A: Not applicable |
||||||
Table 3: Authors and year of publication, key findings, confounder variables, biases, and overall quality.
Thematic Elements
Adherence to guidelines: Most sources addressing ACEIs and ARBs emphasize compliance with clinical guidelines. The ACC, AHA, and ESC provide detailed guidelines that highlight specific criteria for initiating and managing methods to enhance patient outcomes. Maintaining adherence to these established procedures is crucial for attaining optimal clinical outcomes (American College of Cardiology, 2021; ACC & AHA, 2017; ESC, 2021).
Patient Outcomes: The research emphasizes assessing the efficacy and safety of ACE inhibitors and angiotensin receptor blockers. Research published in Diabetes Care and Kidney International highlights the importance of continuous assessment of patient responses, necessitating treatment modifications to ensure safety, particularly regarding renal function and glycemic control in highrisk groups, such as diabetic patients (Diabetes Care, 2018; Kidney International, 2019).
Variations in clinical practice: Articles by Singh et al. (2025) and Circulation (2023) address variations in clinical practice. They discuss practical issues such as patient adherence to medication, the management of side effects, and adjustments based on individual responses. These studies demonstrate that practical implementations may vary based on individual patient conditions and physician discretion, despite published recommendations, leading to variability in practice.
Special Populations: Pregnant and diabetic patients necessitate customized strategies for managing ACEIs and ARBs. The American Journal of Obstetrics and Gynecology (2020) discussed the potential risks associated with the use of certain antihypertensive drugs during pregnancy, specifically ACE inhibitors and ARBs, emphasizing the importance of alternative therapies and careful monitoring to reduce fetal risk. More recent research from the ESC Registry of Pregnancy and Cardiac Disease (ROPAC) raises similar concerns, with van der Zande et al. (2024) documenting the effects of ACE inhibitor and ARB exposure in pregnant women and emphasizing the significance of guideline-directed therapy.
Emerging Evidence and Trends: Recent evidence and trends underscore the changing role of ARBs in managing cardiovascular disorders. Recent studies in Circulation (2023) and the Journal of Clinical Hypertension (2024) suggest a growing body of evidence supporting modifications in the use of these medications. Recent discoveries necessitate the modification of therapeutic approaches in response to the latest clinical developments.
Summary of Key Findings
Appropriateness Before Initiation of ACEIs or ARBs: In their 2021 guidelines, the ACC underscored the importance of evaluating patient-specific characteristics, including blood pressure levels, heart failure, diabetic nephropathy, and the risk of cardiovascular events, prior to using ACEIs or ARBs. Clinical guidelines and patient tolerance should guide the initiation of these medications. These drugs are recommended for patients with hypertension, heart failure, and chronic kidney disease, with special consideration given to those with high cardiovascular risk (American College of Cardiology, 2021). Li et al. (2021) systematically reviewed the suitability of ACE inhibitors and angiotensin receptor blockers, emphasizing the importance of evaluating baseline renal function, blood pressure, and co-morbidities. They emphasized the significance of personalized risk evaluation and clinical discernment. They endorsed the utilization of ACEIs and ARBs under recognized standards, emphasizing patient history and current health condition. Singh et al. (2025) examined the use and management of ACEIs and ARBs, highlighting the need to assess cardiovascular disease history, renal function, and potential drug interactions before commencing treatment. It is advised to initiate therapy following a thorough risk assessment that includes variables such as age, comorbidities, and baseline blood pressure. The 2017 ACC and AHA guidelines established definitive limits for the initiation of ACEIs and ARBs, especially for patients with hypertension and other risk factors like diabetes or cardiovascular disease. These guidelines emphasize attaining target blood pressure objectives and minimizing cardiovascular incidents, incorporating patient-specific modifications as necessary (ACC & AHA, 2017). Finally, the ESC (2021) guidelines for heart failure care specified criteria for initiating ACEIs and ARBs, particularly for patients categorised by the New York Heart Association (NYHA) functional class and left ventricular ejection fraction. These drugs are highlighted for their role in alleviating symptoms and reducing mortality in patients with heart failure (European Society of Cardiology, 2021).
Relevance and Appropriateness During Continuous Treatment: The ACC (2021) advised continuous surveillance for adverse effects, including hypotension, alterations in renal function, and electrolyte disturbances, alongside regular followups and dosage modifications contingent on the patient’s response. Li et al. (2021emphasized the necessity of monitoring adherence, addressing side effects, and conducting periodic assessments of treatment efficacy, guiding mitigating adverse effects and ensuring therapeutic compliance. Singh et al. (2025) further elaborate on techniques to enhance adherence, manage prevalent side effects, and modify medication based on patient input and laboratory findings, emphasising the need for patient education. The ACC and AHA (2017) emphasized the need to adhere to treatment procedures during therapy, concentrating on monitoring side effects, regular blood pressure management, and cardiovascular risk evaluations. Likewise, the ESC (2021) recommended adjusting dosages based on symptom improvement, adverse reactions, and changes in heart failure status. Pregnant patients get special attention, as indicated in a systematic study published by the American Journal of Obstetrics and Gynaecology (2020) (Buawangpong et al., 2020). The review examined the hazards associated with the use of ACEIs and ARBs during pregnancy, advocating for alternate therapies and vigilant oversight (Buawangpong et al.,2020). Buawangpong et al. (2020) conducted a systematic assessment of the teratogenic risks associated with first-trimester exposure to ACE inhibitors and ARBs, highlighting unfavorable pregnancy outcomes and emphasizing the importance of alternate therapy and close clinical monitoring. Regarding long-term monitoring, Kidney International (2019) investigated the effects of ACEIs and ARBs on renal outcomes, emphasising the need for routine kidney function assessments and dosage adjustments. The 2018 Diabetes Care study examined the continuous management of these drugs in patients with diabetes, emphasising glucose control monitoring and potential renal adverse effects. The Journal of the ACC (2022) assessed the efficacy of ACEIs and ARBs in patients with heart failure and preserved ejection fraction (HFpEF). It advised continual modifications based on patient responses. Circulation (2023) examined the evolving role of ARBs in managing cardiovascular disease, emphasising the need for ongoing monitoring and medication adjustments. The Journal of Clinical Hypertension (2024) evaluated contemporary approaches to managing ACEIs and ARBs, identified research gaps, and proposed future avenues to enhance treatment strategies.
Discussion
We assessed eleven studies to underscore the significance of the findings. We summarise the best evidence of the appropriateness of ACEIs and ARBs before initiation and during treatment across the eight main areas addressed in this review. These include (1) effectiveness in cardiovascular disease management, (2) guideline recommendations, (3) comparison of ACEIs and ARBs, (4) safety and tolerability, (5) impact on renal outcomes, (6) clinical practice and management, (7) pregnancy considerations, and (8) future directions. In addition to the interpretation of the results, we also discuss the limitations and strengths.
Eight Main Areas Addressed in this Review
Effectiveness in Cardiovascular Disease Management: ACEIs and ARBs are extensively utilized to treat hypertension, heart failure, and chronic kidney disease, substantiating their efficacy by substantial data (American College of Cardiology, 2021; Circulation, 2023). Research indicates that ACEIs and ARBs significantly diminish cardiovascular morbidity and death by decreasing blood pressure and safeguarding target organs, including the heart and kidneys (Oparil et al., 2018). The efficacy of these medications is improved when treatment is customized to patients’ specific characteristics and comorbidities, such as diabetes or heart failure (Burnier & Egan, 2019).
Guideline Recommendations: Guidelines from significant societies such as the ACC (2017), the AHA (2017), and the ESC (2021) recommend their use as first-line therapies in these conditions, particularly emphasizing individualized treatment plans based on comorbidities and patient response (ACC & AHA, 2017; ESC, 2021). Current clinical guidelines advocate ACEIs and ARBs as first-line treatments for hypertension, heart failure with reduced ejection fraction, and chronic kidney disease (Whelton et al., 2018). These guidelines also recommend regular assessment of renal function and serum potassium concentrations to prevent adverse consequences, such as hyperkalaemia and renal dysfunction (National Institute for Health and Clinical Excellence [NICE], 2019). The incremental titration of these medications from low doses is crucial to mitigate risks and ensure effective therapeutic outcomes. Clinicians must meticulously evaluate these aspects before prescribing these drugs for extended use, with a strong emphasis on patient safety. Assessing baseline renal function and serum electrolytes is essential when initiating ACEIs or ARBs (National Institute for Health and Clinical Excellence [NICE], 2018 and 2019). Follow-up tests should be conducted one to two weeks after initiation and after dose increases (Ritter, 2011). In patients with hypertension, renal function and electrolyte levels should be monitored annually once treatment is established. In patients with CHF, monitoring should occur within days of changes in condition or medication. Once the patient is stable, monitoring should occur at least every six months (NICE 2018 and 2019). Additional monitoring is necessary for patients with comorbidities, those on specific medications, or those receiving ACEIs and ARBs (Ritter, 2011; BNF, 2024). Studies have shown that recommendations for biochemical monitoring are frequently not followed, which can lead to severe adverse events. One study indicated that merely 59% of patients prescribed ACEIs for hypertension underwent baseline monitoring, whereas only 38% received any further monitoring (Coleman et al., 2010).
Comparison of ACEIs and ARBs: The comparative efficacy of ACEIs and ARBs remains under examination. Although both drug classes offer substantial cardiovascular and renal benefits, ARBs may be preferred for patients who exhibit sensitivity to ACEIs, primarily due to the prevalence of cough as a side effect (Diabetes Care, 2018; Journal of Clinical Hypertension, 2024). Their modes of action and side effect profiles differ. ACEIs inhibit the transformation of angiotensin I into angiotensin II, whereas ARBs specifically obstruct the angiotensin II type 1 receptor. This distinction suggests that ACEIs are associated with an increased risk of cough and angioedema resulting from bradykinin accumulation (Yancy et al., 2017). Conversely, ARBs typically exhibit a more advantageous safety profile. They are less prone to induce severe side effects, rendering them an appropriate choice for those who cannot endure ACEIs. Notwithstanding these distinctions, both categories of medications confer comparable long-term cardiovascular and renal advantages, rendering them interchangeable in numerous therapeutic situations (Messerli et al., 2018).
Safety and Tolerability: The safety profiles of these agents differ (Singh et al., 2025), while effective, have been linked to higher rates of adverse events like cough, angioedema, and, less commonly, renal dysfunction. ARBs are generally better tolerated but may still cause hyperkalaemia and renal impairment in highrisk patients, such as those with chronic kidney disease or diabetes. (Fu et al., 2021). Combination therapy utilizing ACEIs and ARBs has been demonstrated to elevate the risk of severe adverse effects, such as hypotension and acute renal injury, without providing substantial extra advantages (Makani et al., 2013). A reduced dose should be sustained if maximum doses are intolerable to guarantee therapeutic benefits (Yancy et al., 2017).
Effects on Kidneys: Notably, both drug classes exhibit beneficial renal results, particularly in individuals with diabetic nephropathy, as they decelerate the progression of kidney disease and diminish the likelihood of end-stage renal failure (Kidney International, 2019). Research indicates that these medications mitigate nephropathy progression by decreasing glomerular filtration pressure and proteinuria (Vejakama et al., 2017), while enhancing renal function and delaying the onset of end-stage renal disease (Pugh et al., 2019). Monitoring renal function and electrolyte levels is crucial to avert problems such as hyperkalemia, especially in patients with compromised kidney function. In patients with renal impairment, fosinopril is an appropriate option because it is excreted through both renal and fecal pathways. Additional ACE inhibitors, including captopril, enalapril, lisinopril, and ramipril, are also applicable for use in dialysis (Singh et al., 2025).
ACEIs and ARBs in Clinical Practice and Management: In clinical practice, determining treatment, including ACEIs and ARBs, frequently relies on renal function, blood pressure regulation, and patient tolerability (Li et al., 2021; Journal of the American College of Cardiology, 2022). Research indicates that compliance with prescribed monitoring techniques, such as routine evaluation of serum creatinine and potassium levels, is essential for enhancing patient outcomes (McMurray et al., 2014). Clinicians are urged to implement a patient-centred approach, adjusting dosages based on individual responses and adapting to changing clinical circumstances. The BNF 2024 recommends that all patients with diabetic nephropathy, proteinuria, or confirmed microalbuminuria should be treated with ACEIs and ARBs (BNF, 2024). Lisinopril and Captopril are unique among ACE inhibitors as they are not prodrugs, making them appropriate for use in patients with hepatic impairment (Singh et al., 2025).
Pregnancy Considerations: These drugs are forbidden during pregnancy because of their teratogenic risks, requiring alternate therapeutic choices (American Journal of (Obstetrics & Gynaecology, 2020; Buawangpong et al., 2020). Studies showed teratogenic effects, such as renal dysgenesis, oligohydramnios, and, in severe cases, fetal death, mainly when used in the second and third trimesters. As a result, healthcare providers must discontinue ACEIs and ARBs in women who are planning to conceive or are already pregnant. Timely identification of pregnancy is essential to mitigate exposure, and regular pregnancy testing may be recommended for women of reproductive capacity who are undergoing these treatments (Countouris et al., 2025; Walfisch et al., 2011). In pregnancy, the use of ACE inhibitors is contraindicated during the second and third trimesters due to established risks of fetopathy. The risks associated with first-trimester exposure remain inadequately characterized. A significant correlation has been identified between exposure to ACE inhibitors during the first trimester and the occurrence of major cardiovascular and neurological malformations (Singh et al., 2025). Breastfeeding considerations: ACE inhibitors exhibit low bioavailability; however, they are metabolized into active metabolites that possess extended half-lives. Evidence suggests that minimal quantities of the parent drug and its metabolite are found in breast milk for other ACE inhibitors. Enalapril is frequently favored based on the extensive published data and minimal infant exposure. ACE inhibitors can be utilized with caution while breastfeeding. [31] ACE inhibitors are generally regarded as safe for breastfeeding mothers, with exceptions for cases of premature birth or renal failure in the infant (Singh et al., 2025).
Future Directions
Subsequent research will probably concentrate on broadening the therapeutic applications of ACEIs and ARBs in novel and evolving clinical scenarios (Journal of Clinical Hypertension, 2024; Schiffrin et al., 2020). Furthermore, current research seeks to elucidate the long-term comparative efficacy of ACEIs against ARBs, which will aid in the refinement of therapeutic guidelines and improve the personalized use of these medications (Packer et al., 2015).
Interpretation of Results
The analysis of the results underscores numerous critical aspects concerning the utilization of ACEIs and ARBs. Adherence to guidelines is essential, as contemporary practices frequently diverge from established standards. Numerous studies have identified deficiencies in monitoring and management, indicating that clinical practice frequently fails to meet guideline standards, especially with baseline monitoring and follow-up for patients taking ACEIs and ARBs. Factors affecting the suitability of commencement and management encompass patient-specific attributes, including renal function, comorbidities, and the risk of side consequences such as hyperkalaemia and renal impairment. These characteristics require personalized treatment approaches and underscore the significance of consistent biochemical monitoring to avert problems. To boost the suitability of ACEIs and ARBs in clinical practice, it is advised that healthcare practitioners bolster adherence to guidelines, particularly by underscoring the significance of thorough risk assessments, personalized treatment approaches, and consistent follow-up monitoring. By addressing these deficiencies, doctors can improve patient outcomes and reduce the likelihood of adverse events.
- A systematic study by Li et al. (2021) assessed the suitability of ACIEs and ARBs, highlighting the necessity for clarity regarding their comparative efficacy and safety. Singh et al. (2025) emphasised the difficulties in administering these drugs in clinical practice, hence underscoring the need for this scoping study.
- The ESC (2021) and recent publications (Circulation, 2023; Journal of Clinical Hypertension, 2024) contribute to ongoing discussions about their roles in managing heart failure and hypertension.
- The ESC 2024 and the ACC and AHA 2024 guidelines emphasize similar principles in managing cardiovascular conditions using ACIEs and ARBs while highlighting critical differences in their recommendations for specific patient populations.
- According to the 2024 ESC guidelines, initiation of ACEIs or ARBs should always begin with a low dose, followed by gradual titration to achieve therapeutic targets, with close monitoring of renal function and electrolyte levels, particularly in high-risk groups such as patients with diabetes or chronic kidney disease. In cases of heart failure with decreased ejection fraction (HFrEF), ACEIs are typically the chosen first-line therapy. Concurrently, ARBs provide an appropriate alternative for individuals who suffer from side symptoms, such as cough, associated with ACEIs. The guidelines advise against the use of combination therapy with both ACEIs and ARBs due to the increased risk of side effects such as hyperkalaemia and renal impairment. The guidelines emphasize the necessity of consistent follow-up, including annual evaluations of renal function and electrolytes, to guarantee the safety and efficacy of the medication (European Society of Cardiology, 2024).
- The 2024 ACC/AHA guidelines offer critical suggestions for using ACEIs and ARBs in treating cardiovascular disorders and prioritizing patient safety. It is recommended to initiate these drugs at low dosages, particularly in patients with heart failure or those at heightened cardiovascular risk, followed by incremental dose escalations. ACEIs are the primary treatment for heart failure with reduced ejection fraction, although angiotensin receptor blockers provide a suitable alternative for those who cannot tolerate ACEIs. The ACC/ AHA guidelines advise against the concomitant use of ACE inhibitors and ARBs due to the heightened risk of adverse consequences, including hyperkalaemia and deteriorating renal function. Consistent evaluation of renal function and electrolytes is essential after initiation, with recommendations for annual or more frequent long-term follow-up examinations based on the patient’s risk factors (American College of Cardiology, 2024; American Heart Association, 2024). Both guidelines emphasise a patient-centred approach, focusing on personalised treatment regimens, vigilant monitoring, and dosage adjustments to optimise therapeutic efficacy while minimising the likelihood of adverse effects.
Limitations include study design and outcome variability, insufficient data on specific patient populations or diseases, and biases. The diversity and variability in research methodologies may affect the generalizability of findings. Ultimately, papers published in languages other than English will be excluded, potentially disregarding relevant findings.
Strengths
We integrated extensive coverage and a wide-ranging reach, encompassing many sources and data from prominent clinical guidelines and governmental authorities. We employed a transparent and reproducible methodology, using a stringent search to identify all pertinent studies and minimise selection bias. The overall quality was examined to help readers assess the reliability and validity of the evidence and consider the robustness of the overall conclusions. An exhaustive review and analysis of recent research pertinent to behavioural change and clinical inertia in diabetes management encompassing a variety of interventions and techniques.
Conclusion
This review aims to consolidate the existing evidence about using ACEIs and ARBs to inform clinical decision-making. The evaluation of these medicines highlights their considerable clinical efficacy and safety in various settings. The results underscore the significance of personalized treatment strategies, especially when commencing these drugs at minimal doses and progressively adjusting them. Guidelines constantly recommend vigilant monitoring to enhance therapy outcomes, especially in high-risk groups. The review outlines current research domains focused on enhancing clinical techniques and addressing emerging issues, particularly in the areas of long-term outcomes and comparative effectiveness.
Recommendations
Healthcare practitioners must acquire comprehensive knowledge from prominent clinical recommendations and governmental authorities to provide these medications appropriately and successfully. Comprehensive recommendations must be developed to address the noted deficiencies. Subsequent research on patientspecific attributes and longitudinal studies will address information deficiencies, enhance therapeutic practices, and assess long-term results and the efficacy of medications.
Policy and Impact on Clinical Practice
Amendments to policy and the implementation of best practices are crucial for effectively and appropriately utilising these drugs. Implementing major clinical guidelines and adhering to recommendations from governmental agencies is crucial for using these medicines appropriately and avoiding harm. Medications must be tailored to individual patient needs, and an individualized approach requires attention to patient-specific factors such as comorbidities, drug interactions, and cardiovascular events, especially before initiation and during treatment. Clinicians should assess baseline renal function and electrolyte levels, particularly in high-risk patients with chronic kidney disease or diabetes. Furthermore, the combination of medications should generally be avoided.
Acknowledgements
We would appreciate the co-authors for Their Continuous Support, Cooperation, and Assistance.
Funding
This research was conducted without funding.
Availability of Data and Materials
The datasets used and analysed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics Approval and Consent to Participate: Not applicable.
Consent for Publication: Not applicable.
Competing Interests
The authors declare that they have no competing interests.
References
- American College of Cardiology (2021) Guidelines for the use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers.
- American College of Cardiology (2024) 2024 ACC/AHA guideline for the management of cardiovascular disease.
- Jones DW, Ferdinand KC, Taler SJ, Johnson HM, Shimbo D, et al. (2025) 2025 AHA/ACC/AANP/AAPA/ABC/ACCP/ACPM/AGS/AMA/ASPC/NMA/PCNA/SGIM Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Hypertension 82: e212-e316.
- McEvoy JW, McCarthy CP, Bruno RM, Brouwers S, Canavan MD, et al. (2024) 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur Heart J 45: 3912-4018.
- American Journal of Obstetrics and Gynecology (2020) ACE inhibitors and ARBs during pregnancy: Teratogenic risks and alternatives. American Journal of Obstetrics and Gynecology 223: 123-130.
- Buawangpong N, Teekachunhatean S, Koonrungsesomboon N (2020) Adverse pregnancy outcomes associated with first-trimester exposure to angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers: A systematic review and meta-analysis. Pharmacol Res Perspect 8: e00644.
- Arksey H, O’Malley L (2005) Scoping studies: Towards a methodological framework. International Journal of Social Research Methodology 8: 19-32.
- Pugh D, Gallacher PJ, Dhaun N (2019) Management of Hypertension in Chronic Kidney Disease. Drugs, 79: 365-379.
- British National Formulary (2024) British National Formulary (87th Edition). BMJ Group and Pharmaceutical Press.
- Burnier M, Egan BM (2019) Adherence in hypertension: A review of prevalence, risk factors, impact, and management. Circulation Research 124: 1124-1140.
- American Heart Association (2023) The evolving role of angiotensin receptor blockers in cardiovascular disease management. Circulation 148: 456-465.
- Coleman JJ, McDowell SE, Evans SJW, Gill PS, Ferner RE (2010) Oversight: A retrospective study of biochemical monitoring in patients beginning antihypertensive drug treatment in primary care. Br J Clin Pharmacol 70: 109-117.
- Diabetes Care (2018) ACE inhibitors versus angiotensin receptor blockers in diabetes: A meta-analysis.
- Wang K, Hu J, Luo T, Wang Y, Yang S, et al. (2018) Effects of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on all-cause mortality and renal outcomes in patients with diabetes and albuminuria: a systematic review and meta-analysis. Kidney Blood Press Res 43: 768-779.
- Li Y, Zhang D, Pang B, Wang J, Chen Y, et al. (2021) Comparative efficacy and safety of ACE inhibitors and ARBs in hypertension and heart failure: A systematic review and meta-analysis. Frontiers in Pharmacology, 12, 627416.
- European Society of Cardiology (2021) 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure.
- European Society of Cardiology (2024) Guidelines for the management of cardiovascular diseases.
- Williams B, Mancia G, Spiering W, Agabiti-Rosei E, Azizi M, et al. (2024) 2024 ESC guidelines for the management of elevated blood pressure and hypertension: Developed by the Task Force on the Management of Elevated Blood Pressure and Hypertension of the European Society of Cardiology (ESC) and endorsed by the European Society of Endocrinology (ESE) and the European Stroke Organisation (ESO). European Heart Journal.
- Fyhrquist F, Saijonmaa O (2008) Renin-angiotensin system revisited. J Intern Med 264: 224-236.
- Johnson L, Lee R (2019) Use and management of ACE inhibitors and ARBs in clinical practice.
- Singh B, Cusick AS, Goyal A, Patel P (2025) ACE inhibitors. In StatPearls. StatPearls Publishing.
- Journal of Clinical Hypertension (2024) ACE inhibitors vs. ARBs: Current evidence and future directions.
- Alcocer LA, Bryce A, De Padua Brasil D, Lara J, Cortes JM, et al. (2023) The pivotal role of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in hypertension management and cardiovascular and renal protection: a critical appraisal and comparison of international guidelines. Am J Cardiovasc Drugs 23: 663-682.
- Journal of the American College of Cardiology (2022) Comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients with heart failure with preserved ejection fraction.
- Xie C, Chen R, Zhou S, Lin Y, Liu J, et al. (2025) Comparative Effectiveness of Angiotensin-Converting Enzyme Inhibitors Versus Angiotensin Receptor Blockers: Multidatabase Target Trial Emulation Studies. Hypertension.
- Kidney International (2019) Long-term effects of angiotensinconverting enzyme inhibitors and angiotensin receptor blockers on renal outcomes.
- Chen JJ, Lee CC, Yen CL, Fan PC, Chan MJ, et al. (2024) Impact of Different Angiotensin-Converting Enzyme Inhibitors or Angiotensin Receptor Blocker Resumption Timing on Post Acute Kidney Injury Outcomes. Kidney Int Rep 9: 3290-3300.
- Makani H, Bangalore S, Desouza KA, Shah A, Messerli FH (2013) Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomised trials. BMJ 346: f360.
- Countouris M, Mahmoud Z, Cohen JB, Crousillat D, Hameed AB, et al. (2025) Hypertension in pregnancy and postpartum: Current standards and opportunities to improve care. Circulation 151: 490-507.
- McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, et al. (2014) Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 371: 993-1004.
- Messerli FH, Bangalore S, Bavishi C, Rimoldi SF (2018) Angiotensinconverting enzyme inhibitors in hypertension: To use or not to use? J Am Coll Cardiol 71: 1474-1482.
- National Institute for Health and Care Excellence (2025) Chronic heart failure in adults: Diagnosis and management (NICE Guideline NG106).
- National Institute for Health and Clinical Excellence (2019) Hypertension: Clinical management of primary hypertension in adults (NICE Clinical Guideline 127).
- Oparil S, Acelajado MC, Bakris GL, Berlowitz DR, Cífková R, et al. (2018) Hypertension. Nat Rev Dis Primers 4: 18014.
- Packer M, McMurray JJV, Desai AS, Gong J, Lefkowitz MP, et al. (2015) Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 131: 54-61.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, et al. (2021) The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372: n71.
- Ritter JM (2011) Angiotensin converting enzyme inhibitors and angiotensin receptor blockers in hypertension. BMJ 342: 1673-1680.
- Schiffrin EL, Touyz RM, Pimenta E (2020) Heart failure and hypertension: The links. Hypertension 76: 333-341.
- Walfisch A, Al-maawali A, Moretti ME, Nickel C, Koren G (2011) Teratogenicity of angiotensin converting enzyme inhibitors or receptor blockers. J Obstet Gynaecol 31: 465-472.
- Fu EL, Clase CM, Evans M, Lindholm B, Rotmans JI, et al. (2021) Comparative Effectiveness of Renin-Angiotensin System Inhibitors and Calcium Channel Blockers in Individuals With Advanced CKD: A Nationwide Observational Cohort Study. Am J Kidney Dis 77: 719-729.e1.
- Whelton PK, Carey RM, Aronow WS, Casey Jr DE, Collins KJ, et al. (2018) 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/ NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71: 1269-1324.
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey Jr DE, et al. (2017) 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 136: e137-e161.
- Vejakama P, Ingsathit A, McKay GJ, Maxwell AP, McEvoy M, et al. (2017) Treatment effects of renin-angiotensin aldosterone system blockade on kidney failure and mortality in chronic kidney disease patients. BMC Nephrol 18: 342.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.