Efficacy of Hybrid Cooperative Complexes of Hyaluronic Acid for Treating Hand Skin Laxity and Thickness: An Ultrasound-Based Evaluation
by Agnaldo G de Castro Filho1*, Juliana S Palo1, Gladstone EL Faria1, Luciana Zattar2, Clara Cigni3, Franco Grimolizzi3, Ricardo F Boggio1
1Cosmiatry Department, Instituto Boggio, São Paulo, SP, Brazil
2Diagnostic Imaging Center, RADIODERM-SP and Sírio-Libanês Hospital, São Paulo, SP, Brazil
3IBSA Farmaceutici Italia Srl, Lodi, Italy
*Corresponding author: Agnaldo G de Castro Filho, Cosmiatry Department, Instituto Boggio, São Paulo, SP, Brazil
Received Date: 09 June 2025
Accepted Date: 13 June 2025
Published Date: 16 June 2025
Citation: de Castro Filho AG, Palo JS, EL Faria G, Zattar L, Cigni C, et al. (2025). Efficacy of Hybrid Cooperative Complexes of Hyaluronic Acid for Treating Hand Skin Laxity and Thickness: An Ultrasound-Based Evaluation. 10: 2320. https://doi.org/10.29011/25747754.102320
Abstract
Background: There is a recent growing interest in hand rejuvenation treatments. Objective: This study aimed to assess the efficacy and safety of hybrid cooperative complexes (HCC) of hyaluronic acid (HA) for addressing hand skin laxity and roughness. Materials and methods: This single-centre case series enrolled women aged 35 to 55 years with hand skin laxity and roughness (N=10). Treatment involved administering 1.5 mL of HCC of HA (3.2% concentration) using a 22G x 50mm microcannula at baseline (T0) and four-weeks (T1). Outcomes were evaluated at T1 and 4-months (T2) through hand grading, the Global Aesthetic Improvement Scale (GAIS), and skin thickness measured via ultrasound. Results: Comparing baseline with T1 and T2, the mean hand grade score (±standard deviation) was found to decrease (i.e. improve) when rated by a plastic surgeon (2.1±1.0 vs 2.0±1.0 vs 1.3±0.5, respectively [p<0.05]) or dermatologist (2.8±0.6 vs 2.2±0.6 vs 2.1±0.4 [p<0.001]). Skin thickness (epidermis plus dermis) and total thickness (skin plus subcutaneous layer) increased at T1 and T2. GAIS scores were lower at T2 vs T1, indicating a trend for aesthetic improvement over time. Conclusion: This case series suggests that HCC of HA effectively improve skin laxity and roughness, highlighting their potential as a treatment option.
Keywords: Hyaluronic acid; Hybrid cooperative complexes; Hands; Skin laxity; Ultra-sound evaluation
Introduction
In recent years, as the demand for facial rejuvenation treatments has increased, there has also been growing interest in hand rejuvenation. Hands, like the face, are prone to visible signs of aging, including skin laxity and roughness, and can betray one’s age despite facial interventions. [1-3] the anatomy of the hand is characterized by a complex structure composed of layers including the epidermis, dermis, and deeper fascial planes that separate various fatty laminae (Figure 1). The hand’s dorsal area, in particular, has a relatively thin dermis, which typically ranges in thickness from 0.2 to 0.9 mm. [2] As the hands age, the skin becomes more attenuated, may lose elasticity, and can become rougher and more translucent, with features such as veins, joints, tendons, and bones becoming more prominent with a decrease in subcutaneous fat. [4-6] Skin aging is influenced by intrinsic factors, such as genetics, as well as extrinsic factors like sun exposure, environmental pollutants, smoking, and major weight fluctuations. [1,7,8] The dermis itself undergoes structural changes, with a decline in the skin’s levels of hyaluronic acid (HA) and collagen, leading to laxity, wrinkling, and a more fragile appearance, which further accelerates the aging process. [9] Hyaluronic acid-based injectable treatments have become increasingly popular in aesthetic medicine for addressing wrinkles, skin sagging, and roughness in various body areas, including the face, inner arms, abdomen, knees and hands. [3,1014] Hyaluronic acid treatments work by restoring lost volume, improving skin elasticity and hydration, and stimulating collagen production to counteract the effects of aging [13].
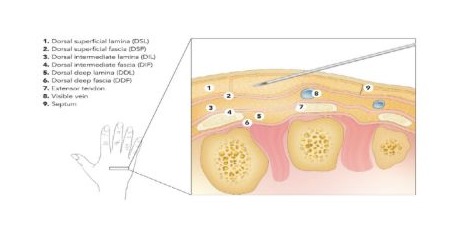
Figure 1: Hand anatomy.
Recent studies have demonstrated the efficacy and safety of HA in improving the appearance of the hands, providing a minimally invasive option for patients seeking hand rejuvenation. A study by Sparavigna et al. [3] used hybrid cooperative complexes (HCC) of high- and low-molecular-weight HA to counteract skin roughness and laxity on the back of the hands. Statistically significant improvements were observed for skin laxity, roughness, wrinkle depth and plastoelasticity; the main treatment side effect was transient light bruising at the injection site, with no serious AEs reported.
HA products help combat aging by restoring lost volume, enhancing skin elasticity and hydration, and promoting collagen production. Additionally, HA is biocompatible, non-immunogenic, and can be degraded by the enzyme hyaluronidase. [13, 15-17] HA has a beneficial effect on a range of physiological processes and pathways. In laboratory settings, wound healing and bioremodelling effects were observed, including increased expression of collagen (type I, III, IV and VII) and elastin, and reduced expression of inflammatory biomarkers. This bioremodelling action is able to induce tissue restoration through a physiological improvement of extracellular matrix homeostasis and cellular viability [17].
Several studies have demonstrated the potential of various synthetic biomaterials, such as poly-L-lactic acid (PLLA), calcium hydroxylapatite (CaHa), polycaprolactone (PCL) and carboxymethylcellulose (CMC), to stimulate collagen and elastin production when injected into the dermis layer of the skin. However, these products primarily function through a biostimulatory mechanism. Biostimulation is the process that leads to tissue augmentation by fibroblast activation and neocollagenesis induction (predominantly type I collagen production) through a subclinical inflammatory response. [17, 18] The biostimulation process initiated by synthetic biomaterials like PLLA typically involves an immune-mediated response, which can subsequently lead to the activation of fibrotic pathways and inflammatory cytokine production. While some research suggests that CaHabased fillers can promote tissue regeneration without inducing inflammation, the scientific consensus recognizes that these products generally trigger collagen production via an immunemediated pathway [17-24].
Given the benefits of HA for treatment of skin aging and the growing interest hand rejuvenation, this study sought to evaluate the efficacy and safety of HCC of HA for the treatment of skin laxity and skin roughness of the back of the hands.
Material and Methods
Study design
This case series was conducted at a single centre (Cosmiatry Department, Instituto Boggio, São Paulo, Brazil) enrolling 10 patients with skin roughness and laxity on the back of the hands. Eligible patients were women aged between 35 and 55 years, without interventions on their hands in the last 6 months (surgical or non-surgical). Patients were excluded if they had previous local surgery, injectable- or energy-based procedures for hand rejuvenation in the last 6 months, known allergy to the product, active autoimmune diseases, current pregnancy or lactation, or active skin diseases on their hands.
Approval for the treatment of skin laxity and roughness on the back of the hands with HCC of HA was previously obtained from a local ethics committee. The study was performed in accordance with the Consolidated Standards of Reporting Trials and the Declaration of Helsinki. All patients signed an informed consent after receiving detailed explanations of the procedure and possible side effects and complications.
Procedures
Eligible patients were treated with HCC of HA for skin roughness and laxity on the back of the hands. The HCC of HA was provided in prefilled syringes of containing 3.2% HA for intradermal use.
Treatment was performed at baseline (T0) and at the second visit (T1), 4 weeks later (Figure 2). 1.5 mL was administered into each hand using a blunt tip 22G x 50mm microcannula. Using a single-entry point, a fanning technique with 5 passages was implemented, injecting 0.3 mL per passage.
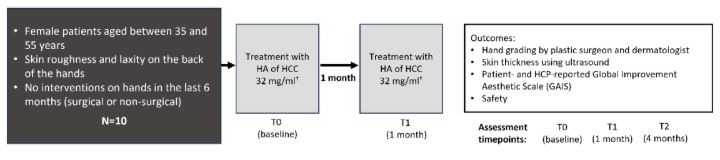
Notes: † The HCC of HA was provided in prefilled syringes of 3 mL containing 3.2% HA for intradermal use.
Abbreviations: HA: Hyaluronic acid; HCC: Hybrid Cooperative Complexes; N: Number of patients; T: Timepoint.
Outcomes, Data Collection and Follow Up
Patients received treatment at baseline (T0) and at 1 month (T1), with a follow up assessment conducted at 4 months (T2); patients were assessed at each visit (T0, T1 and T2). Assessments conducted at each timepoint included hand grading by a plastic surgeon or dermatologist (using a validated hand grading scale, [25] (see Table 1), patient- and HCP-reported improvement (Global Improvement Aesthetic Scale [GAIS]), skin thickness for both hands (assessed using ultrasound) and safety. Skin thickness (epidermis plus dermis) and total skin thickness (skin plus subcutaneous layer) was assed using ultrasound [L4-20t-RS 5 to 20 MHz linear array transducer with 11.6 x 48.4mm footprint, 38.4mm field of view and 256 elements. GE Medical Systems Ultrasound and Primary Care Diagnostics LLC, 9900 W Innovation Drive WAUWATOSA WI 53226, United States of America]. The Global Improvement Aesthetic Scale is a five-point scale that measures the perceived aesthetic improvement of a subject’s appearance (rated on a 5-point scale: 1, very much improved; 2, much improved; 3, improved; 4, no change; 5, worse), was assessed at T1 and T2. For the hand grading and skin thickness analyses, measurements for the left and right hand were considered together (N=20).
|
Grade 0 |
No loss of fatty tissue |
|
Grade 1 |
Mild loss of fatty tissue; slight visibility of veins |
|
Grade 2 |
Moderate loss of fatty tissue; mild visibility of veins and tendons |
|
Grade 3 |
Severe loss of fatty tissue; moderate visibility of veins and tendons |
|
Grade 4 |
Very severe loss of fatty tissue; marked visibility of veins and tendons |
Table 1: Validated hand grading scale.
Notes: Validation of the hand grading scale was published by Carruthers et al. [25].
Statistical Analysis
Data were reported as mean and standard deviation (SD). Where applicable, statistically significant differences between assessment periods were assessed using the Kruskal-Wallis test (used for non-parametric data) or an ordinary one-way analysis of variance (ANOVA; used for normally distributed data).
Results
Hand grading
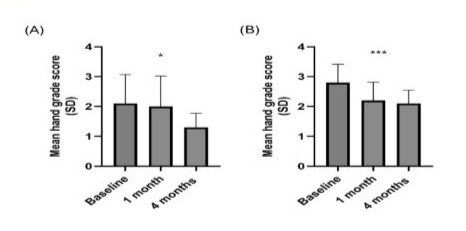
Patient hands were graded by a plastic surgeon and a dermatologist according to a previously published hand grading scale. [25] Comparing baseline with the 1-month and 4-month assessment periods, mean hand grade score (±SD) was found to decrease (i.e. improve) when rated by a plastic surgeon (2.1±1.0 vs 2.0±1.0 vs 1.3±0.5, respectively [Kruskal-Wallis test p<0.05]) or dermatologist (2.8±0.6 vs 2.2±0.6 vs 2.1±0.4 [p<0.001]) (Figure 3).
Global Improvement Aesthetic Scale and Treatment tolerability
Patient- and HCP-reported GAIS results are presented in Table 2. Overall, GAIS scores were lower at T2 vs T1, indicating a trend for aesthetic improvement over time. Scores were similar for both hands, with patient-reported scores slightly higher compared with HCP-reported scores (Table 2).
Tolerability to the treatment was judged as good by the patients and no serious AEs were recorded during this case series.
|
Assessment time |
Mean GAIS score (SD) |
|||
|
Patient-reported score |
HCP-reported score |
|||
|
Left hand |
Right hand |
Left hand |
Right hand |
|
|
T1 (1 month) |
2.6 (1.1) |
2.5 (0.8) |
2.1 (0.7) |
2.2 (0.6) |
|
T2 (4 months) |
2.1 (0.7) |
2.1 (0.7) |
1.5 (0.7) |
1.6 (0.7) |
Table 2: Patient- and HCP-reported GAIS
Notes: Rated on a 5-point scale: 1, very much improved; 2, much improved; 3, improved; 4, no change; 5, worse. N=10 for left hand and N=10 for right hand
Abbreviations: GAIS: Global Improvement Aesthetic Scale; HCP: Healthcare practitioner; SD: Standard deviation.
Skin thickness and total skin thickness
Skin thickness was assessed using ultrasound. Comparing baseline with T1 and T2, skin thickness (epidermis plus dermis) (±SD) was found to increase (7.6 mm [±1.1] vs 7.9 mm [±1.5] vs 9.4 mm [±1.6], respectively; one-way ANOVA test p<0.001) (Figure 4A). Similarly, total thickness (skin plus subcutaneous layer) was also found to increase (31.9 mm [±5.3] vs 33.10 mm [±6.2] vs 34.7 mm [±5.5]) (Figure 4B). Visuals examples of hand improvements over time are presented in Figure 4C.

Figure 4: Ultrasound assessment of (A) skin thickness (epidermis plus dermis), (B) total thickness (skin plus subcutaneous layer), (C) Images of treated hands across assessment periods.
Notes: Ordinary one-way ANOVA test: ***, p<0.001. Figure A: N=20 (n=10 for left hand and n=10 for right hand).
Abbreviations: SD: Standard deviation.
Discussion
This prospective case series demonstrated that in females with skin laxity and roughness on the back of the hands, treatment with HCCs of HA led to significant improvements in hand grade score, hand aesthetics, and skin thickness. Hand grading was determined using a previously validated scale, [25] and treatment was found to reduce (i.e. improve) the mean hand grade score over the course of the study, with mean hand grade scores of approximately 1 (representing mild loss of fatty tissue, slight visibility of veins) or 2 (representing moderate loss of fatty tissue, mild visibility of veins and tendons), indicating an improvement in features when compared with baseline. It was notable that there were differences in hand grading scores by rater, with scores reported by the dermatologist rater greater compared with the plastic surgeon rater. Although not further explored in this study, variable factors such as individual clinical experience and training may contribute to the observed difference. Evidence of a trend for aesthetic improvement was indicated by the patient- and HCP-reported GAIS scores, with patient-reported scores marginally higher compared with HCPreported scores.
Importantly, skin thickness was found to increase with HA treatment, for both skin thickness (epidermis plus dermis) and total skin thickness (skin plus subcutaneous layer). Overall, results across all measured outcomes were similar for both hands treated.
Statistically significant results between assessments periods were observed for some outcomes, specifically for dermatologist rated hand grading and skin thickness (epidermis plus dermis, left hand only). These positive results are in keeping with previous studies investigating treatment of skin laxity and roughness of the hands with HCC of HA, which showed statistically significant improvements in skin laxity, roughness, wrinkle depth and plastoelasticity. [3] However, it must be noted that this study had a low number of participants, which may have contributed to statistical differences not being observed across all groups for these outcomes, including the total skin thickness outcome. Consequently, a larger study with more participants is required to confirm these preliminary positive results.
This study had planned to assess the impact of treatment on photodamage and echogenicity, however this was not possible due to limited patient numbers and severity of photodamage. To appropriately assess the impact of HA on photodamage, correct selection of patients (i.e. those mild to moderate photodamage) is required to optimally determine treatment effect. This is of interest for future studies as HA treatment has been shown to improve hyperpigmentation and melisma [26].
The efficacy and safety of HCC of HA has been established in several studies across different body areas, including treatment of the face, inner arms, abdomen, knees and hands. [3,10-14] For treatment of skin laxity and roughness of the hands, HCC of HA was associated with a good or excellent tolerability profile; nearly all patients (88%) reported light bruising at the injection site which completely faded within 5-7 days, with no serious AEs reported. The excellent tolerability profile has also been confirmed by this case series, as reported by the patients. It is important to note that this study used a specific injection procedure to minimise the risk of safety events. Specifically, a fanning injection technique with a single-entry point was used to reduce the risk of bruising, while a blunt cannula was used to reduce the risk of blood vessel puncture.
In contrast to HA, other dermal fillers have more burdensome safety profiles. CaHa is associated with oedema and ecchymosis that persist approximately 1 to 3 weeks after injection, in addition to nodules (some of which may require surgical correction). One of the most common side effects associated with PLLA is the occurrence of subcutaneous papules and nodules, which can persist for years in rare cases. Furthermore, delayed onset of granulomas are associated with CaHa and PLLA treatment [27].
The biostimulatory effects of specific dermal fillers may influence their safety profiles. HA is associated with a bioremodelling action, inducing tissue restoration through a physiological improvement of extracellular matrix homeostasis and cellular viability. [17] In contrast, dermal fillers containing synthetic biomaterials rely on biostimulation, a process that leads to tissue augmentation by fibroblast activation and neocollagenesis induction through a subclinical inflammatory response. [17,18] While biostimulation leads to collagen production, it is an immune-mediated response which can subsequently activate fibrotic pathways and inflammatory cytokine production.17-24 These aspects are crucial to be considered by physicians when selecting a treatment to ensure the best and most respectful approach for their patients [28].
While the results of this prospective study are encouraging, it is important to note several limitations, such as the small sample size, absence of a comparative treatment, and the lack of longterm outcome data.
Conclusion
This prospective case series demonstrated that treatment with Hybrid Cooperative Complexes of Hyaluronic Acid improved skin laxity and roughness on the back of the hands, as evidenced by enhancements in hand grade score, hand aesthetics, and skin thickness. Further studies with a larger patient cohort are needed to confirm and expand upon these treatment benefits.
Acknowledgments
Authors are grateful to Éanna Connaughton, Aran Scientific Communications for medical writing support with developing the first draft of the manuscript.
Disclosure
A.G. F., J.S.P., G.EL.F., R.F.B. declare no conflict of interest. C.C. and F.G. are employees of IBSA Farmaceutici Italia Srl.
Funding
Medical writing and statistical analysis have been supported by IBSA Farmaceutici Italia Srl.
References
- McGuire C, Boudreau C, Tang D. (2022). Hand Rejuvenation: A Systematic Review of Techniques, Outcomes, and Complications. Aesthetic Plast Surg. 46: 437-449.
- Har-Shai L, Ofek SE, Lagziel T. (2023). Revitalizing Hands: A Comprehensive Review of Anatomy and Treatment Options for Hand Rejuvenation. Cureus. 15: e35573.
- Sparavigna A, Grimolizzi F, Cigni CRL, Bellia G. (2023). Use of intradermic injection of hybrid cooperative complexes of hyaluronic acid to counteract Skin Roughness and Laxity on the Back of the Hands. Journal of Plastic and Pathology Dermatology. 19: 53-59.
- Bains RD, Thorpe H, Southern S. (2006). Hand aging: patients’ opinions. Plast Reconstr Surg. 117: 2212-2218.
- Fabi SG, Goldman MP. (2012). Hand rejuvenation: a review and our experience. Dermatol Surg. 38: 1112-27.
- Guinot C, Malvy DJ, Ambroisine L. (2002). Relative contribution of intrinsic vs extrinsic factors to skin aging as determined by a validated skin age score. Arch Dermatol. 138: 1454-60.
- Ganceviciene R, Liakou AI, Theodoridis A, Makrantonaki E, Zouboulis CC. (2012). Skin anti-aging strategies. Dermatoendocrinol. 4: 308-19.
- Farage MA, Miller KW, Elsner P, Maibach HI. (2008). Intrinsic and extrinsic factors in skin ageing: a review. Int J Cosmet Sci. 30: 87-95.
- Papakonstantinou E, Roth M, Karakiulakis G. (2012). Hyaluronic acid: A key molecule in skin aging. Dermatoendocrinol. 4: 253-8.
- Fagien S, Monheit G, Jones D. (2018). Hyaluronic Acid Gel With (HARRL) and Without Lidocaine (HAJU) for the Treatment of Moderateto-Severe Nasolabial Folds: A Randomized, Evaluator-Blinded, Phase III Study. Dermatol Surg. 44: 549-556.
- Jones D, Murphy DK. (2013). Volumizing hyaluronic acid filler for midface volume deficit: 2-year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 39: 1602-12.
- Hoffmann K. (2009). Volumizing effects of a smooth, highly cohesive, viscous 20-mg/mL hyaluronic acid volumizing filler: prospective European study. BMC Dermatol. 9: 9.
- Bukhari SNA, Roswandi NL, Waqas M. (2018). Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int J Biol Macromol. 120: 1682-1695.
- Sparavigna A, Grimolizzi F, Cigni C, Lualdi R, Bellia G. (2023). Profhilo Body ® for tackling skin roughness and laxity of inner arm, abdomen and knees. Journal of Plastic and Pathology Dermatology. 19: 31-38.
- Cavallini M, Gazzola R, Metalla M, Vaienti L. (2013). The role of hyaluronidase in the treatment of complications from hyaluronic acid dermal fillers. Aesthet Surg J. 33: 1167-74.
- Kontis TC. (2013). Contemporary review of injectable facial fillers. JAMA Facial Plast Surg. 15: 58-64.
- Humzah D, Molina B, Salti G, Cigni C, Bellia G. (2024). Intradermal Injection of Hybrid Complexes of High- and Low-Molecular-Weight Hyaluronan: Where Do We Stand and Where Are We Headed in Regenerative Medicine? Int J Mol Sci. 25.
- da Cunha MG, da Cunha AG, Garcia M, da Silva Pinhal M. (2022). Biostimulators and their mechanisms of action. Arch Clin & Experim Dermatol. 4: 130.
- Nowag B, Schäfer D, Hengl T, Corduff N, Goldie K. (2024). Biostimulating fillers and induction of inflammatory pathways: A preclinical investigation of macrophage response to calcium hydroxylapatite and poly-L lactic acid. J Cosmet Dermatol. 23: 99-106.
- Lin SL, Christen MO. (2020). Polycaprolactone-based dermal filler complications: A retrospective study of 1111 treatments. J Cosmet Dermatol. 19: 1907-1914.
- Vleggaar D. (2005). Facial volumetric correction with injectable poly-Llactic acid. Dermatol Surg. 31: 1511-18.
- Sánchez O, Rodríguez-Sureda V, Domínguez C. (2012). Study of biomaterial-induced macrophage activation, cell-mediated immune response and molecular oxidative damage in patients with dermal bioimplants. Immunobiology. 217: 44-53.
- Loghem JV, Yutskovskaya YA, Philip Werschler W. (2015). Calcium hydroxylapatite: over a decade of clinical experience. J Clin Aesthet Dermatol. 8: 38-49.
- Kim JS. (2019). Changes in Dermal Thickness in Biopsy Study of Histologic Findings After a Single Injection of Polycaprolactone-Based Filler into the Dermis. Aesthet Surg J. 39: Np484-np494.
- Carruthers A, Carruthers J, Hardas B. (2008). A validated hand grading scale. Dermatol Surg. 34: S179-83.
- Siquier-Dameto G, Boisnic S, Boadas-Vaello P, Verdú E. (2023). AntiAging and Depigmentation Effect of a Hyaluronic Acid Mechanically Stabilized Complex on Human Skin Explants. Polymers (Basel). May 24 2023;15.
- Luebberding S, Alexiades-Armenakas M. (2013). Critical appraisal of the safety of dermal fillers: a primer for clinicians. Current Dermatology Reports. 2: 150-157.
- da Prato EB, Cartier H, Margara A, Molina B, Tateo A, et al. (2024). The ethical foundations of patient-centered care in aesthetic medicine. Philos Ethics Humanit Med. 19: 1.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.