Encephalic Radiation Treatment During Tyrosine Kinase Inhibitors (TKIs) in Advanced Non-Small Cell Lung Cancer (NSCLC): Retrospective RealWorld Analysis of a Single Centre.
by Bianca Santo1,#,*, Elisa Ciurlia1,#, Maria Lucia Reale2, Maria Cristina Barba1, Elisa Cavalera1, Paola De Franco1, Sara De Matteis1, Giuseppe Di Paola1, Angela Leone1, Antonella Papaleo1, Dino Rubini3, Donatella Russo1, Silvana Leo2, Giuseppe Rubini4, Angela Sardaro1
1Radiation Oncology Unit, Department of Onco-Hematology, “Vito Fazzi” Hospital, Lecce, Italy
2Medical Oncology, “Vito Fazzi” Hospital, Lecce, Italy
3Radiation Therapy Unit, Department of Precision Medicine, Università Degli Studi Della Campania Luigi Vanvitelli, Italy
4Nuclear Medicine Unit, Interdisciplinary Department of Medicine, University of Bari, Italy
*Corresponding author: Bianca Santo, Radiation Oncology Unit, Department of Onco-Hematology, “Vito Fazzi” Hospital, Lecce, Italy
Received Date: 25 July 2025
Accepted Date: 29 July 2025
Published Date: 31 July 2025
Citation: Santo B, Ciurlia E, Reale ML, Barba MC, Cavalera E, et al (2025). Encephalic Radiation Treatment During Tyrosine Kinase Inhibitors (TKIs) in Advanced Non-Small Cell Lung Cancer (NSCLC): Retrospective Real-World Analysis of a Single Centre. Ann Case Report. 10: 2356. DOI: https://doi.org/10.29011/2574-7754.102356
Abstract
Background: This study aimed to evaluate the outcomes of non-small cell lung cancer (NSCLC) patients with central nervous system (CNS) metastases treated with radiotherapy and targeted tyrosine kinase inhibitors (TKIs); Methods: The study reviewed 14 symptomatic NSCLC patients treated with either whole-brain radiotherapy (WBRT) or stereotactic radiosurgery (SRS) while undergoing TKI therapy. Results: The median time from TKI initiation to radiotherapy was 16.4 months. No acute toxicity was reported. After a median follow-up of 2.1 months, the intracranial objective response rate (ORR) was 64.3%, with five patients achieving complete cerebral response, four showing partial response, and four having stable disease. Nine patients died due to systemic progression, though they maintained intracranial responses; Conclusion: The study suggests that concurrent radiotherapy and TKIs is safe and effective, with survival more influenced by systemic disease progression than intracranial factors.
Keywords: Brain Metastases; Oncogene Addicted Lung Cancer; Targeted Tyrosine Kinase Inhibitors.
Introduction
Brain metastases (BMs) are an area of concern in the management of patients with non-small cell lung cancer (NSCLC). In fact, BMs occur in roughly 40% of individuals with stage IV NSCLC and about 10–20% of cases had BMs at the time of diagnosis [1-4]. The incidence of BMs is higher among patients with oncogeneaddicted NSCLC compared to those with wild-type tumors in relation to the longer survival particularly in patients who have ALK and EGFR drivers, whose overall survival (OS) is increased by five years [5,6]
BMs in patients with advanced oncogenic NSCLC by mutation type have higher rates in those with epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), human epidermal growth factor receptor 2 (HER2), Proto-oncogene tyrosine-protein kinase ROS (ROS1), Ret proto-oncogene (RET), and Kirsten rat sarcoma viral oncogene homolog (KRAS), MET exon 14 skipping alterations(METex14). The increased prevalence of cranial magnetic resonance imaging (MRI) screening at the diagnosis of NSCLC has also resulted in increased diagnosis of patients with asymptomatic BMs. [7-10].
Some exceptions (as for KRAS G12C or MET ex14skipping NSCLC), first-line treatment for oncogene-addicted disease is represented by targeted therapies characterized by high penetrability of the blood-brain barrier with effective and prolonged CNS disease response [11].
There are currently no established protocols for BMs treatment of NSCLC oncogene-addicted patients and BMs may be treated with local therapy including surgery or stereotactic radiosurgery (SRS). With a few exceptions, whole brain radiation therapy (WBRT) is becoming decreasingly common [12,13]. For a long time, WBRT has long been used to control brain metastases. The greatest restriction of this therapeutic option is neurocognitive toxicity, despite several studies have been conducted to determine the longterm benefits of hippocampal avoidance without conclusive results [14]. Compared to WBRT, SRS reduces the risk of neurotoxicity by delivering a high dose of radiation to the target tumor while minimizing the dose to normal tissue, either in a single fraction or in multiple fractions. [2-5].
Although SRS has less neurological toxicity, it is often associated with radionecrosis [15]. Data currently available in the literature have shown promising results in the control of BMs. The role of radiotherapy is debated especially with regard to timing. The aim of our study is to review the studies in the literature and to report our experience of irradiation in oncogene-addicted patients with symptomatic BMs.
EGFR
At baseline, the incidence of BMs in EGFR-mutated patients is approximately 23-20%. Over time, this percentage tends to increase. [3,16–19]. During the course of the disease, the percentage of BMs in oncogene-addicted NSCLC can reach up to 70% for the bone marrow and 10% for the spinal cord [17,20–22]. The risk of brain progression during TKIs is also higher in those with BMs at baseline and correlates with a worse prognosis.
In addition, there are conflicting data in the literature as to which subtype is associated with a higher risk of the development of BMs, and there is no evidence that this subtype is associated with a higher risk of the development of BMs.; in fact, some studies report worsening data for the DEL19-mutated variant [21], while others for the L858R-mutated tumors [23,24].
Erlotinib and Gefitinib have been shown to be active in EGFRmutant BMs patients in retrospective observational and phase II investigations. [25-30] when compared to first-generation TKIs, Osimertinib’s ability to cross the blood-brain barrier (BBB) and significantly improve icPFS has revolutionized clinical practice. However, at the 2-year mark, nearly half of the patients experience another recurrence [31].
Numerous Osimertinib resistance pathways have been found, including histologic change, secondary EGFR mutations, and MET proto-oncogene amplification. Patients with BMs upon diagnosis still constitute a challenging-to-treat minority in this situation, which could have an impact on quality of life in the event of local development and result in poorer outcomes [32]. (Figure 1)
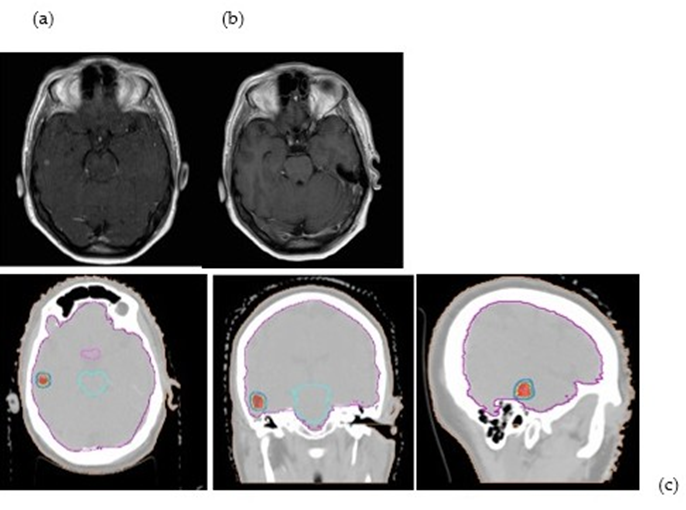
Figure 1: The figure shows an SRS (21 Gy/1fx) of a patient with EGFR t790MUT with a single brain metastasis on pre-RT MRI a) in complete response 45 days post-treatment b) The treatment plan c) shows the dose distribution (95%-130%)
In a recent meta-analysis, Nepote et al. demonstrated a benefit in iPFS and OS in patients with EGFR-mutant NSCLC metastatic to the BM treated with Osimertinib and concurrent brain radiotherapy, showing a significant synergy between the two regimens. Considering this association a first-line treatment option [33].
Anaplastic Lymphoma Kinase
Anaplastic Lymphoma Kinase (ALK) rearrangements result from inversions or translocations on chromosome 2 and are present in 5% of NSCLC tumors. Second- and third-generation TKIs (Lorlatinib,Alectinib, Critinib, Brigatinib, Iruplinalkib) designed to cross the BBB have shown intracranial activity in patients with advanced NSCLC and BMs with ALK rearrangement. This ability to cross the BBB has been found to be superior compared to firstgeneration TKIs [20,34-42]. The ALTA-1L study, demonstrate that in patients with advanced ALK-positive NSCLC and asymptomatic/stable BMs, naive to TKIs, the median intracranial PFS of patients with baseline BMs was significantly longer with Brigatinib compared to Crizotinib (24.0 vs 5.5 months, P<0.0001) (20).
A 97% reduction in intracranial progression was demonstrate in long-term data from phase 3 CROWN study, which analyzed patients with new or progressive BMs receving Lorlatinib or Crizotinib, a first generation TKI [43]. However, neurocognitive damage is higher in patients treated with lorlatinib compared to Crizotinib (35% vs 11%) [44].
Others
About 1-2% of patients with NSCLC had the ROS1 rearrangement. The risk of brain metastases is higher, even if the percentage of BMs upon diagnosis is lower than that of ALK [45,46]. The RET rearrangement is also present in 1-2% of NSCLC patients [47,48]. It is more common in adenocarcinomas and in non-smoking patients.
The incidence of BMs is 27%, regardless of age, smoking, and type of fusion; this incidence is 49% over the lifetime of patients with NSCL RET rearranged [49]. Activating BRAF mutations occur in approximately 2-4% of patients with NSCLC. The most common BRAF mutation is V600E [50] First-line therapy in V600Epositive patients involves the combination of the BRAF inhibitor (Dabrafenib) and the MEK inhibitor (Trametinib) [51,52].
Although data on intracranial disease control are not available, this combination of TKIs has reported efficacy at the central nervous system level in BRAF V600E-positive melanoma, suggesting the same efficacy in NSCLC [53]. Roughly 13% of NSCLC cases have been found to have the KRASG12C mutation, which changes glycine 12 to cysteine.
The KRAS G12C mutation has recently been identified as a targetable oncogenic mutation. It confers sensitivity to covalent inhibitors. [54,55]. Sotorasib is a selective, irreversible targeted drug that the FDA has approved in second-line treatment for KRAS G12C.
The intracranial efficacy of Sotorasib in KRAS G12C mutated NSCLC patients with BMs previously operated on or undergoing radiotherapy was highlighted in the post-hoc analysis of the phase 1/2 CodeBreaK 100 study [56]. The Real-World data collected by the Italian group has also validated this efficacy [57]. NTRK gene fusions are rare (0.1-1% of cases). Oral TRK inhibitors (Larotrectinib and Entrectinib) are FDA-approved for advanced or
metastatic NTRK fusion-positive NSCLC. Entrectinib has shown durable systemic and intracranial activity in 67% of patients in a small number of NTRK fusion-positive NSCLC cases. [58].
The incidence of BMs in patients with MET exon 14 (METex14)– altered at diagnosis is 17% and increases to 36% over a lifetime; Crizotinib can ensure the control of BMs in this setting; however, selective MET inhibitors such as the type Ib inhibitor capmatinib ensure better control of intracranial disease [59]. In (Table 1), we have reported the main studies on the concomitance between RT and molecular-targeted drugs in patients with oncogene-addicted NSCLC that have been published.
|
Study tyoe |
Mutations |
Drugs |
n° patients |
WBRT |
SRS/ SRT |
PFS (months) |
extracPFS (months) |
iPFS |
OS |
|
|
Takeda et al (60) |
RS |
ALK– rearrangement– positive |
Crizotinib |
7 |
4 |
3 |
5.5 (2.6- 17.2) |
4.8 (4.4-7.5) |
- |
- |
|
Zhao et al (61) |
RS |
EGFR- mutations |
1st gen TKIs |
265 |
36 |
58* |
||||
|
Osimertinib |
91 |
22 |
24* |
|||||||
|
Zhou et al (62) |
RS |
EGFR- mutations |
3rd genTKIs |
83 |
36 |
47 |
32.3 |
37.8 |
44.5 |
|
|
Zhai et al (63) |
RS |
EGFR- activating mutations |
Osimertinib |
21 |
14+5 (a) |
2 |
9 |
16.67 |
29.2 |
|
|
Yu et al (64) |
RS |
EGFR- mutations |
Osimertinib |
48 |
12.9 |
27.8 |
||||
|
Xie et al (65) |
RS |
EGFR- mutations |
Osimertinib |
9 |
- |
9 |
NR |
16.2 |
||
|
Tozuka et al (66) |
RS |
EGFR- mutations |
Osimertinib |
42 |
7+1(b) |
32+2 ( c ) |
NR |
- |
.- |
NR |
|
Thomas et al (67) |
RS |
EGFR- mutations |
Osimertinib/ Rocilentinib |
43 |
9 |
34 |
6.9 |
NR |
20.5 |
NR |
|
ALK positive |
Alectinib/ Brigantinib/ Lorlatinib/ Ensartinib |
20 |
4 |
16 |
13.4 |
NR |
21.8 |
NR |
||
|
Gu et al (68) |
PSRS |
EGFR- mutations |
1st-2nd-3rd gen TKIs |
60 |
ns |
ns |
12.8(9.3- 16.3) |
16.3(13.1- 19.6) |
28.9(13.8- 21.2) |
42.7 |
|
Niu et al (69) |
RS |
EGFR- mutations |
3rd gen TKIs |
28 |
14 |
14 |
14(12- 21) |
- |
- |
43 |
RS Retrospective study; PS Prospective study
* upfront stereotactic radiosurgery and/or surgery; gen generation x HA WBRT, SRS Radiosurgery
(a)WBRT+SIB (b) surgery+WBRT (c) Surgery+SRS
NR not reached
Table 1: Main studies on the interaction of TKIs and encephalic RT
Materials and Methods
We retrospectively identified symptomatic patients treated at our institution who received either WBRT or SRS while undergoing TKIs between January 2021 and June 2024. We evaluated intracranial response and analyzed the interval from initial diagnosis to radiotherapy. All patients had to have undergone systemic staging with CT and brain staging with CT or MRI with contrast. The toxicities were evaluated according to the Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) [60]. All patients were taking full-dose targeted molecular drugs and were re-evaluated systemically and cerebrally after radiotherapy
Results
The analysis included 14 consecutive symptomatic patients on TKIs therapy: 10 with EGFR and 2 with KRAS mutations, 1 with ALK and 1 with RET rearrangement. Eight patients with EGFR mutation were taking Tagrisso, one patient Monocertinib, and one Afatinib. Two KRAS-mutated patients were taking Sotorasib, one RET-rearranged patient was taking Selperbatinib, and one ALK-rearranged patient was undergoing ongoing treatment with Lorlatinib. Nine patients received WBRT (30 Gy in 10 fractions), of which one with integrated simultaneous boost (SIB). Five patients received SRS. Radiotherapy was administered at a median time of 16.4 months from TKIs initiation.
No acute toxicity was reported during treatment.
After a median follow-up of 2.1 months, the intracranial objective response rate (ORR) was 64.3%. Specifically, five patients achieved a complete cerebral response (CR), four showed a partial response (PR), and four demonstrated stability of cerebral disease (SD). One patient had extra-lesional progression (PD) at 45.7 months post-treatment. At the time of analysis, five patients were alive with disease, including two with a complete cerebral response, one with partial response, one with stable disease, and one with exclusively intracranial extra-lesional progression (median followup of 21.4 months). Nine patients died due to systemic progression while maintaining intracranial response (median follow-up of 2.8 months after radiotherapy). We have summarized the treatment response characteristics in (Table 2). Radionecrosis has not been recorded.
CR | P | R | S | D | PD | |||||
WBRT | SRS | WBRT | SRS | WBRT | SRS | WBRT | SRS | |||
EGFR | 2(1+1×) | 2*+ | 4 | 2 | ||||||
Ex19, ex21, ex 18 | -14.30% | -14.30% | -28.70% | -14.30% | ||||||
KRAS | 1 | 1# | ||||||||
-7.10% | -7.10% | |||||||||
ALK | (7.1%) | |||||||||
RET | 1 | |||||||||
-7.10% | ||||||||||
PD Progression Disease; CR Complete Response; SD Stable Dis WBRT: 30 Gy in 10 fractions *SRS: 21Gy in single fraction +SRS: 30/25/27 Gy in 5/5/3 fractions (3 metastases) #SRS: 24Gy in 3 fractions-PD in other site ×WBRT+Boost+ Hippocampal avoidance: 30/40 Gy in 10 fracti | ease ons | |||||||||
Table 2: Brain responses in relation to the molecular driver
Discussion
The role of brain metastases radiotherapy in oncogene addicted NSCLC have shown promising results but the data regarding the association of radiotherapy with TKIs are not strong. Conceptually, brain radiotherapy can disrupt the BBB and increase TKI concentration, improving the local control rate [61]. In the last decade, several studies have demonstrated the effectiveness of radiotherapy combined with EGFR-TKI [62,63]. However, the use of brain radiotherapy combined with EGFR-TKI is still controversial, especially in first-line treatment.
EGFR TKIs combined with brain radiation (both SRS and WBRT) may significantly improve overall survival (46 vs 30vs 25 months, P<0.001), according to an international retrospective study [64]. Another study compared the efficacy of EGFR TKIs + WBRT with EGFR TKIs alone and found that WBRT did not improve intracranial local control or long-term survival in patients with EGFR-sensitive mutations [65]. Another study demonstrated the efficacy of first-generation EGFR-TKI combined with brain radiotherapy as a first-line treatment for patients with EGFR mutations and BMs [29,66]. The introduction of Osimertinib has completely changed clinical practice due to its ability to overcome the BBB, with a significant improvement in iPFS compared to first-generation TKIs. In 2024 the meta-analysis regarding the role of upfront brain RT in patients with BMs from NSCLC undergoing third-generation TKIs was published [33], showing an iPFS and OS benefit.
These considerations should also be extended to NSCLC with ALK rearrangement. ALK inhibitors have improved control of systemic and CNS disease and may have a role in the delay of local therapies in some circumstances. Second- and third-generation ALK inhibitors are now considered first-line therapy for ALKrearranged NSCLC. However, surgery and RT remain important for the control of large, extensive and symptomatic intracranial disease. Furthermore, ALK inhibitor resistance development will continue to impact progression-free survival.This study has several shortcomings. First main limitation is the mono-institutional and retrospective nature.
The reported experience has some limitations. Firstly, the small number of patients and the retrospective nature of the analysis. However (Table 1) most of the experiences reported in the studies are retrospective and with the exception of studies [67,68] the other experiences are more limited [69-76]. Secondly, this analysis was conducted on a very heterogeneous population both in terms of the present mutation and the radiotherapy treatment performed.
With the exception of four patients who were treated with SRS (1 - 3 fr), the remaining patients performed whole brain treatment (8 patients) and the remaining two patients performed moderate hypofractionated RT. The number of lesions and the total volume dictated the choice of treatment. In all patients, intracranial response was recorded and PFS was dictated by extracranial disease progression. Thus, albeit with the limitation of the short follow-up, dose and fractionation did not influence the control of intracranial disease.
This finding coupled with the systemic progression recorded in the deceased patients suggests a negative selection of patients: firstly, the patients were symptomatic; secondly, they had already developed resistance to the TKI (which they had been taking for 16.4 months). The choice of proposing RT at the onset of BMs would make it possible to prevent neurological symptoms and reduce treatment volumes by favouring synergic action with TKIs, in the face of our experience suggesting that late RT is proposed at a time of resistance to target therapy.
In line with the meta-analysis by Nepote et al [33], radiotherapy could be proposed as an upfront treatment, in light of its synergistic action with TKIs, in order to avoid WBRT treatments by favouring stereotactic radiotherapy. The lack of standardized follow-up, even in relation to the limited monitoring time for death-related events, could have influenced the response rate and the interactions with TKIs concerning toxicities.
This latter point is crucial for defining the appropriate timing. Future prospective studies examining the combination of TKIs will be essential to demonstrate the potential efficacy and safety of this combination.
Conclusions
This single-institution experience suggests that concurrent encephalic radiotherapy and TKIs therapy is safe and effective in NSCLC patients with CNS metastases. Although neurological symptoms are typically associated with poor survival, in this cohort, survival was more strongly influenced by systemic disease progression than by intracranial factors. The high intracranial response rate (64.3%) in contrast with the short overall survival, suggests the potential benefit from an earlier or upfront radiotherapy approach. Considering the retrospective design, limited sample size, and patient heterogeneity, further studies are needed to investigate these findings and to determine optimal timing for encephalic radiotherapy in brain-metastatic NSCLC patients receiving TKIs.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Cagney DN, Martin AM, Catalano PJ, Redig AJ, Lin NU, et al. (2017) Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: A population-based study. Neuro Oncol. 19: 1511-1521.
- Villano JL, Durbin EB, Normandeau C, Thakkar JP, Moirangthem V, et al. (2015) Incidence of brain metastasis at initial presentation of lung cancer. Neuro Oncol. 17: 122-128.
- Hendriks LEL, Smit EF, Vosse BAH, Mellema WW, Heideman DAM, et al. (2014) EGFR mutated non-small cell lung cancer patients: More prone to development of bone and brain metastases? Lung Cancer. 84: 86-91.
- Riihimäki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, et al. (2014). Metastatic sites and survival in lung cancer. Lung Cancer. 86: 78-84.
- Lin JJ, Cardarella S, Lydon CA, Dahlberg SE, Jackman DM, et al. (2016) Five-year survival in EGFR-mutant metastatic lung adenocarcinoma treated with EGFR-TKIs. J Thorac Oncol. 11: 1556-1565.
- Duruisseaux M, Besse B, Cadranel J, Pérol M, Mennecier B, et al. (2017) Overall survival with crizotinib and next-generation ALK inhibitors in ALK-positive non-small-cell lung cancer (IFCT1302 CLINALK): A French nationwide cohort retrospective study. Oncotarget. 8: 21903-21917.
- Steindl A, Brunner TJ, Heimbach K, Schweighart K, Moser GM, et al. (2022) Changing characteristics, treatment approaches and survival of patients with brain metastasis: data from six thousand and thirty-one individuals over an observation period of 30 years. Eur J Cancer. 162: 170-181.
- Hendriks LE, Kerr KM, Menis J, Mok TS, Nestle U, et al. (2023) Nononcogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 34: 358-376.
- Hendriks LE, Kerr KM, Menis J, Mok TS, Nestle U, et al. (2023). Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 34: 339-357.
- Le Rhun E, Guckenberger M, Smits M, Dummer R, Bachelot T, et al. (2021) EANO–ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann Oncol. 32: 1332-1347.
- Mantovani C, Gastino A, Cerrato M, Badellino S, Ricardi U, et al. (2021) Modern radiation therapy for the management of brain metastases from non-small cell lung cancer: Current approaches and future directions. Front Oncol. 11: 1-24.
- Karschnia P, Le Rhun E, Vogelbaum MA, van den Bent M, Grau SJ, et al. (2021) The evolving role of neurosurgery for central nervous system metastases in the era of personalized cancer therapy. Eur J Cancer. 156: 93-108.
- Vogelbaum MA, Brown PD, Messersmith H, Brastianos PK, Burri S, et al. (2022) Treatment for brain metastases: ASCO-SNO-ASTRO Guideline. J Clin Oncol. 40: 492-516.
- Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, et al. (2016) Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA. 316: 401-409.
- Minniti G, Clarke E, Lanzetta G, Osti MF, Trasimeni G, et al. (2011). Stereotactic radiosurgery for brain metastases: Analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 6: 1-9.
- Iuchi T, Shingyoji M, Itakura M, Yokoi S, Moriya Y, et al. (2015) Frequency of brain metastases in non-small-cell lung cancer, and their association with epidermal growth factor receptor mutations. Int J Clin Oncol. 20: 674-679.
- Rangachari D, Yamaguchi N, VanderLaan PA, Folch E, Mahadevan A, et al. (2015). Brain metastases in patients with EGFR-mutated or ALKrearranged non-small-cell lung cancers. Lung Cancer. 88: 108-111.
- Omuro AMP, Kris MG, Miller VA, Franceschi E, Shah N, et al. (2005) High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer. 103: 2344-2348.
- Lee YJ, Choi HJ, Kim SK, Chang J, Moon JW, et al. (2010) Frequent central nervous system failure after clinical benefit with epidermal growth factor receptor tyrosine kinase inhibitors in Korean patients with nonsmall-cell lung cancer. Cancer. 116: 1336-1343.
- Camidge DR, Kim HR, Ahn MJ, Yang JCH, Han JY, et al. (2020). Brigatinib versus crizotinib in advanced ALK inhibitor–naive ALKpositive non–small cell lung cancer: Second interim analysis of the phase III ALTA-1L trial. J Clin Oncol. 38: 3592-3603.
- Heon S, Yeap BY, Britt GJ, Costa DB, Rabin MS, et al. (2010) Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res. 16: 5873-5882.
- Ramsay HM, Fryer AA, Hawley CM, Smith AG, Nicol DL, et al. (2002) Non-melanoma skin cancer risk in the Queensland renal transplant population. Br J Dermatol. 146: 950-956.
- Ma X, Zhu H, Guo H, Han A, Wang H, et al. (2016) Risk factors of brain metastasis during the course of EGFR-TKIs therapy for patients with EGFR-mutated advanced lung adenocarcinoma. Oncotarget. 7: 81906-81917.
- Patel SH, Rimner A, Foster A, Zhang Z, Woo KM, et al. (2017) Patterns of initial and intracranial failure in metastatic EGFR-mutant non-small cell lung cancer treated with erlotinib. Lung Cancer. 108: 109-114.
- Porta R, Sánchez-Torres JM, Paz-Ares L, Massutí B, Reguart N, et al. (2011) Brain metastases from lung cancer responding to erlotinib: The importance of EGFR mutation. Eur Respir J. 37: 624-631.
- Wu YL, Zhou C, Cheng Y, Lu S, Chen GY, et al. (2013) Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: A phase II study. Ann Oncol. 24: 993-999.
- Park SJ, Kim HT, Lee DH, Kim KP, Kim SW, et al. (2012) Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer. 77: 556-560.
- A TI, B MS, A TS, C KH, E ON, et al. (2013) Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer. 82: 282-287.
- Zhang Q, Zhang X, Yan H, Jiang B, Xu C, et al. (2016) Effects of epidermal growth factor receptor-tyrosine kinase inhibitors alone on EGFR-mutant non-small cell lung cancer with brain metastasis. Thorac Cancer. 7: 648-654.
- Hotta K, Kiura K, Ueoka H, Tabata M, Fujiwara K, et al. (2004) Effect of gefitinib (“Iressa”, ZD1839) on brain metastases in patients with advanced non-small-cell lung cancer. Lung Cancer. 46: 255-261.
- Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, et al. (2020) Overall survival with osimertinib in untreated, EGFRmutated advanced NSCLC. N Engl J Med. 382: 41-50.
- Roper N, Brown AL, Wei JS, Pack S, Trindade C, et al. (2020) Clonal evolution and heterogeneity of osimertinib acquired resistance mechanisms in EGFR mutant lung cancer. Cell Reports Med. 1: 100007.
- Nepote A, Poletto S, Bertaglia V, Carnio S, Piumatti C, et al. (2025) Role of osimertinib plus brain radiotherapy versus osimertinib single therapy in EGFR-mutated non-small-cell lung cancer with brain metastases: A meta-analysis and systematic review. Crit Rev Oncol Hematol. 205: 104540.
- Camidge DR, Kim DW, Tiseo M, Langer CJ, Ahn MJ, et al. (2018) Exploratory analysis of brigatinib activity in patients with anaplastic lymphoma kinase-positive non–small-cell lung cancer and brain metastases in two clinical trials. J Clin Oncol. 36: 2693-2701.
- Crinò L, Ahn MJ, De Marinis F, Groen HJM, Wakelee H, et al. (2016) Multicenter phase II study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non-small-cell lung cancer previously treated with chemotherapy and crizotinib: Results from ASCEND-2. J Clin Oncol. 34: 2866-2873.
- Gadgeel SM, Gandhi L, Riely GJ, Chiappori AA, West HL, et al. (2014) Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): Results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 15: 1119-1128.
- Novello S, Mazières J, Oh IJ, de Castro J, Migliorino MR, et al. (2018) Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: Results from the phase III ALUR study. Ann Oncol. 29: 1409-1416.
- Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, et al. (2017) Alectinib versus crizotinib in untreated ALK-positive non–small-cell lung cancer. N Engl J Med. 377: 829-838.
- Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, et al. (2020) Firstline lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 383: 2018-2029.
- Shi Y, Chen J, Yang R, Wu H, Wang Z, et al. (2024) Iruplinalkib (WX0593) versus crizotinib in ALK TKI-naive locally advanced or metastatic ALK-positive NSCLC: Interim analysis of a randomized, open-label, phase 3 study (INSPIRE). J Thorac Oncol. 19: 912-927.
- A PBJSMP, B TMBM, C PTSKMM, D PGLM, E PJMM, et al. (2023) Efficacy and safety of first-line lorlatinib versus crizotinib in patients with advanced, ALK-positive non-small-cell lung cancer: Updated analysis of data from the phase 3, randomised, open-label CROWN study. Lancet Respir Med. 11: 354-366.
- Solomon BJ, Besse B, Bauer TM, Felip E, Soo RA, et al. (2018) Lorlatinib in patients with ALK-positive non-small-cell lung cancer: Results from a global phase 2 study. Lancet Oncol. 19: 1654-1667.
- Solomon BJ, Liu G, Felip E, Mok TSK, Soo RA, et al. (2024) Lorlatinib versus crizotinib in patients with advanced ALK-positive non–small cell lung cancer: 5-year outcomes from the phase III CROWN study. J Clin Oncol. 10: 3400-3409.
- Solomon BJ, Bauer TM, Ou SHI, Liu G, Hayashi H, et al. (2022) Post hoc analysis of lorlatinib intracranial efficacy and safety in patients with ALK-positive advanced non-small-cell lung cancer from the phase III CROWN study. J Clin Oncol. 40: 3593-3602.
- Gainor JF, Tseng D, Yoda S, Dagogo-Jack I, Friboulet L, et al. (2017) Patterns of metastatic spread and mechanisms of resistance to crizotinib in ROS1-positive non–small-cell lung cancer. JCO Precis Oncol. 1001: 1-13.
- Preusser M, Streubel B, Birner P. (2014) ROS1 translocations and amplifications in lung cancer brain metastases. J Neurooncol. 118: 425-426.
- Gautschi O, Milia J, Filleron T, Wolf J, Carbone DP, et al. (2017) Targeting RET in patients with RET-rearranged lung cancers: Results from the global, multicenter RET registry. J Clin Oncol. 35: 1403-1410.
- Ferrara R, Auger N, Auclin E, Besse B. (2018) Clinical and translational implications of RET rearrangements in non–small cell lung cancer. J Thorac Oncol. 13: 27-45.
- Ramalingam S, Skoulidis F, Govindan R, Velcheti V, Li B, et al. (2021) Efficacy of sotorasib in KRAS p.G12C-mutated NSCLC with stable brain metastases: A post-hoc analysis of CodeBreaK 100. J Thorac Oncol. 16: S1123.
- Villaruz LC, Socinski MA, Abberbock S, Berry LD, Johnson BE, et al. (2016) Clinicopathologic features and outcomes of patients with lung adenocarcinomas harboring BRAF mutations in the Lung Cancer Mutation Consortium. Cancer. 121: 448-456.
- Subbiah V, Lassen U, Élez E, Italiano A, Curigliano G, et al. (2020) Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 21: 1234-1243.
- Planchard D, Besse B, Kim TM, Quoix EA, Souquet PJ, et al. (2017) Updated survival of patients with previously treated BRAF V600E– mutant advanced non-small cell lung cancer who received dabrafenib or dabrafenib plus trametinib in the phase II BRF113928 study. J Clin Oncol. 35
- Yamamoto G, Sakakibara-Konishi J, Ikari T, Kitai H, Mizugaki H, et al. (2019) Response of BRAF V600E-mutant lung adenocarcinoma with brain metastasis and leptomeningeal dissemination to dabrafenib plus trametinib treatment. J Thorac Oncol. 14: e97-e99.
- Ogawa M, Kosaka N, Choyke PL, Kobayashi H. (2008) Frequency and distinctive spectrum of KRAS mutations in never smokers with lung adenocarcinoma. Cancer Res. 14: 5731-5734.
- Biernacka A, Tsongalis PD, Peterson JD, de Abreu FB, Black CC, et al. (2016) The potential utility of re-mining results of somatic mutation testing: KRAS status in lung adenocarcinoma. Cancer Genet. 209: 195-198.
- Ramalingam S, Skoulidis F, Govindan R, Velcheti V, Li B, et al. (2021) Efficacy of sotorasib in KRAS p.G12C-mutated NSCLC with stable brain metastases: A post-hoc analysis of CodeBreaK 100. J Thorac Oncol. 16: S1123.
- Passiglia F, Reale ML, Lo Russo G, Pasello G, Minuti G, et al. (2024) Sotorasib in KRAS p.G12C mutated advanced NSCLC: Real-world data from the Italian expanded access program. Lung Cancer. 187
- Demetri RD, Lassen U, Elez E, Italiano A, Curigliano G, et al. (2019) Entrectinib in NTRK-fusion positive non-small cell lung cancer: Integrated analysis of patients enrolled in three trials (STARTRK-2, STARTRK-1 and ALKA-372-001). Cancer Res. 79
- Luo J, Guo R, Lyo JK, Falcon C, Dienstag J, et al. (2020) CNS metastases in patients with MET exon 14–altered lung cancers and outcomes with crizotinib. Cancer Res. 871-876.
- Cox JD, Stetz JA, Pajak TF. (1995) Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 31: 1341-1346.
- Liu Y, Wang J, Wu J, Yang Q, Zeng Y, et al. (2021) The efficacy of firstgeneration EGFR-TKI combined with brain radiotherapy as the firstline treatment for lung adenocarcinoma patients with brain metastases and EGFR sensitive mutations: A retrospective study. Technol Cancer Res Treat. 20: 1-8.
- Xu Q, Zhou F, Liu H, Jiang T, Li X, et al. (2018) Consolidative local ablative therapy improves the survival of patients with synchronous oligometastatic NSCLC harboring EGFR activating mutation treated with first-line EGFR-TKIs. J Thorac Oncol. 13: 1383-1392.
- Weickhardt AJ, Scheier B, Gan G, Lu X, Bunn PA, et al. (2012) Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene addicted non-small cell lung cancer. J Thorac Oncol. 7: 1807-1814.
- Magnuson WJ, Lester-Coll NH, Wu AJ, Yang TJ, Lockney NA, et al. (2017) Management of brain metastases in tyrosine kinase inhibitor– naïve EGFR-mutant non–small-cell lung cancer: A retrospective multiinstitutional analysis. J Clin Oncol. 35: 1070-1077.
- Jiang T, Su C, Li X, Zhao C, Zhou F, et al. (2016) EGFR TKIs plus WBRT demonstrated no survival benefit other than that of TKIs alone in patients with NSCLC and EGFR mutation and brain metastases. J Thorac Oncol. 11: 1718-1728.
- Khalifa J, Amini A, Popat S, Gaspar LE, Faivre-Finn C. (2016) Brain metastases from NSCLC: Radiation therapy in the era of targeted therapies. J Thorac Oncol. 11: 1627-1643.
- Zhao Y, Li S, Yang X, Chu L, Wang S, et al. (2022) Overall survival benefit of osimertinib and clinical value of upfront cranial local therapy in untreated EGFR-mutant nonsmall cell lung cancer with brain metastasis. Int J Cancer. 150: 1318-1328.
- Zhou J, Zhou Y, Sun Y, Xiao L, Lu H, et al. (2023) The efficacy of upfront craniocerebral radiotherapy and epidermal growth factor receptortyrosine kinase inhibitors in patients with epidermal growth factor receptor-positive non-small cell lung cancer with brain metastases. Front Oncol. 13: 1-11.
- Takeda M, Okamoto I, Nakagawa K. (2013) Clinical impact of continued crizotinib administration after isolated central nervous system progression in patients with lung cancer positive for ALK rearrangement. J Thorac Oncol. 8: 654-657.
- Zhai X, Li W, Li J, Jia W, Jing W, et al. (2021) Therapeutic effect of osimertinib plus cranial radiotherapy compared to osimertinib alone in NSCLC patients with EGFR-activating mutations and brain metastases: A retrospective study. Radiat Oncol. 16: 1-13.
- Yu F, Ni J, Zeng W, Zhou Y, Guo T, et al. (2021) Clinical value of upfront cranial radiation therapy in osimertinib-treated epidermal growth factor receptor–mutant non-small cell lung cancer with brain metastases. Int J Radiat Oncol Biol Phys. 111: 804-815.
- Xie L, Nagpal S, Wakelee HA, Li G, Soltys SG, et al. (2019) Osimertinib for EGFR-mutant lung cancer with brain metastases: Results from a single-center retrospective study. Oncologist. 24: 836-843.
- Tozuka T, Noro R, Mizutani H, Kurimoto F, Hakozaki T, et al. (2024) Osimertinib plus local treatment for brain metastases versus osimertinib alone in patients with EGFR-mutant non-small cell lung cancer. Lung Cancer. 191
- Thomas NJ, Myall NJ, Sun F, Patil T, Mushtaq R, et al. (2022) Brain metastases in EGFR- and ALK-positive NSCLC: Outcomes of central nervous system-penetrant tyrosine kinase inhibitors alone versus in combination with radiation. J Thorac Oncol. 17: 116-129.
- Gu Y, Xu Y, Zhuang H, Jiang W, Zhang H, et al. (2021) Value and significance of brain radiation therapy during first-line EGFR-TKI treatment in lung adenocarcinoma with EGFR sensitive mutation and synchronous brain metastasis: Appropriate timing and technique. Thorac Cancer. 12: 3157-3168.
- Niu L, Wu H, Gao R, Chen L, Wang J, et al. (2024) Optimal sequence of LT for symptomatic BM in EGFR-mutant NSCLC: A comparative study of first-line EGFR-TKIs with/without upfront LT. J Cancer Res Clin Oncol. 150: 1-13.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.