Endocrine Disrupting Chemicals and their Role in the Genesis of Neoplasms
by Mauri José Piazza*
Department of Tocogynecology, Universidade Federal do Paraná, UFPR, Curitiba (PR), Brazil
*Corresponding author: Mauri José Piazza, Department of Tocogynecology, Universidade Federal do Paraná – UFPR - Curitiba (PR), Brazil
Received Date: 17 July 2025
Accepted Date: 21 July 2025
Published Date: 23 July 2025
Citation: Mauri José Piazza (2025) Endocrine Disrupting Chemicals and their Role in the Genesis of Neoplasms J Surg 10: 11391 https://doi.org/10.29011/2575-9760.011391
Abstract
The goal of this review is to address all agents and mechanisms capable of inducing and characterizing Endocrine-disrupting chemicals. Such agents are known to cause harmful effects to humans and wildlife. There is a wide range of chemicals developed for commercial use mainly in agriculture and plastic industries, which may cause endocrine disruption and affect human’s health, inducing many diseases and even tumours. Numerous studies correlate toxic endocrine disruptors and environmental contamination; however, no liability is being taken for the damages. A specific group of these substances can induce neoplasms called hormone dependent-tumours on the ovary,uterus,breasts, prostate and thyroid. Our proposal is to review the correlation between this wide range of abnormalities and their carcinogens agents.
Keywords: Breast Cancer; Endocrine-Disrupting Chemicals; Prostate Neoplasia; Thyroid Cancer; Uterine-Endometrium Neoplasia; Vaginal Neoplasia
Introduction
A growing number of scientific evidence has been collected over the past few years suggesting that human capacity has been affected by a wide range of recurrent substances found in everyday products.Several indicators are showing an increased incidence of cardiovascular disorders,obesity, hormone-dependent cancers and chronic diseases,not to mention early puberty development,pregnancy length disorders and other reproductive and neoplasm abnormalities.Those acting agents have been defined as Endocrine-disrupting chemicals (EDCs).Endocrine disruptors are chemical products that may interfere and cause adverse effects on the endocrine system at any life-stage,given their resemblance to endogenous steroid hormones.Their actions were defined as agonist or antagonist against several hormones (oestrogens or androgens) [1]. Some EDCs can disturb proteins involved in the transport of hormones and disrupt the delivery of endogenous hormones to target cells.Usually, the molecular structure consists of a phenol group on the first ring and one group replacement by chlorine or bromine and mimics steroids hormones.These events interfere with synthesis, secretion, transport and metabolism of many hormones and, because of their similarities,induce a destabilization of hormonal homeostasis.In 2013, Birnbaum showed that, between 1947 and 2007, the global production of these chemicals has increased 23.5 times, whereas in 2012 only, the US produced 9.5 trillion pounds or 2.09 trillion kilograms of such products including pesticides, plastics, chemical drugs or even personal hygiene products [2]. Deserving more attention is DDT and its by-products, such as atrazine and 2,4-dichlorophenoxyacetic acid found in toys, or containing plumb and cadmium,chemicals used for the production of plastic bottles containing Bisphenols A, phthalates and several other substances employed in the textile and apparel industries [1]. Among acting agents are substances like Bisphenol A and its by-products, such as Bisphenol B, Tetrabromobisphenol A and Bisphenol F and S [2-4].Some years ago, the first disruptor among EDCs considered a special hormone, was named Diethylbestrol (DES) causing disorders in the genital tract of female and male offsprings [5]. Our proposal is to evaluate the capacity of these agents to affect and interfere with the genesis of different neoplasms in women.
|
Several Endocrine-Disrupting chemicals and their Effects |
||
|
Atrazine/Alachlor |
Herbicides Cadmium chloride/Methiran |
Fungicides |
|
Carbaryl/Chlordane/DDT, etc. |
Inseticides |
|
|
Aldicarb/DBCP |
Nematocides |
|
|
Acrylamide |
Water treatment-paper manufacturing |
|
|
Ascarel (PCB) |
Adhesives/paints-silo storage coatings |
|
|
Benzoanthracene Benzopyrene |
Tar-asphalt-grease-mineral oils |
|
|
Bisphenols A, etc. |
Epoxy resin-plastics-can coatings |
|
|
Plumbs |
Bateries-paints/pigments |
|
|
Polychlorinated Dibenzodioxin |
Pesticide-burn/residues-PVC-diesel |
|
|
DBPC (Dibromochloropropane) |
Nematicide |
|
|
Carbon Disulfide |
Celophane manufacture-rayon-solvents/wax |
|
|
Sthyrene |
Plastic and glasses-rubber manufacturing |
|
|
Phenilphenols |
Desinfecting products |
|
|
Phthalates |
Plastificants/varnish-cosmetics/inseticides |
|
|
HCB ( Hexachlorobenzene ) |
Organochlorinated processes |
|
|
Manganese |
Iron-paint-fertilizers manufacturing |
|
|
Mercury |
Agrotoxics-paints-soda industry |
|
|
Ethylene Chloride |
Surgical equipment sterilization |
|
|
Pentachlorophenol |
Paints-timber conservants-fungicide |
|
|
Welding |
Metalsmithing and Weldings-Boilermaking |
|
|
Trichlorfon |
Anthelmintics |
|
Endocrine-Disrupting Chemicals
In the last few decades,a growing number of neoplasms called hormone dependent-tumours on the breast, endometrium,ovaries,testis,prostate and thyroid have been reported.Although early diagnosis has been increasingly common,several other etiological and causal factors such as nutrition, pharmaceutical drugs or chemicals (including Endocrine-disrupting chemicals) are also held responsible.The purpose of this review is to address the harmful effects of these potential carcinogenic agents involved in the genesis of human and wildlife tumours. Among several Endocrine-disrupting chemicals are:
Bisphenols
Bisphenol A (BPA)was first synthesized in 1891,but evidences show oestrogen activity since 1936 [1]. Its annual production has steadily increased given its multiple applications in the manufacturing of toys, plastics, epoxy resin and food packaging.Exposure to BPA can occur by oral ingestion or transdermal/sublingual absorption, suffering a fast hepatic breakdown.Because of its lipophilicity, BPA can easily build-up in the adipose tissue. Since 1950, it was observed that BPA could be polymerized for the manufacturing of polycarbonate plastics given its malleability, lightweight, transparency and resistance to heat and other chemicals [3,4]. Five different types of Bisphenols are currently being employed in the industry such as Bisphenol B (BPB),Bisphenol F (BPF),Bisphenol S (BPS),Bisphenol AF (BPAF) and Tetrabromobisphenol (TBBPA) (Figure 1).
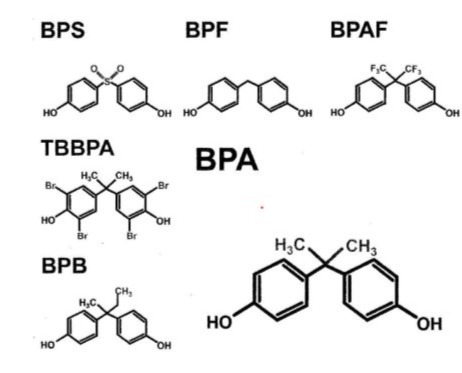
Figure 1: Structural Formulas of different Bisphenols [2].
Phthalates
Phthalates and phthalates esters are substantially used in the industries of plastics, cosmetics and toys, or in the manufacturing of medical equipment like blood bags. Its global dissemination can be verified in the food industry including fruit juices, sports drinks, food supplements and frozen products like ice-cream [6] (Figure 2).
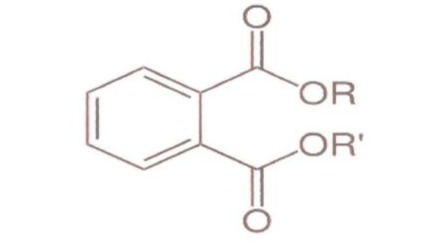
Figure 2: Structural formulas of Phthalates.
Atrazine
Atrazine (2-chloro-4-ethylamino-6-isopropylamino-1,3,5-s-triazine) (ATR) is commonly used in agriculture as herbicide to reduce the growth of weeds in corn,soy and sugar cane cultures.Atrazine remains active for long periods of time contaminating water tables and causing anomalies in many aquatic organisms, according to previous studies by Solomon et al [7] (Figure 3).
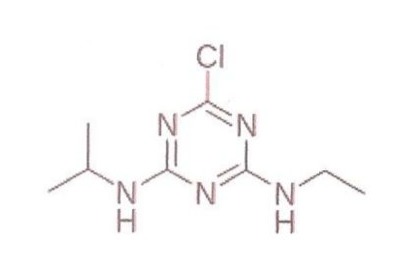
Figure 3: Structural formula of Atrazine.
Polychlorinated Bisphenols (PCB) and Polybrominated Diphenyl Ethers (PBDE)
This group of aromatic chemicals have phenolic ring with chlorine or bromine radicals and extremely high levels of toxicity.They have been manufactured since 1920,but some were banned in 1979 because of their toxic effects.However,because of its multiple applications in the industry of plastics,rubber,adhesives,paints and resins.Many PCBs are still being used. Some of these chemicals have thyrogenic, estrogenic and anti-androgenic activity [8,9]. Polybrominated Diphenyl Ethers (PBDE) were first used as flame retardants for the manufacturing of mattresses and apparel.
DDT (Dichlorodiphenyltrichloroethane), DDE (Dichlorodiphenyldichloroethylene) and DDD (Dichlorodiphenyldichloroethane)
DDT is an insecticide with long average lifetime and lipophilicity;but, unfortunately it is considered a major environmental contaminant.DDT was banned from the USA market in 1972,even though it has been used to control insects that carry malaria and typhoid fever [10,11]. DDD (Dichlorodiphenyldichloroethane) was associated to the genesis of endocrine disorders like diabetes type 2,endometrial,pancreatic and breast cancer [18-20]. Other DDT's metabolites include DDE (Dichlorodiphenyldichloroethylene) and DDD (Dichlorodiphenyldichloroethane).
Diethylstilboestrol (DES)
It is a powerful non-steroidal oestrogen, first synthesized in 1938,which was previously used in the USA to prevent miscarriage and/or its potential complications.Initially,low doses of 5mg/day were administered,which were progressively increased to 125mg/day or more, summing up to an average dose of 3650-4000mg.In 1953,Dieckmann et al. proved this treatment to be ineffective [5].Yoshida et al.(2011) described adverse effects of perinatal exposure and their dose dependency in female rats [12]. In 1971, Herbst et al assessed young women (where mothers had been treated with DES during gestation)having noticed a correlation between the use of DES and the appearance of clear cell vaginal adenocarcinoma,while in 1976, the same author described other abnormalities in the genital tract of these young women [13,14].In 2012, Harris and Waring, and Troisi et al., in 2014, have noticed a correlation between the increased number of disorders in the reproductive system of the offspring (where mothers had been treated with DES during gestation).Male offspring’s developed cryptorchidism, and female offspring showed uterine abnormalities like T-shaped uterus and some types of hormone-dependent cancers [15,16] (Figure 4).
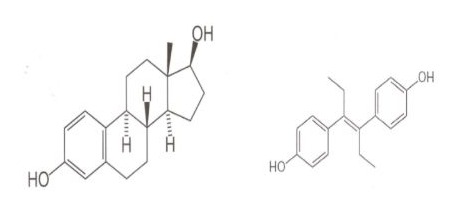
Figure 4: Structural formulas of Oestradiol(E2) and Diethylstilboestrol.
Endocrine-Disrupting Chemicals and their Effects on the Development of Tumors
Uterine and Endometrial Tumors/Vaginal Neoplasias
Endometrial tumours are the most common type of neoplasms found in the female genital tract,second to breast tumours,and the rise in life expectancy has contributed to their incidence increase.There are two distinctive types,type I being frequently reported following a higher oestrogenic activity and characterized by the development of different cases of endometrial hyperplasia.Type II being associated to elderly women with atrophic endometrium and several cancer-causing agents.Distinct epidemiological studies have been correlating these tumours and exposure to Endocrine Disruptors. Kogevinas et al. (1997) showed that exposure to dioxin increases the risk of endometrial tumour in a group of female workers [17]. Sturgeon et al (1998) have found no significant correlation between increased levels of serum DDT and endometrial cancer. Hiroi et al. (2004) have not detected increased Bisphenol A concentrations in patients with endometrial hyperplasia as compared to a control group. However, lower BPA concentrations were reported in the patients with hyperplasia and a distinct tendency to undergo malignant as compared to those patients with endometrial neoplasm [18,19]. There is limited evidence correlating ovarian neoplasms and Endocrine-Disrupting Chemicals while the number of exposed women is insignificant. Donna et al.(1989) showed a 2.7 increased risk of ovarian neoplasms in women exposed to chlorothiazide [20]. An epidemiological study by Young et al.(2005)revealed limited correlation between ovarian cancer and occupational exposure to chlorothiazide [21].In a large study group Alavanja et al.(2005)observing female applicators exposed to pesticides in Iowa and North Carolina, including two groups of women (in private and commercial areas), it was observed that women working in private areas had increased risk of developing epithelial neoplasm in the ovaries [22]. Finally, a study conducted by Vieira et al.in 2013, revealed that high levels of perfluorooctanoic acid were detected in a population living next to Dupont facilities, in West Virginia, increasing the incidence of ovarian cancer [23]. In the vagina, evidences show that diethylstilboestrol, a nonsteroidal oestrogen, plays an important part in the carcinogenesis of female foetuses,where mothers had been treated to prevent miscarriages.Further studies by Herbst et al.(1971-1976),Harris et al.(2012) and Troisi et al.(2013)described the incidence of vaginal adenosis and clear cell adenocarcinoma in young women, where mothers had been exposed to DES under several conditions during gestation [13-16].
Breasts Cancer and Chemicals
The mammary gland,as part of the mammal’s reproductive system,is responsible for lactation and particularly sensitive to Endocrine-Disrupting Chemicals because it involves growth and differentiation systems,secretion and regression,all under the influence of hormones and growth factors.Thus,the mammary tissue is strongly influenced by these factors during 3 typical life stages:puberty,gestation and lactation. During gestation, when the mammary buds begin to form the small ducts and extend down to the subjacent adipose tissue, many Endocrine-disrupting Chemicals might end up altering the development of the mammary structures. During puberty, mammary growth is exponential and proliferative when a fast division of the terminal mammary ducts and mammary buds occurs, which can be influenced by the adverse effects of the EDCs. Starting puberty and menarche at a younger age, late menopause, nulliparity, first pregnancy later in life and perimenopausal obesity have been associated with increased risk for breast cancer. A growing number of chemicals have been correlated to the development and growth of the mammary tissue increasing the risk for the development of breast neoplasms. In 1982,a study conducted by the United States National Toxicology Program with rodents has established that 75-150mg/kg daily intake levels of BPA would be enough to have carcinogenic effects.With limited evidences, this study raised many questions as it failed to review and include rodents in the perinatal period [24].Previous studies by Timms et al.(2005) and Moral et al.(2008)., included gestation and lactation periods with oral doses of 10-250ug/kg/day, reporting proliferative lesions in breasts duct epithelium and squamous metaplasia of the prostate in new-born rats,which are lesions with a predisposing condition for neoplasia [25,26].Jenkins et al.(2009)and Prins et al.(2011) showed these chemicals would promote an early development of breast neoplasms as well as prostate intraepithelial neoplasia in rats [27,28].However, animal experiments evaluating exposure to BPA during specific life periods presented deficient design failing to have a conclusive oncogenic potential due to a reduced number of animals included and insufficient exposure time and/or lack of additional treatment. Despite the great variety of existing sexual hormone-mimicking chemicals, organochlorine compounds have been held responsible for the harmful effects observed in many sites. Among them are DDT (1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane) and its isomer p’-DDT both having oestrogenic properties; DDE (1,1-dichloro-2,2-di-p-chlorophenylethylene)with mechanism of antiandrogen action;TCDD (tetrachlorodibenzo-p-dioxin) which is considered antiestrogenic;and PCB (polychlorinated biphenyl)and its congeners,having oestrogenic and antiestrogenic action [29-32].
Dioxins and Breast Cancer-Actions
There are over 400 different types of dioxins (30 of them are toxic to humans),the most toxic being TCDD (tetrachlorodibenzo-p-dioxin).TCDD is a chemical compound with strong lipophilicity and long average life (7-11 years)that has been commonly used as herbicide and pesticide.It reduces plasmatic levels of oestradiol and affects breasts development given its antiestrogenic properties.Studies in humans by Den Hond et al. (2002)in Belgium, and Leijs et al.(2008) in the Netherlands, observed children living in polluted areas exposed to dioxin, showing evident signs of late breast development [33,34]. A historical accident happened in 1976, in the chemical plant of Seveso,Italy, releasing a significant amount of TCDD into the environment.Later studies observing residents exposed in the nearby zones(divided according to contamination levels),indicated a possible relationship between high levels of exposure over the years and increased incidence of neoplasms. A 10 and 15-year follow-up of this population group (starting in 1989 and 1997),by Bertazzi et al. failed to reveal increased neoplasm or mortality rates [35,36].However, a 15-20-year follow-up of the most vulnerable and exposed population group revealed a discrete increase in neoplasms [36,37].In 2002, the same population group of Seveso was reassessed by Warner et al. in the Women’s Health Study,to further evaluate the increased incidence of breasts neoplasia.In the group of women exposed to dioxin in early childhood,with plasma concentrations exceeding by 10 times the acceptable levels, it was observed twice as many cases of breast cancer (considering that this group was not yet aged 40-55, an age group with increased risk for developing this kind of neoplasia) [38].An epidemiological study conducted in Russia by Revich et al.in the region of Chapayevsk, where another episode of dioxin contamination occurred, revealed increased incidence of breast cancer among women living near contaminated sites [39].
Dichlorodiphenyltrichloroethane(DDT) - Dichlorodiphenyldichloroethylene(DDE) Dichlorodiphenyldichloroethane (DDD) and Breast Actions
According to previous studies in humans by Rogan et al. (1987) and Karmaus et al. (2005) exposure to DDT and DDE might interfere with women’s ability to breastfeed [40,41]. References and evaluations of the National Toxicology Program evidenced that DDE has oestrogenic or antiandrogen-like activities, eventually interfering with hormone levels during lactation [10]. In the latest years, numerous studies have raised concerns about the effects of exposure to Endocrine-Disrupting Chemicals, that already exist in the environment or are being manufactured, which may contribute to the development of breast cancer.Most of these studies are considered ‘control cases’, showing inconsistent results and failing to reach conclusions with statistical significance.Another issue is the potential association with other types of existing chemicals that could be equally harmful, preventing the progress of research toward conclusive results. Before 1995, at least 7 “CONTROL-CASE” studies were conducted to assess EDC’s concentrations in tissues or in the serum of different organochlorine compounds. The first experiment conducted by Wasserman et al.(1976)observed fragments of formalin-fixed mammary tissue from 9 patients with breast cancer and 5 control women, revealing the presence of DDT’s or PCB’s (Poly(bisphenol) B) metabolites.High concentrations of PCB were found in the lipids of tumoral mammary fragments of the female patients as compared to the mammary tissue of the control group.Despite that, the highest p,p’-DDE level, the main stored metabolite of DDT,was significantly higher in the control group [42]. The second and third studies,led by Unger et al.(1984)and Mussalo-Rauhamaa et al.(1990) evaluated the presence of DDE or DDT’s metabolites in fragments of mammary tissue collected from recently deceased patients with neoplasia as compared to normal control cases.No evidences of altered concentrations were found in both studies,although β-hexachlorocyclohexane levels were increased.These results were inconclusive considering the fragments were obtained from deceased female patients [43,44].
Falck et al.(1992)conducted the fourth study,detecting 7 organochlorine compounds in the mammary tissue of women diagnosed with breast cancer in a control group.High concentrations of DDE and PCB were found in the mammary tissue of the patients with neoplasms,although no statistical significance was found when the patients were classified by age group or as smokers or non-smokers [45]. In the fifth study,Dewailly et al.(1993)found oestrogen-receptor-positive or negative (ER+ or ER-)in the tissue of breast neoplasms and detected increased PCB and DDE concentrations(depending on the group selected),although a small sample was considered in this study,leading to inconclusive results [46]. A mammography screening of 14290 participants living in the surrounding areas of New York was conducted as a cohort study (the sixth study) by Wolff et al. from 1985-1991;where 58 patients were diagnosed with breast cancer. 80% were Caucasian women, average age of 51, with significantly high serum PCB and DDE levels [47].Another significant contribution was a study arm (the seventh study) including Californian women evaluated from 1964-1971 by Krieger et al. Among participants, 150 patients developed breast cancer and other 150 were selected for the control group. They were divided into three distinct racial groups:Asian,white and black women all within the same age range.An individual assessment of the racial groups revealed white and black presented high serum DDE levels as compared to the control group.Lower serum DDE levels were evidenced in Asian women in relation to the control group. A multivariable control system was developed to control body mass indexes, age of menarche onset, gestations and menopause which were used for the proper evaluation of potential risks. PCB levels were lower in Asian and white women in comparison to the control group. Odds ratio statistical analysis showed no correlation between serum concentrations of organochlorine and increased risk of developing breast cancer.In addition,serum concentrations varied according to the geographic area [48]. Wolf et al(1993) showed the control group had 7.7ng/ml serum DDE levels,while Kriger et al.(1994)observed white women had 35.0ng/ml,black women had 43.4ng/ml and Asian women had 50.8ng/ml.Black and Asian women had higher seric levels when compared to white women [47,48]. Studies conducted before 1995 showed no correlation between exposure to PCB or DDT and its metabolites with increased risk for developing breast cancer.Since then,other signi9ficant researches correlating PCB or DDT and its metabolites were targeting the adipose tissue or the serum levels of the patients,most of them clsssified as Control-case studies.Van’t Verr et al.(1997)detected DDE concentrations in tissue samples of female patients across 5 European countries associated to increased incidence of breast cancer. They were post-menopausal patients, 265 diagnosed with cancer and 341 in the control group. Logistic regression analysed with age, sex, body mass, index, alcohol consumption and age by the end of the last pregnancy showed DDE levels varied according to country of origin,but levels lower than 1,9ug/g were found in the control groups.These findings showed no association between the level of exposure to DDE and increased risk of developing breast cancer [49]. Liljegren et al.(1998) in Sweden and Guttes et al.(1998)in Germany,evidenced no correlation between DDE and PCB levels and the development of breast cancer in the adipose tissue of humans [50,51]. According to previous studies by Sutherland et al(1996) a variety of chemicals were assessed in a cohort study called ‘Charleston Heart Study’, including 405 black and white women detecting 32.0ng/ml serum DDE levels, following the same line of thought and findings of Krieger et al [48].These patients were followed until 1994, and 20 participants developed breast cancer, however no evidences were found of increased breast neoplasms associated to DDE levels using regression models and other variables [52].
In a cohort study by Hunter et al. (1997) called the ‘Nurses Health Study’, including 120000 nurses followed since 1976, DDE and PCB blood levels were assessed in 240 women with breast cancer and the same number of control participants. Average serum DDE levels were 6.01ng/ml in the patients with breast cancer and 6.97ng/ml in the control group, while PCB levels were 5.08ng/ml and 5.16ng/ml, respectively. No association was made between the highest plasma levels of organochlorines with an increased risk for breast cancer based on multivariable adjustments and comparison of both groups [53]. According to the ‘City Heart Study’ conducted in Copenhagen, by Hoyer et al. (1998) and subsequently by the Danish Cancer Center Registry, 240 from 7712 women, who had their serum stored, were diagnosed with breast cancer and 447 patients were in the control group. 18 different pesticides or its metabolites and 28 variations of PCB were detected in the serum samples of these participants.No relation between DDT and its isomers or any kind of PCB congeners was evidenced using statistical analysis and regression models.Among all chemical compounds evaluated, only Dieldrin was found using the odds ratio of 1.96 and 2.05 and its potential correlation with breast cancer [54]. Later studies have shown inconsistencies in the association between DDT and its potential influence in the increased rate of breast cancer.Cohn et al.’s case-control study(2007)noted that increased disease incidence was related to the duration of exposure to DDT and its metabolites, like DDE. High serum p,p’-DDT levels are age-related, particularly for those patients born before 1931 (exposed before the age of 14), showing 5 times more chances of developing breast cancer [55]. Another study was conducted in the Canary Islands, Spain, by Boada et al(2012) to assess the multiple effects of human exposure to organochlorine pesticides.After evaluating multiple variables,they observed the serum levels of DDE, DDD and Aldrin insecticide (hexachlorocyclopentadiene)were extremely high in women diagnosed with breast cancer as compared to healthy women [56]. The ‘Long Island Breast Cancer Study,by White et al.(2013) observed a group of female patients aged less than 20 years,following an intensive exposure to DDT under the presence of oestrogen and progesterone receptors showing an increased risk for developing breast neoplasia in relation to a control group that was not exposed [57]. Ingber et al.(2013)found there are significantly conflicting meta-analyses showing results differ greatly when DDT and DDE levels are correlated to increased incidence of breast neoplasms.Variations in data including age, menopausal state, study designs and variable considerations make this association even more inconsistent [58].
Bisphenol A And Breast Actions
Bisphenol A has structural similarity to oestradiol and binds to the oestrogen receptor alpha with lower levels of activity but has a strong affinity with Gama receptor and G-protein, being capable of inducing the proliferation of breast cancer epithelial cells by stimulating the oestrogen receptor alpha [59-61]. Several in vitro studies and other studies carried out with rodents have been showing BPA might alter breast development and growth and increase the likelihood of tumour development. Markey et al. (2001), Munoz-de-Toro et al. (2015) and Acevedo et al. (2013) evidenced that exposure to low doses of BPA during foetal, prenatal and puberty periods are associated to the development of preneoplastic lesions, with different forms of hyperplasia. And yet, when doses higher than 2.5ug/kg of BPA are administered, this exposure is correlated to the development of ductal adenocarcinomas [62-64].
Phatalates and Actions on the Breast
In 2014, in a study by Wolf et al. observing late pubertal development and pubic hair growth in 1200 prepuberal girls were detected in these participants high molecular weight phthalates associated to the antiandrogenic properties in their urine samples.Delayed breast growth wasw also observed in these participants [65]. Lopez-Carrilo et al. study (2010), in Northern Mexico, correlated the urinary concentrations of phthalates metabolites (found in 82% of the female patients) and breast cancer showing that concentrations of MEP (monoethyl phthalate) were higher in patients diagnosed with breast cancer than in control cases. The study concluded that exposure increased 2.5 times the risk of tumour development as compared to the control group [66]. Animal experiments showed by Moral et al (2011) that DBP (dibutyl phtalate) adversely affects rodents’reproductive system having a weak binding affinity with oestrogen receptors.Exposure of pregnant rats to DBP could induce (through nursing mothers) a hypoplasia of the alveolar buds in their female offspring [67].
Actions on the Breast by Atrazine
Epidemiological studies have found limited or no correlation between atrazine (ATR) exposure in agriculture and increased incidence of breast cancer.A study conducted in Kentucky, USA,showed that the contamination of superficial waters with atrazine, from 1991-1992 has significantly affected the incidence of breast cancer in exposed women.When deeper waters were included in the analysis, from 1993-1994, no correlation was found [68,69]. A populational study conducted by Muir et al.(2004)in the urban and rural areas of Lincolnshire and Leicestershire Counties,England,from 1989-1991,revealed a positive association between the exposure to atrazine(found in pesticides) and increased incidence of breast cancer [70]. These experiments are limited, and no correlation has been found between exposure to atrazine and increased risk of developing breast cancer.Many animal experiments show that early exposure of pregnant rats to ATR might interfere with the development of the mammary glands of their female offspring [70]. Although not considered carcinogenic, extensive and chronic use of ATR increased mammary adenocarcinoma in Sprague-Dawley rats and high doses this chemical induced breast hyperplasia in male rats in a study by Gammon et al.(2005) [71].
Diethylstilboestrol (DES) and the Breast Actions
DES, a non-steroidal oestrogen formerly used to prevent miscarriages (in variable dosages of 5mg/day-125mg/day), can cause several anomalies.Herbst et al. observed anomalies in the female offspring of pregnant women exposed,having later developed vaginal adenosis or clear cell adenocarcinoma of the vagina [14]. Harris and Waring (2012) and Troisi et al.(2013) have correlated the exposure to DES and many disorders such as: cryptorchidism in boys, uterine anomalies (T-shaped uterus) and hormone-dependent tumours.Animal experiments showed pregnant or nurturing rats exposed to high doses of DES stimulated mammary growth causing anomalies Christenesen CH, et al. (2010). When those animals were exposed to DES during prenatal period, it was observed an increased risk for developing mammary tumours related to a significant increase in EZH2 protein or histone methyltransferase linked to the genesis of hormone-dependent breast cancer [72,73].
Perfluorooctanoic Acid (PFOA)and Breast actions
PFOA is a surfactant widely used in the cleaning of water and grease, and as water repellent or firefighting foam. Also used in dental products or food packaging with average life of 16-22 days in rats and 2-4 years in humans. Combining in-vitro PFOA and oestradiol activates oestrogen and antioestrogen properties [74]. Such properties are known to cause late pubertal development and increase risk to developing breast cancer. The ‘Breast Cancer and Environment Research Program’showed a direct association between serum PFOA levels and lactation period (considering breastfed girls aged 6-8).Also, a significant correlation was made by Kale et al.(2015)between contaminated water sources in North Kentucky and lactation history of the exposed breastfed girls, causing late breast development during pubertal period [75]. Several animal experiments also evidenced a direct correlation between PFOA levels and functional changes in breast development. Gestational exposure to PFOA can delay the epithelial development of the mammary glands and result in a rise in mortality rate of new-borns. Those mammary abnormalities can increase mammary hyperplasia and stromal density, both associated to a greater risk of developing breast cancer [76].
Endogenous and Exogenous Steroid Hormones and the Breast actions
Growing concerns have been raised about the harmful effects of steroid hormones used as hormone replacement therapy or associated to oral contraceptive methods. Endogenous production of numerous hormones affects different tissues with variable response levels to cyclic alternation from the beginning of the administration of these exogenous therapies. Action potential varies widely and depends upon many factors including dosage, growth factors and type of acting hormones, etc.Hormone influence on breasts differs from uterine endometrial action.Endogen oestrogens affect cellular proliferation in the mammary gland and, under progesterone influence (in the 2nd phase of the cycle),maturation and structural gland alterations can be observed.Replacement hormone therapy has been considered in many experiments and the ‘Nurse Health Study’ evidenced the relative risk for developing breast cancer increases 1.3 times (oestrogen use only)and 1.4 times (when oestrogen is associated to progestogens) [77]. In 2002, the ‘Women Health Initiative’ (WHI)investigated the risks involved in the hormonal therapy during climaterium including a large randomized group (16608 women, 290 of them diagnosed with breast cancer).After a 5.2-year follow-up,an increased risk for developing this type of neoplasm was observed when oestroprogestative replacement was used in comparison to oestrogen only therapy [78]. Another relevant study, conducted from 1996-2001,was the ‘Breast Cancer and Hormone Replacement Therapy in the Million Women Study’ following-up a group of 1084110 women (9364 diagnosed with breast cancer and 637 cancer-related deaths after a 2.6 and 4.1-year follow-up).Patients that underwent the hormonal therapy had 1.66 increased risk for developing this pathology as compared to the patients that did not undergo such therapy.In the same way,increased risk was related to oestrogen-progesterone hormone preparations in comparison to oestrogens-only preparations.The results showed little variation depending on the oestrogen-progesterone doses administered,or upon the continuous or sequential treatment schemes [79]. In two epidemiological studies, conducted from 1991-1996, a discrete 1.2-1.5 increased risk for developing breast cancer was observed in the group of contraceptive pills users (including 50000 users and 100000 control patients).
However, among women using hormonal contraceptive methods for more than ten years, no increased risk was noticed [80,81]. According to a recent study by Manson et al.(2017) there was a correlation between menopausal hormone therapy versus placebo during a 5-7-year follow-up and mortality risk up to 18 years (WHI initiative randomized trials) being followed in 40 centers.Among 27347 women with average age of 63.4, 80.6% white,7489 deaths were reported; in 8506 cases, 0.625mg conjugated oestrogens + medroxyprogesterone acetate were used versus placebo (8102 cases during a 5.6-year follow-up)and 5310 cases using conjugated oestrogens-only versus placebo (5429 with a 7.2-year follow-up).Total mortality causes were 27.1% in the hormone therapy group as compared to 27.6% in the placebo group; while in the cancer-related (hormone therapy), total mortality rate was 8.2% versus 8.5% in the placebo group.This study showed that among post-menopausal women, hormone therapy associated to conjugated oestrogens + medroxyprogesterone and a 5.6-year follow-up or conjugated oestrogen-only for 7.2 years were not associated to all causes of cardiovascular diseases and even to cancer mortality during an 18-year follow-up [82].This wide range of chemical substances used in different industries for the manufacturing of pesticides, plastic and resin compounds, paint and ink, among others, include numerous examples of contaminants that are potentially harmful to the environment and human health. They are also considered co-authors and facilitators for the development of numerous disorders such as breast carcinomas.
Analysed different papers our conclusions are: There has been a progressive increase in breast cancer incidence; There are critical periods of breast development during windows of susceptibility to endocrine-disrupting chemicals’ action; Many rodents experience critical periods of breast development making them predisposed to developing neoplasms; Dioxins are chemical disruptors that may delay pubertal breast development in young girls and rodents; Epidemiological studies emphasize the importance of evaluating the effects of endocrine disruptors in women that are predisposed to developing breast cancer; Further research is needed to shed more light on different endocrine disruptors combinations based on their chemical structures, on the evaluation of cell lines of pre-cancerous tissues to establish potential mechanisms of action related to the origin of breast cancer.
Prostatic Neoplasia
The prostate is an accessory gland of the male genital tract producing part of the seminal fluids and facilitating sperm transport. It is dependent on testes androgen hormones in early stages of its embryogenesis and growth. The 5-alpha-reductase enzyme regulates prostate development metabolizing testosterone to DHT.In addition to being androgen-dependent, the prostate is affected by many disorders including neoplasia and benign hyperplasia not to mention the influence of other steroids such as oestrogens or protein hormones.
Among several agents that can cause adverse effects to the prostate are:
Pesticides
Pesticides are commonly used by farmers and farm workers on a large scale presenting potential health hazards.In 1993,a follow-up of 57000 farm workers was conducted in the ‘Agricultural Health Study’(AHS)observing an increased risk for developing prostate cancer within this populational group.Early analysis in 2003, showed exposure to methyl bromides could induce this increased risk,but subsequent analysis observed organophosphates(malathion, aldrin, etc.)and organochlorines would increase risk for developing prostate cancer in patients with family history [83-86]. These chemicals induce alterations metabolizing enzymes in liver, testosterone, estradiol and estrone.Malathion is known to reduce FSH, LH and testosterone serum levels while aldrin can rise aromatase activity [87,88]. After investigating farmers in British Columbia, Canada,Band et al.(2011)have correlated a significant increase in prostate cancer with Endocrine-Disrupting chemicals(EDCs) such as Endosulphan(oestrogen agonist and androgen antagonist activities and aromatase activity),DDT(xenoestrogen activity binding to oestrogen receptors),diazinon(estrogenic activity)and malathion (antiandrogenic properties) [89]. Based on these reviews,e agree that several pesticides can influence the development and incidence of prostate cancer.Among the organochlorine insecticides that might be held responsible for the genesis of this neoplasms are dieldrin, endosulfan, lindane, toxaphene, dicofol and heptachlor.According to previous studies by Koutros et al.(2011)and Karami et al.(2013) the gene expression of TXNRD2 may also be altered by these pesticides [87]. The RXR complex, involved in the metabolism of vitamin D, has a protective effect and its reduction could increase the risk for developing prostate cancer [90,91]. Initially,the agent orange and other toxins had no correlation to the etiopathogeny of this disorder.Later, as a mixture with equal parts of 2,4-Dichlorophenoxyacetic acid and 2,4,5-Trichlorophenoxyacetic acid,the agent orange was widely used as defoliant chemical during Vietnam war.Veterans exposed to this agent showed a significant 2.3-6 increase in the incidence of prostate cancer in comparison to unexposed veterans [92-94]. In addition, it was observed an early, more aggressive progression and recurrence of this disease in the exposed group [90,91]. A meta-analysis of cohort data (40286 participants) conducted by Leng et al. (2014) verified this association related to the genesis of this disorder [95].
Bisphenols
Several studies have indicated that bisphenols may have carcinogenic properties for the development of prostate cancer.Tarapore et al.study(2014)involved 60 urology patients showing increased serum BPA levels in the patients with positive prostate biopsy as compared to negative prostate biopsy.Cancer development was also observed at a comparatively early age in patients with increased serum BPA levels suggesting that increased levels were correlated to life style, although a more comprehensive follow-up is still needed to better assess patients.96 Even lower doses of BPA are capable of inducing cell proliferation and cell lineage replication, increasing the chances of migration and invasion of LNCaP cells [97]. Animal experiments also showed that mesenchymal cell lines in cultures of foetal rats prostate have a similar response to estradiol in relation to androgen receptors and oestrogen receptors alpha, based on a dose-dependent stimulation [98].
Polychlorinated biphenyl (PCB)
Only a few references show that PCB153 and PCB180 have been associated with increased risk of prostate cancer.A 35-year follow-up including a cohort of 24865 employees relates exposure to these chemicals and prostate cancer-related mortality, in studies conducted by Ruder et al.(2014) [99]. However, no association was made between prostate cancer and organochlorinated chemicals (such as p’-DDT, p,p’-DDT and p,p’-DDE) in Sawada et al.’s case/control study(2010) with 14203 male patients and 12.8-year follow-up [100]. In Canada,Aronson et al.also failed to make an association between chemicals and increased incidence of prostate cancer after examining urology patients and detecting plasma levels of PCB153 and PCB180 [101]. It seems a potential correlation depends on exorbitant concentrations of polychlorinated chemicals and the increased incidence of PCa in elderly men.Animal experiments or studies with cell lineages evidenced PCB’s effects on prostate cancer cell line LNCaP can reduce cell proliferation and slowdown 5 alpha-reductase activity and PSA secretion. Other PCBs such as PCB153 and PCB118 may induce cell proliferation and increase PSA concentrations even though low doses are used.
Viclozin
Viclozin is a fungicide with antiandrogenic properties and androgen receptors antagonists. Positive responses were evidenced in male adult rats, but similar findings were not observed in humans by Cowin et al. (2010) [102].
Heavy Metals
Arsenic and cadmium are involved in the genesis of many neoplasms including prostate cancer. Inorganic arsenic found in the soil of contaminated areas has toxic effects that can lead to oxidative stress causing damage to DNA and might disrupt steroids receptors.Previous studies by Garcia-Esquinas et al.(2013) evidenced a high prostate-cancer-related mortality rate in a group of 4000 American Indians (20 years follow-up) exposed to inorganic arsenic [103]. Environmental concerns have been raised in relation to cadmium exposure (as smokers or via food intake) once heavy metals mimic and induce oestrogen receptors’ activation. Inconsistent findings by Mullins et al. (2012) and Julin et al. (2012) fail to verify a positive or negative relationship between prostate cancer and cadmium [104,105].
Thyroid Neoplasms
Thyroid hormones are crucial to regulate the homeostasis and control several physiological processes in humans and vertebrates.Thyroid hormones’ control depends on the hypothalamic release of TRH-releasing factor, Thyroid Stimulating Hormone (TSH) and secretion of 2 types of Thyroid Hormones: T4 (the most important) and T3.Several Endocrine-disrupting chemicals might affect the entire chain of the hypothalamic–pituitary–thyroid axis including the synthesis, release and transport of thyroid hormones, as well as its metabolism and mechanism of action on target tissues. Many chemical substances found in the environment may adversely affect iodine uptake and consequently disrupt thyroid function.Among them are: perchlorate,chloride,nitrate and thiocyanates.Chlorides and nitrates are commonly found in high concentrations on the water supply chain while thiocyanates are toxic components of tobacco.Perchlorate is widely used in the manufacturing of explosives and car automobile airbag inflation systems.These are all capable of inhibiting iodine’s uptake leading to low or insufficient absorption levels [106]. Perchlorates have a complex mechanism of action on thyroid function, but a recent study by Taylor et al.(2014) evidenced exposure of pregnant women with borderline thyroid function to high concentrations of this substance reduced cognitive function in their offspring [107]. Thyroperoxidase is an enzyme that plays an important role in the synthesis of the thyroid hormones which may be disrupt under the influence of isoflavone or thiocyanate. Other phenolic compounds, such as Bisphenols, might have a binding affinity with plasma proteins hence reducing circulating thyroid hormones and their tissue-specific actions in a Cao et al. study (2010) [108]. A great number of chemical compounds have been shown to interfere with, potentially reducing, thyroid function, although it has been hard to establish consistent results on humans.
Among them are:
Perchlorates
Experimental studies show perchlorates have a half-life of about 8 hours and an exposure level of about 5.2ug/Kg/day would be enough to reduce iodine intake and consequently affect the synthesis of thyroid hormones [109]. Gisnberg et al.(207) and Steinmaus et al.(NHANES Survey)(2013)observed children are highly sensitive to thyroid hormone deficiency and when perchlorate levels found in breast milk are high they may interfere with children’s thyroid hormones and affect their development [110,111].
Polychlorinated Biphenyls (PCB)
Prenatal or postnatal exposure to Polychlorinated Biphenyls B (PCBs)has been associated to a wide range of cognitive disorders in children, even though accurate measurements for these functions are hard to establish by Schantz et al. (2003) and Majidi et al. (2014) [112,113].
Polybrominated Diphenyl Ethers (PBDEs)
PBDEs have been found in the blood of pregnant women,in cord blood and breast milk while high PBDE concentrations detected and have been associated to impaired cognitive functions as well as deficient verbal and IQ performance of the exposed children by Frederiksen et al (2009)and Herbstman et al (2010) [114,115]. This association was also verified in animal experiments,particularly rodents’ exposure to polyphenols B or PBDE [116]. In Erikson et al (2006)high levels of PBDE causes a decrease in thyroid hormone levels and consequently disrupt neurodevelopment and induce cognitive deficit.
Phthalates
Evidences from animal, biochemical and human studies show that Phthalates can interfere with free and total Thyroid Hormones(T4). After evaluating NHANES Survey’s data, Meeker et al. (2011) observed a negative association between MEHP urinary levels and free and total T4 and a positive association between MEHP urinary levels and TSH levels [117]
Bisphenol A (BPA)
Several epidemiological studies in humans have evidenced a correlation between exposure to BPA and the rise in levels of thyroid hormones. Meeker et al. have also observed an association between total and free T4 and the levels of exposure to BPA, showing that these chemicals have weak binding affinity and are indirect antagonists of the thyroid hormone receptors [117]. Weiss et al. (1997) observed that BPA creates a disruptive action which mimics the resistance thyroid hormone [118].
Conclusions
In the latest years, several experiments have brought to our attention that toxic chemicals play a specific part in the genesis of tumours. Previous researches show those chemicals have different degrees of toxicity and carcinogenicity. Based on that information, safety measures must be established and followed in our daily routines to ensure low exposition of pregnant women and prevent damage to intrauterine foetal development.
With this purpose in mind, the International Federation of Gynaecology and Obstetrics (FIGO) recommends the following:
- Common awareness that global exposure to toxic compounds, associated to the genesis of many pathologies, may harmfully affect human population;
- Avoiding exposure to these chemicals must be prioritized by all parts involved;
- Toxic chemicals have a global effect crossing frontiers between countries via food, water, wind and many business relationships;
- Corrective and preventive actions must be taken to avoid continuous release of these chemicals into the environment with negative impacts on vulnerable populations [119].
References
- Dodds EC, Lawson W (1936) Synthetic oestrogen agentes without the phenanthrene nucleus. Nature 137: 996.
- Sartain CV, Hunt PA (2016) An old culprit but a new story: Bisphenol A and next gen bisphenols. Fertil & Steril 106: 820-826.
- Vom Saal FS, Welshons WV (2014) Evidence that bisphenol A (BPA) can be accurately measured without contamination in human serum and urine, and that BPA causes numerous hazards from multiple routes of exposure. Moll Cell Endocrinol 398: 101-113.
- Volkel W, Colnot T, Csanady GA, Filser JG, Dekant W (2002) Metabolism and kinetics of bisphenol A in humans at low doses following oral administration. Chem Res Toxicol 15: 1281-1287.
- Dieckmann WJ, Davis ME, Rinkiewicz LM, Pottinger RE (1953) Does the administration of diethylbestrol during the pregnancy have therapeutic value? Amer J Obstet Gynecol 66: 1062-1081.
- Wu CF, Chang-Chien GP, Su SW, Chen BH, Wu MT (2014) Findings of 2731 suspected phtalate-painted foodstuffs during the 2011 phtalates incident in Taiwan. J Formos Med Assoc 113: 600-605.
- Solomon KR, Giesy JP, LaPoint TW, Giddings JM, Richards RP (2013) Ecological risk assessment of atrazine in North American surface waters. Environ Toxicol Chem 2: 10-11.
- Portigal CL, Cowell SP, Fedoruk MN, Butler CM, Rennie OS (2002) Polychlorinated biphenyls interfere with androgen-induced transcriptional activation and hormone biding. Toxicol Appl Pharmacol 179: 185-194.
- Agency for Toxic Substances and Disease registry (2004) Toxicological Profile for Polybrominated Biphenyls and Polybrominated Diphenyl Ethers (PBBs and PBDEs). Atlanta GA: US Department of Health and Human Services, Public Health Service;2004
- National Toxicology Program (2011) Report on Carcinogens,12th Edition. Washington DC: US Department of Health and Human Services, Public Health Service 12: iii-499.
- Dodds EC, Lawson W, Robison R (1938) Estrogenic activity of certain synthetic compounds. Nature 141: 247-288.
- Yoshida M, Takahashi M, Inone R, Hayashi S, Makaekawa A (2011) Delayed adverse effects of neonatal experience to diethylstilbestrol and their dose dependency in female rats. Toxicol Pathol 19: 823-834.
- Herbst AL, Ulfelder H, Postkanger DC (1971) Adenocarcinoma of vagina. Association of maternal stilbestrol therapy with tumor appearance in Young women. N Engl J Med 284: 878-881.
- Herbst AL (1976) Summary of changes in human female genital tract as a consequence of maternal diethylstilbestrol therapy. J Toxicol Environ Health Suppl 1: 13-20.
- Harris RM, Waring RN (2012) Diethylstilbestrol-a long term legacy. Maturitas 72: 108-112.
- Troisi R, Hyer M, Hatch EE (2013) Medical conditions among adults offspring prenataly exposed to diethylstilbestrol. Epidemiology 24: 430-43825.
- Kogevinas M, Becher H, Benn T (1997) Cancer mortality in workers exposed to phenoxy herbicides, chlorophenols and dioxins. On expanded and updated international cohort study. Am J Epidemiol 145: 1061-1075.
- Sturgeon SR, Brock JW, Potischman N (1998) Serum concentrations of organochlorine compounds and endometrial cancer risk (USA). Cancer causes Control 9: 417-424.
- Hiroi H, Sutsumi O, Takeuchi T (2004) Differences in serum bisphenol A concentrations in premenopausal normal women and women with endometrial hyperplasia. Endocr J 51: 595.
- Donna A, Rosignani P, Robutti F (1989) Triazine herbicides and ovarian epithelial neoplasms.Scand J Work Environ Health 15: 47-53.
- Young H A, Mills PK, Riordan DG, Cress RD (2005) Triazine herbicides and epitelial ovarian cancer risk in central California. J Occup Environ Med 47: 1148-1156.
- Alavanja MC, Sandler DP, Lynch CF (2005) Cancer incidence in the Agricultural Health Study. Scand J Work Environ Health 31: 39-45.
- Vieira VM, Hoffman K, Shin HM, Weinberg JM, Webster TF (2013) Perfluorooctanoic acid exposure and cancer outcomes in a contaminated Community: a geographic analysis. Environ Health Perspect 121: 318-323.
- NTP (National Toxicology Program)1982 (1982) Carcinogenesis bioassay of Bisphenol A in F344 Rats and B3C3F (mice feed study) NTP-80-35. National Toxicology Programa.Research Triangle Park, NC and Bethesda MD.
- Tims BG (2005) Estrogenic chemicals in plastic oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proceedings of National Academy of Health USA 102: 7014-7019.
- Moral R (2008) Effects of prenatal exposure to the endocrine disrupt Bisphenol A on mammary gland morphology and gene expression. Signature. J Endocrinol 196: 101-112.
- Jemkins S (2009) Oral exposure to Bisphenol A increases dimethylbenzantracene induced mammary câncer in rats. Environ Health Perspect 117: 910-1528.
- Prins GS (2011) Serum Bisphenl A pharmacokinetics and prostate neoplastic responses following oral and subcaneous exposure in neonatal Sprague Dawley rats.Reprod Toxicol 31: 1-9.
- Soto AM Chung S, Sonnenschein C (1994) The pesticides endosulfan, toxaphene and dieldrin have estrogenic effects on human estrogenic-sensitive cells. Environ Health Perspect 102: 380-383.
- Kelce WR, Stone CR, Laws SC, Gray LE, Kemppainen JA (1995) Persistent DDT metabolite p.p’-DDE is a potente androgen receptor antagonist.Nature 375: 581-585.
- Moore M, Mustain M, Daniel K, Chen I, Safe S, et al. (1997) Antiestrogenic activity of hidroxilated polychlorinated biphenyl congeners identified in human serum. Toxicol Appl Pharmacol 142: 160-168.
- Astroff B, Safe S (1990) 2,3,7,8-tetrachlorodibenzo-p-dioxin as an antiestrogen: effect on rat uterine peroxidase activity. Biochem Pharmacol 39: 485-488.
- Den Hond E, Roels HA, Hoppenbrouwers K (2002) Sexual maturation in relation to polychlorinated aromatic hydrocarbons: Sharpe and Skakkebaek’s hypothesis revisited. Environ Health Perspect 110: 771-776.
- Leijs MM, Koppe JG (2008) Delayed initiation of breast development in girls with higher prenatal dioxin exposure: a longitudinal cohort study. Chemosphere 73: 999-1004.
- Bertazzi PA, Zocchetti C, Pesatori AC, Guercilena S, Sanarico M (1989) Ten-year mortality study of the population involved in the Seveso-incident in 1976. Am J Epidemiol 129: 1187-1200.
- Bertazzi PA, Zocchetti C, Guercilena S (1997) Dioxin exposure and cancer risk: a 15-year mortality study after the Seveso-incident. Epidemiology 8: 646-652.
- Pesatori AC, Consonni D, Rubagotti M, Grillo P, Bertazzi PA (2009) Cancer incidence in the population exposed to dioxin after the Seveso accident: twenty years of follow-up. Environ Health 8: 39.
- Warner M, Eskenazi B, Mocarelli P (2002) Serum dioxin concentrations and breast cancer risk in the Seveso Women’s Health Study.Environ Health Perspect 110: 625-628.
- Revich B, Aksel E, Ushakova T (2001) Dioxin exposure and public health in Chapayevsk, Russia.Chemosphere 43: 951-1066.
- Rogan WJ, Gladen BC, Mckinney JD (1987) Polychlorinated biphenyls (PCBs) and dichlorodiphenyldichloroethene (DDE) in human milk: effects on growth, morbidity and duration of lactation. Am J Publ Health 77: 1294-1297.
- Karmaus W, Davis S, Fussman C, Brooks K (2005) Maternal concentrations of dichlorodiphenyl dichloroethylene (DDE) and initiation and duration of breast feeding. Paediatr Perinat Epidemiol 19: 338-398.
- Wasserman M, Nogueira L, Tomatis AP, Mirra H, Shibata G (1976) Organochlorine compounds in neoplastic and apparently normal breast tissue. Bull Environ Contam Toxicol 15: 478-484.
- Unger M, Kiaer M, Blichert-Toft P, Olsen J, Clausen J (1984) Organochlorine compounds in human breast fat from deceased with and without breast cancer and in a biopsy material from newly diagnosed patients undergoing breast surgery. Environ Res 1984; 34: 24-28.
- Mussalo-Rauhamaa H, Hasanen E, Pyysalo K, Amervo R, Kauppila R (1990) Occurrence of beta-hexachlorocyclohexane in breast câncer patients. Cancer 66: 2124-2128 .
- Falck A, Ricci A Jr, Wolff MS, Godbold, Deckers P (1992) Pesticide and polychlorinated byphenils residues in human breast lipids and their relation to breast cancer. Arch Environ Health 47: 143-146.
- Dewailly E, Bruneau S, Ayotte P, Laliberte C, Muir DCG (1993) Health status at birth of Inuit newborns prenatally exposed to organochlorines. Chemosphere 27: 359-366.
- Wolff MS, Toniolo PG, Lee EW, Rivera M, Dubin N (1993) Blood levels of organochlorine residues and risk of breast câncer. J Natl Can Inst 85: 648-652.
- Krieger N, Wolff MS, Hiatt RA, Rivera M, Vogelman J (1994) Breast câncer and serum organochlorines: a prospective study among white, black and asian women. J Natl Cancer Inst 86: 589-599.
- van’t Veer P, LobbezooI E, Marin-Moreno JM, Guallar E, Gomez-Aracena J (1997) DDT (dicophane)and postmenopausal breast cancer in Europe:Case control study. BMJ 315: 81-85.
- Liljegren G, Hardell L, Lindstrom G, Dahl P, Magnuson A (1998) Case-control study on breast câncer and adipose tissue concentrations of congener specific polychlorinated biphenyls DDE and hexachlorobenzene. Eur J Cancer Prev 7: 135-140.
- Guttes SK, Failing K, Neumann K, Kleinstein J, Georgii S (1998) Chlororganic pesticides and polychlorinated biphenyls in breast tissue of women with benign and malignant breast disease. Arch Environ Contam Toxicol 35: 140-147.
- Sutherland SE, Bernard VB, Keil JE, Austin H, Hoel DG (1996) Pesticides and twenty years risk of breast câncer. 29th Annual Meeting of the Society for Epidemiologic Research, Boston MA. Am J Epidemiol (Abstracts) 143: 133.
- Hunter DJ, Hankinson SE, Laden F, Colditz GA, Manson JE (1997) Plasma organochlorine levels and the risk of breast cancer. N Engl J Med 337: 1253-1258.
- Hoyer AP, Grandjean P, Jorgensen T, Brock JW, Hartvig HB (1998) Organochlorine exposure and risk of breast câncer. Lancet 352: 1816-1820.
- Cohn BA, Wolff MS, Cirillo PM, Sholtz RI (2007) DDT and breast cancer in young women: new data on the significance of age at exposure. Environ Health Perspect 115: 1406-1414.
- Boada LD, Zumbado M, Henriques-Hernadez LA (2012) Complex organochlorine pesticide mixtures as determinant fator for breast cancer risk: a population-based case-control study in the Canary Islands (Spain) Environ Health 11: 28.
- White AJ, Teitelbaum SL, Wolff MS, Stellman SD, Neugut AI (2013) Exposure to fogger trucks and breast cancer incidence in the Long Island Cancer Study Project; a case-control study. Environ Health 12: 24.
- Ingber SZ, Buser MC, Pohl HR, Abadin HG, Murray HE (2013) DDT/DDE and breast câncer: a meta-analysis. Regul Toxicol Pharmacol 67: 421-433.
- Pupo M, Pisano A, Lappano R (2012) Bisphenol A induces gene expression. Changes and proliferative effects through GPER in breast cancer cells and câncer-associated fibroblastos. Environ Health Perspect 120: 1177-1182.
- Takayanagi S, Tokunaga T, Liu X, Okada H, Matsushima A (2006) Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor ERR with high constitutive activity. Toxicol Lett 167: 95-105.
- Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D (1993) Bisphennol A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology 132: 2279-2286.
- Markey CM, Luque EH, Munoz-de-Toro M, Sonnenschein C, Soto AM (2001) In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biol Reprod 65: 1215-1223.
- Munoz-de-Toro M, Markey CM, Wadia PR, Luque EH, Rubin BS (2005) Perinatal exposure to bisphenol A alters peripubertal mammary gland development in mice. Endocrino0logy 146: 4138-4147.
- Acevedo N, davis B, Schaeberle CM, Sonnenschein C, Soto AM (2013) Perinatally administered bisphenol A as a potent mammary gland carcinogen in rats. Environ Health Perspect 121: 1040-1046.
- Wolff MS, Teitelbaum SL, McGovern K (2014) Phtalate exposure and pubertal development in a longitudinal study of US girls. Hum Reprod 19: 1558-1566.
- Lopez-Carrillo L, Hernandez-Ramos RU, Calafat AM (2010) Exposure to phtalates and breast cAncer risk in northern Mexico. Environ Health Perspect 118: 539-544.
- Moral R, Santucci-Pereira J, Wang R, Russo IH, Lamartiniere CA (2011) In útero exposure to butyl benzyl phtalate induces modifications in the morphology and gene expression. Profile of the mammary gland:an experimental study in rats. Environ Health 10: 5.
- Kettles MK, Browning SR, Prince TS, Horstman SW (1997) Triazine herbicide exposure and breast cancer incidence: na ecologic study of Kentucky counties. Environ Health Perspect 105: 1222-1227.
- Hopenhayn-Rich C, Stump ML, Browning SR (2002) Regional assessment of atrazine exposure and incidence of breast and ovarian cancer in Kentucky. Arch Environ Contam Toxicol 42: 127-136.
- Muir K, Rattanamongkolgul S, Smallman-Raynor M, Thomas M, Downer S (2004) Breast cancer incidence and its possible spatial association with pesticide application in two counties of England. Public Health 118: 513-520.
- Gammon DW, Aldous CN, Carr WC, Sanborn JR, Pfeifer KF (2005) A risk assessment of atrazine use in California: human health and ecological aspects. Pest Manag Sci 61: 331-355.
- Hovey RC, Asai-Sato M, Warri A (2005) Effects of neonatal exposure to diethylstilbestrol, tamoxifen, and toremifene on the BALB/c mouse mammary gland. Biol Reprod 72: 423-435.
- Doherty LF, Bromer JG, Zhou Y, Aldad TS, Taylor HS (2010) In útero exposure to diehylstilbestrol (DES) or bisphenol A (BPA) increases EZH2 expression in the mammary gland: an epigenetic mechanism linking endocrine disruptor to breast cancer. Horm Cancer 1: 146-155.
- Lopez-Espinosa MJ, Fletcher T, Armstrong B (2011) Association of perfluoroctanoic acid (PFOA) and perfluoroctane sulfonate (PFOS) with age of puberty among children living near a chemical plant. Environ Sci Technol 45: 8160-8166.
- Kale A, Deardorff J, Lahiff M (2015) Breastfeeding versus formula-feeding and girls pubertal development. Matern Child Health 19: 519-527.
- White SS, Calafat AM, Kuklenyik Z (2007) Gestaional PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol Sci 96: 133-144.
- Colditz GA, Hankinson SE, Hunter DJ, Willett WC, Manson JE (1995) The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Eng J Med 332:1589-1593.
- Women’s Health Initiative Group (2002) Risks and Benefits of Estrogen plus Progestin in Healthy postmenopausal women-Principal results from Women’s Health Initiative randomized controlled trial. JAMA 288: 321-333.
- Beral V (2003) Breast cancer and hormone-replacement therapy in the million women study. Lancet 362: 419-427.
- IOM (Institute of Medicine) (1991) Oral contraceptives and breast cancer. National Academy Press Washington DC.
- Collaborative group on hormonal factors in breast cancer (1996) Breast cancer and hormonal contraceptives: Collaborative reanalysis of individual data on 53297 women with breast cancer and 100239 women without breast câncer form 54 epidemiological studies. Lancet 347: 1713-1727.
- Manson JE, Aragaki AK, Rossouw JE (2017) Menopausal Hormone Therapy and long term All-cause and specific mortality. JAMA 318: 927-938.
- Alavanja MC, Samanic C, Dosemeci M (2003) Use of agricultural pesticides and prostate cancer risk in the Agricultural Health Study cohort. Am J Epidemiol 157: 800-814.
- Barry KH, Koutros S, Lubin JH (2012) Methyl bromide exposure and cancer risk in the Agricultural Health Study. Cancer causes Control 23: 807-818.
- Koutros S, Beane Freeman LE, Lubin JH (2013) Risk of total and agressive prostate cancer and pesticide use in the Agricultural Health Study. Am J Epidemiol 177: 159-174.
- Christenesen CH, Platz EA, Andreotti G (2010) Coumaphos exposure incident cancer among male participants in the Agricultural Health Study (AHS). Environ Halth Pespect 118: 92-96.
- Uzun FG, Kalender S, Durak D, Demir F, Kalender Y (2009) Malathion-induced testicular toxicity in male rats and the protective effect of vitamin C and E. Food Chem Toxicol 47: 1903-1908.
- Laville N, Balaguer P, Brion F (2006) Modulation of aromatase activity and mRNA by various selected pesticides in the human chroriocarcinoma JEG-3cell line. Toxicology 228: 98-108.
- Band PR, Abanto Z, Bert J (2011) Prostate cancer risk and exposure to pesticides in British Columbia farmers. Prostate 71: 168-183 .
- Koutros S, AndreottiG, Berndt SI (2011) Xenobiotic-metabolizing gene variants, pesticide use and the risk of prostate câncer. Pharmacogenet Genomics 21: 615-623.
- Karami S, Andreotti G, Koutros S (2013) Pesticide exposure and inherited variants in vitamin D pathway genes in relation to prostate cancer. Cancer Epidemiol Biomarkers Prev 22: 1557-1566.
- Akhtar FZ, Garabrandt DH, Ketchum NS, Michalek JE (2004) Cancer in US Air Force veterans of the Vietnan war. J Occup Environ Med 46: 123-136.
- Chamie K, DeVere White RW, Lee D, Ok JH, Ellinson LM (2008) Agent orange exposure, Vietnan War veterans and the risk of prostate cancer. Cancer 113: 2464-2470.
- Shah SR, Freedland SJ, Aronson WJ (2009) Exposure to Agent Orange is a significant predictor of prostatic-specific antigen (PSA)-based recurrence and rapid PSA doubling time after radical prostatectomy. BJU Int 103: 1168-1172.
- Leng L, Chen X, Li CP, Luo XY, Tang NJ (2014) 2,3,7,8 tetrachlorodibenzo-p-dioxin exposure and prostate cancer: a meta-analysis of cohort studies. Public Health 128: 207-213.
- Tarapore P, YingJ, Ouyang B, Burke B, Bracken B, et al. (2014) Exposure to bisphenol A correlates with early-onset prostate cancer and promotes centrosome amplification and anchorage-independent growth in vitro. PLoSOne 9: e903-932.
- Derouiche S, Warnier M, Mariot P (2013) Bisphenol A stimulates prostate cancer migration via remodelling of calcium signalling. Springerplus 2: 54-59.
- Richter CA, Taylor JA, Ruhlen RL, Welshons WV, Vom Saal FS (2007) Estradiol and bisphenol A stimulate androgen receptor and estrogen receptor gene expression.In fetal mouse prostate mesenchyme cells. Environ Health Perspect 115: 902-908.
- Ruder AM, Hein MJ, Hopf NB, Waters MA (2014) Mortality among 24865 workers exposed to polychlorinatedbiphenyls (PCBs) in three eletrical capacitor manufacturing plants: a ten-year update. Int J Hyg Environ Health 217: 176-187.
- Sawada N, Iwasaki M, Inoue M (2010) Plasma organochlorines and subsequent risk of prostate cancer in Japanese men: a neted case-control study. Environ Health Perspect 118: 659-665.
- Aronson KJ, Wilson JW, Hamel M (2010) Plasma organochlorine levels and prostate cancer risk. J Expo Environ Epidemiol 20: 434-445.
- Cowin PA, Gold E, Aleksova J (2010) Vinclozolin exposure in utero induces postpubertal prostatis and reduces sperm production via a reversible hormone-regulated mechanism.Endocrinology 151: 783-792.
- Garcia-Esquinas E, Pollan M, Umans JG (2013) Arsenic exposure and cancer mortality in a US-based prospective cohort:the Strong Heart Study. Cancer Epidemiol Biomarkers 22: 1944-1953.
- Mullins JK, Loeb S (2012) Environmental exposure and prostate cancer. Urol Oncol 30: 216-219.
- Julin B, Wolk A, Johansson JE, Anderson S O, Andren O, et al. (2012) Dietary cádmium exposure and prostate cancer incidence: a population-based prospective cohort-study.Br J Cancer 107: 895-890.
- Council on Environmental Health, Rogan WJ, Paulson JA, Baum C (2014) Iodine deficiency, pollulant chemicals and the thyroid: new information and old problem. Pediatrics 133: 1163-1166.
- Taylor PN, Okosieme OE, Murphy R (2014) Maternal perchlorate levels in women with borderline thyroid function during pregnancy and the cognitive development of their offspring:data from the Controlled Antenatal Thyroid Study J Clin Endocrinol Metab 99: 4291-4298.
- Cao J, Lin Y, Guo LH, Zhang AQ, Wei Y, et al. (2010) Structure-based investigation on the binding interaction of hydroxilated polybrominated diphenyl ethers with thyroxine transport proteins. Toxicology 277: 20-28.
- Greer MA, Goodman G, Pleus RC, Greer SE (2002) Health effects assessment for environmental perchlorate contamination: the dose response for inhibition of thyroidal radioiodine uptake in humans. Environ Health Perspect 110: 927-937.
- Steinmaus C, Miller MD, Cushing L, Blount BC, Smith AH (2013) Combined effects of perchlorate, thiocyanate and iodine on thyroid function in the National Health and Nutrition Examination Survey 2007-08. Environ Res 123: 17-24.
- Ginsberg GL, Hattis DB, Zoeller RT, Rice DC (2007) Evaluation of the U.S. EPAO/OSWER preliminar remediation goal for perchlorate in groundwater: focus on exposure to nursing infants. Environ Health Perspect 115: 361-369.
- Schantz SL, Widholm JJ, Rice DC (2003) Effects of PCB exposure on neuropsychological function in children. Environ Health Perspect 111: 357-576.
- El Majidi N, Bouchard M, Carrier G (2014) Systematic analysis of the relationship between standardized biological levels of polychlorinated biphenyls and thyroid function in pregnant women and newborns. Chemosphere 98: 1-17.
- Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE (2009) Human internal and external exposure to PBDEs- a review of levels and sources. Int J Hyg Environ Health 212: 109-134.
- Herbstman JB, Sjodin A, Kurzon M (2010) Prenatal exposure to PBDE and neurodevelopment. Environ Health Perspect 118: 712-719.
- Eriksson P, Discher C, Frederiksson (2006) A Polybrominated diphenyl ethers, a group of brominated flame retardants can interact with polychlorinated biphenyls in enhancing developmental neurobehavioral defects. Toxicol Sci 94: 302-309.
- Meeker JD, Ferguson KK (2011) Relationship between urinary phtalate and bisphenol A concentrations and serum, thyroid measures in US adults and adolescentes from the National Health and Nutrition Examination Survey (NHANES) 2007-2008. Environ Health Perspect 119: 1396-1402.
- Weiss RE, Stein MA, Refetoff S (1997) Behavioral effects of liothyronine (L-T3) in children with attention deficit hyperactvity disorder in the presence and absence of resistance to thyroid hormone. Thyroid 7: 389-393.
- Di Renzo GC, Conry JA , Blake J, DeFrancesco MS, DeNicola N (2015) International Federation of Gynecolgy and Obstetrics opinion on reprodutive health impacts of exposure to toxic environmental chemicals. Int J Gynecol Obstet 131: 219-225.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.