Efficacy of Cosmerna ARI in Improving Androgenetic Alopecia in Europeans: A Double-Blind, Randomized, Placebo-Controlled Clinical Trial over 6 months
by Jiwon Han1, Woorim Choi 1, Seung Sik Lim1, Werner Voss2 Tanja Emmler2, Janina Tiemann2, Han-Oh Park1*
1Bioneer Corporation 71, Techno 2-ro, Yuseong-gu, Daejeon 34013, Korea
2Dermatest, Korea
*Corresponding Author: Han-Oh Park, Ph.D. Chief executive officer; Bioneer Corporation 71, Techno 2-ro, Yuseong-gu Daejeon 34013, Republic of Korea.
Received Date: 05 December, 2025
Accepted Date: 23 December, 2025
Published Date: 26 December, 2025
Citation: Han J, Choi W, Lim SS, Voss W, Emmler T, et al. (2025) Efficacy of Cosmerna ARI in Improving Androgenetic Alopecia in Europeans: A Double-Blind, Randomized, Placebo-Controlled Clinical Trial over 6 months. Clin Exp Dermatol Ther 10: 244. https://doi.org/10.29011/2575-8268.100244
Abstract: Cosmerna-ARI is developed with the key ingredient SAMiRNA-AR68, which effectively reduces androgen receptor levels in hair follicles. Background: AGA needs to be managed for a lifetime. Cosmerna-ARI, a weekly hair tonic, has been shown to be effective and safe in clinical trials among Korean AGA patients. The aim of this study is to conduct clinical trials to verify the effect on Europeans. Methods: The efficacy and safety of 120 patients in Germany undergoing AGA were evaluated in a double-blind, randomized, placebo-controlled trial over a period of 6 months. Results: Compared to the placebo group, the Cosmerna-ARI treatment group experienced a significant reduction in hair shedding, an increase in terminal hair density, and an improvement in terminal hair density. Additionally, no pathological skin lesions were identified, and both the test and placebo groups exhibited healthy skin at the test site before and after the application period. Conclusion: CosmeRNA-ARI demonstrated that AGA patients in Germany improve AGA without side effects, as was done in previous Korean patients. These results suggest that Cosmerna-ARI can be an effective and convenient RNAi-based tonic for AGA management, suitable for both Asian and European populations.
Keywords: Androgenetic alopecia; Androgen receptor; SAMiRNA; Cosmerna; terminal hair; Vellus hair; European population; RNAi hair tonic.
Introduction
Androgenetic Alopecia (AGA) is the most common type of hair loss in both men and women, characterized by its progressive nature and the significant role of genetic factors [1]. The prevalence of AGA varies among different ethnic groups due to genetic factors. In Caucasian populations, it affects approximately 50% of males [2,3] and 19% of females. In contrast, among Koreans, the prevalence is reported to be 14.1% in males and 5.6% in females [4]. One of the key pathological mechanisms of AGA is the follicular miniaturization process induced by the interaction between Dihydrotestosterone (DHT) and Androgen Receptor (AR) [5]. Overexpression of AR, along with DHT, serves as a major cause of AGA, with studies reporting approximately three times higher AR expression in the balding ar-eas of patients compared to normal scalp [6]. AR functions as a crucial regulatory factor in the pathogenesis of AGA, suggesting that even with low DHT concentrations, overexpres-sion of AR enhances the mechanism of hair loss by increasing AR binding to chromatin [7]. To reduce this overexpressed AR, we have developed and reported a novel hair loss improvement tonic cosmetic utilizing RNA interference (RNAi). We developed SAMiRNA (Self-Assembled Micelle inhibitory RNA), a supramolecular structure of approximately 100 nm that effectively penetrates hair follicles, reducing AR mRNA levels and improving hair loss. SAMiRNA-AR68, the main component of CosmeRNA ARI, is a functional RNAi cosmetic that downregulates AR expression without the innate immune stimulation commonly associated with siRNAs [8].
When applied once weekly to Korean subjects, SAMiRNA-AR68 demonstrated a reduction in hair loss and an increase in hair density, with no adverse reactions observed during the test period [8]. A key advantage of SAMiRNA technology is its safety profile; it does not induce inflammatory cytokines at effective doses and has demonstrated stability in hair tonic formulations for over six months. This makes it a promising alternative to current treatments that often require frequent administration and may cause systemic side effects [8].
The SAMiRNA-AR68 treatment has been commercially released in Europe in May 2023 under the brand name CosmeRNA, making it one of the first hair loss treatments utilizing RNA technology. Its mechanism of specifically targeting the androgen receptor presents a novel approach to addressing AGA at the translation level, potentially offering a more targeted and effective solution than traditional treatments [9].
Given that the prevalence of AGA varies among different ethnic groups due to genetic differences, its efficacy may also differ across ethnicities. Therefore, we conducted a clinical study targeting European individuals. This study performed a doubleblind, randomized, placebo-controlled clinical trial to evaluate the efficacy and safety of CosmeRNA-ARI in German subjects. Through a six-month treatment regimen, the study verified the safety and efficacy of SAMiRNA-AR68 in European AGA patients by demonstrating a reduction in shed hair count, an increase in hair density, and confirming its safety profile.
Materials and Methods
Subjects
Although 120 participants were initially enrolled and randomized into four groups, the final efficacy analysis was conducted on a subset of 41 subjects 21 in the test group and 20 in the control group. This reduction was due to two main factors: (1) the lack of scalp tattooing led to inconsistent alignment of TrichoScan imaging across timepoints, potentially introducing variability in hair density measurement; and (2) outlier values ex-ceeding 3 standard deviations above the group mean were excluded to minimize analytical distortion from measurement artifacts or improper self-application. As the topical solutions were selfapplied at home, inconsistent application techniques and dosage deviations were also considered a significant source of variability.
Participants who showed values exceeding 3 standard deviations were excluded from the analysis. As a result, the treatment group included 21 participants (17 males and 4 females), with a mean age of 47 years and an average hair loss stage of 4.5, as assessed by the Norwood/Ludwig classification scheme. Among them, 9 males belonged to the 1-week treatment group, while 8 males and 4 females belonged to the 2-week treatment group, making a total of 21 subjects included in the final treatment analysis. The control group included 20 participants (12 males and 8 females), with a mean age of 43 years and an average hair loss stage of 4.0, also based on the Norwood/Ludwig classification.
The trial was conducted in a total of 30 participants per group and a total of 120 participants in four groups. The mean age of the subjects participating in the clinical trial was 43.7 years, and the male-to-female ratio was 40 females and 80 males, with a sex ratio of 1:2. The first group consisted of 7 women and 21 men in the 1-week treatment group, the second group 9 women and 21 men in the 2-week treatment group, the third group 10 women and 20 men in the 4-week
Materials
For the control group, a solution was prepared with the following composition: 1% (w/v) niacinamide, 1% (w/v) betaine, 0.02% (w/v) biotin, 0.05% (w/v) L-Menthol, 0.2 (w/v) D-Panthenol and 15% (v/v) ethanol. The test solution was created by adding 5 mg/ ml of SAMiRNA-AR68 to the control solution. Both the control and test solutions were packaged in the same case.
In the clinical study, the test groups were randomly assigned. The control group were applied by themselves once a week. The solutions were applied by themselves at intervals of 1, 2, and 4 weeks across three groups. After shampooing and drying the hair, the test and control solutions were applied, and the scalp was massaged for 30 seconds by themselves.
Efficacy assessment
The one-week trial and control groups were randomized, doubleblind, placebo controlled design and performed. Subjects visited the study site at weeks 0, 16 and 24, and quantified the number of shedding hairs that fell out through combing before image measurement. In addition, the density (1/cm2) of terminal hair and Vellus hair was measured by shaving 2cm2 area and taking pictures with Trichoscan at 0 weeks, 16 weeks, and 24 weeks, and analyzing with Trichogram.
Statistical analysis
The data were analyzed statistically and verified using SPSS 20.0. Before and after using the test product, we identified the significance between the test and control sites, along with intragroup and between-group comparisons, using a hypothetical mean difference of 5% (p < 0.05). After conducting a normalization test for homogeneity on the measured values from both the test site and the control site, as well as performing an intragroup comparison between the two, Repeated Measures ANOVA was applied to determine if normality was satisfied. In cases where normality was not met, the analysis was verified using Generalized Estimating Equations (GEE). If the sphericity test is satisfied, it confirms the sphericity assumption for the effect test within individuals. If sphericity is not satisfied, the Greenhouse-Geisser correction is applied. Each time point’s differences are assessed using the intraindividual contrast test.
Results
Of the 120 participants originally enrolled, 41 subjects (21 in the test group and 20 in the control group) were included in the final efficacy analysis. Subjects were excluded if their hair density measurements showed extreme deviations (defined as more than 3 standard deviations from the group mean) or if TrichoScan image data lacked consistency due to the absence of scalp tattooing, which impaired accurate localization and comparison.
The study involved 120 European men and women, aged 22 to 63, who were diagnosed with moderate androgenetic alopecia. It was conducted by Dermatest in Germany. Participants were randomly assigned to either a control group or a test group that received treatment with Cosmerna ARI (SAMiRNA-AR68 5 mg/ml). The clinical study concluded with an analysis of 21 participants from the test group and 20 from the control group, ex-cluding any values that were more than 2 standard deviations above the mean. For each outcome, we conducted before-and-after comparisons, as well as within-group and be-tween-group comparisons, using repeated measures ANOVA (RM ANOVA).
In the group that used CosmeRNA ARI for 24 weeks, significant efficacy was observed both within the group and between groups starting at 16 weeks, specifically in terms of the amount of hair loss. By 24 weeks, there was a notable improvement in the density of terminal hair within and between groups. Although there was no significant effect on the density of vellus hair, there was a trend suggesting gradual improvement over time.
In the analysis of fallen hair, the control group did not exhibit any significant changes. In contrast, the test group demonstrated a 48% improvement, with the average hair loss reducing from 19.7 at week 0 to 9.5 at week 16, and further decreasing to 8.5 by week 24. This improvement was statistically significant, with p-values less than 0.01 at both week 16 and week 24, underscoring the effectiveness of CosmeRNA ARI over time. Furthermore, a comparison between the test group and the control group revealed a statistically significant reduction in hair loss in the CosmeRNA ARI treatment group. The results indicated p-values less than 0.05 at both week 16 and week 24 when comparing these two groups (Figure 1).
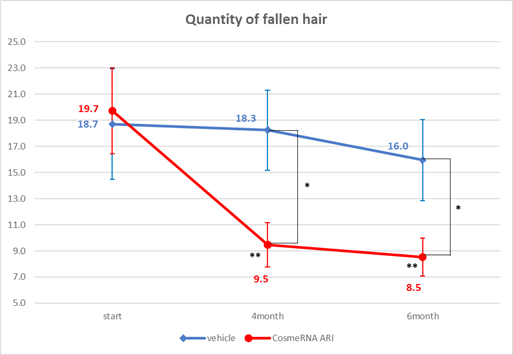
Figure 1: Quantity of fallen hair.
The control group did not show any significant changes in terminal hair density. In contrast, the test group exhibited a notable improvement, increasing from 99.7 at 0 weeks to 127.8 at 24 weeks, with a p-value of less than 0.01. This suggests that CosmeRNA ARI enhances terminal hair density over time. Furthermore, the difference between the control and test groups decreased significantly to a p-value of less than 0.05 at 24 weeks (Figure 2).
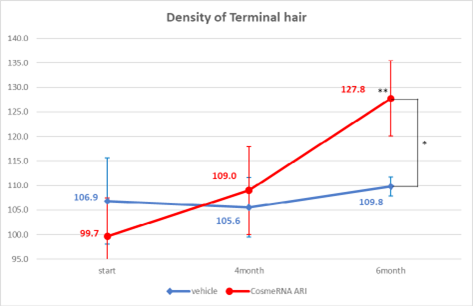
Figure 2: Density of Terminal hair.
The control group showed no significant changes in the density of vellus hair. In contrast, the test group exhibited a notable improvement in hair density, increasing from 32.5 at week 0 to 42.2 after 24 weeks, with a statistical significance at p < 0.05. This indicates that CosmeRNA ARI effectively enhances hair density over time. However, although the test group demonstrated a trend toward increased density compared to the control group, this difference did not reach statistical significance (Figure 3).
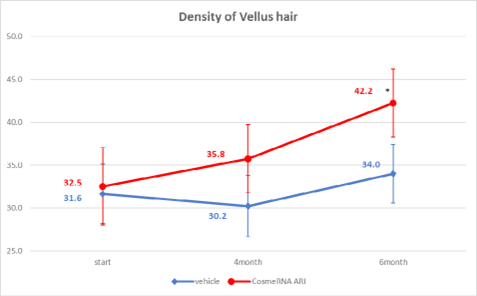
Figure 3: Density of Vellus hair.
Dermatological examinations were conducted based on established clinical-dermatological evaluation criteria. In a study involving 120 Europeans, participants were treated with CosmeRNA once a week for 24 weeks. All subjects maintained healthy skin conditions at the test site before, during, and after the application period, with no pathological skin lesions observed. Throughout the 24-week clinical trial, there were no issues regarding discontinuation of the product or adjustments in the dosage, as confirmed by the dermatologist, and no additional dermatological treatments were required (Table 1).

Table 1: Dermatological examination results means no pathological findings.
Discussion
Despite the initial enrollment of 120 participants, only 41 subjects were included in the final analysis due to inconsistencies in application and imaging. Since product application was selfadministered by the participants at home, there was a lack of control over dosage accuracy, application area, and massage intensity. Moreover, without scalp tattooing, precise realignment of TrichoScan imaging areas was difficult to achieve, resulting in data variability. To ensure data robustness, values exceeding 3 standard deviations from the group mean were excluded to mitigate the impact of outliers likely caused by improper application or measurement noise.
Moving forward, future clinical trials will adopt a more controlled design wherein product application will be performed on-site by trained professionals. This approach is expected to reduce individual variation, ensure consistent dosing, and improve the reliability of imaging-based assessments such as TrichoScan.
The CosmeRNA ARI utilized in this study is an advanced cosmetic designed for hair loss relief, featuring SAMiRNA, a supramolecular siRNA. SAMiRNA-AR68 is included in the Cosmetic Ingredient Dictionary (ICID) maintained by the Personal Care Products Council (PCPC) in Europe, which allows it to be used as a cosmetic product. The application process is straightforward: simply apply it to the scalp and massage for 30 seconds.
Recent studies have demonstrated that the pathogenesis of androgenetic alopecia is not solely related to androgens, but also to the extent of androgen receptor expression. It has been discovered that the overexpression of androgen receptors in the human hair papilla triggers paracrine signaling. This process leads to the secretion of signaling pathway inducers such as TGF-beta 1, resulting in the miniaturization of the hair papilla. The active ingredient SAMiRNA-AR68 in the CosmeRNA ARI product used in this study reduces the level of androgen receptor protein by decreasing the mRNA of overexpressed androgen receptors. Consequently, the paracrine signaling caused by this oversxpression is inhibited. This inhibition appears to prevent TGF-beta 1 from suppressing the Wnt signaling pathway, thus helping to maintain the anagen phase in the affected areas. As a result, this process reduces hair loss. The results of this study demonstrated that CosmeRNA ARI significantly reduced hair shedding at week 16 and increased hair density by week 24 in patients with androgenetic alopecia (AGA). Additionally, analysis of the test groups treated once a week(A1-26), once every two weeks(A2-17), and once every four weeks(A4-08) revealed no significant differences; however, a trend toward reduced hair loss was noted (Table 2).
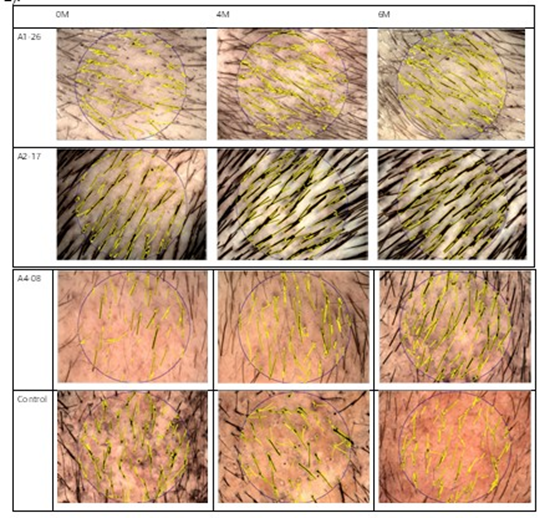
Table 2: The sustained effectiveness of CosmeRNA ARI.
The sustained effectiveness of siRNA in SAMiRNA 68, the key active ingredient in CosmeRNA ARI, is achieved after only one application every four weeks. This effectiveness is due to the siRNA’s ability to form an RNA-induced silencing complex (RISC) within cells, continuously degrading the target mRNA. The siRNAs loaded into the RISC can effectively reduce AR mRNA by acting repeatedly until the RISC is either detached or degraded. Research indicates that the effect is more pronounced with treatments every two weeks compared to every four weeks. The duration of efficacy for SAMiRNA 68 is shorter than that of typical siRNA therapeutics because it has a natural RNA structure, which is more vulnerable to hydrolysis compared to the modified RNA used in therapeutic siRNA. Additionally, with the reduced number of applications (six times application for the four-week regimen as opposed to twelve times application for the two-week regimen), it is likely that delivery to all hair follicles affected by hair loss during the self-application and massage phase is less effective than when treatments are applied more frequently.
CosmeRNA ARI provides significant convenience with a usage protocol of once every week to two weeks, offering a substantial advantage in long-term hair loss management compared to daily treatments like minoxidil. It is noteworthy that subjects corresponding to Norwood Hamilton Scale VII showed significant improvement (Table 3).
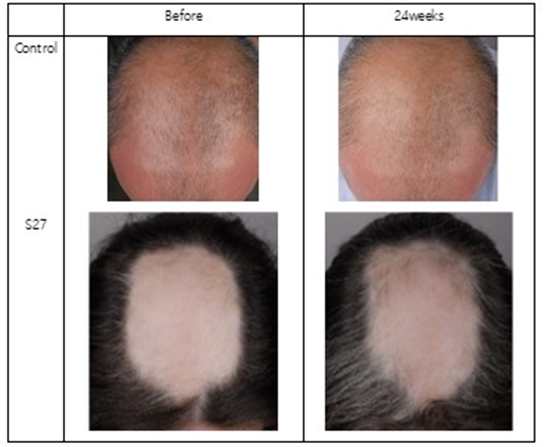
Table 3: Significant improvement of CosmeRNA ARI.
Conclusions
In this European clinical study, Cosmerna demonstrated significant efficacy in re-ducing hair shedding and increasing hair density, with no adverse effects observed during a six-month long-term safety test. These findings are comparable to previous studies conducted in Korea, suggesting that CosmeRNA ARI is a promising new alternative for patients with androgenetic alopecia (AGA), regardless of race. Traditional medications for androgenetic alopecia (AGA) that lower dihydrotestos-terone (DHT) can have side effects due to their mechanism of action. Moreover, these medications are not appropriate for treating female pattern hair loss (FPHL) because the adverse effects linked to DHT inhibition can be more critical to women.This study involves the direct delivery of CosmeRNA ARI to the hair follicle through application and massage. It works by reducing the androgen receptors in the hair follicle cells via topical delivery, and it does not affect other areas of the body, making it safe for women to use. The results of this clinical trial support its potential benefits. The study had a limited number of women participants, which restricts its ability to draw definitive conclusions. Follow-up studies exclusively focused on women are currently underway. If these studies demonstrate a clear improvement in clinical outcomes for women with androgenetic alopecia and achieve sufficient statistical significance, CosmeRNA ARI could become a viable solution for managing female hair loss, with the added convenience of application once every week to two weeks.
Author Contributions
Conceptualization, methodology, Han-Oh Park; Investigation, Woorim Choi, Jiwon Han, Tanja Emmler, Janina Tiemann; writingoriginal draft preparation, Jiwon Han; writing-review and editing, Han-Oh Park; supervision, Werner Voss, Han-Oh Park; project administration, Seung Sik Lim; funding acquisition, Han-Oh Park, All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by BIONEER.
Institutional Review Board Statement
This research was conducted in compliance with the guidelines of the P&K Skin Research Center Institutional Review Board (approval number: P2407-6702) for human subject studies.
Informed Consent Statement
Written informed consent for participation was secured from all study subjects, and separate consent for publication was obtained from any participants whose identities could be recognized.
Data Availability Statement
All original findings of this study are presented in this article, and any further inquiries should be addressed to the corresponding author.
Conflicts of Interest
Jiwon Han and Seung Sik Lim are employees of Bioneer
Corporation. Han-Oh Park is the chief executive officer of Bioneer Corporation.
References
- Piraccini BM, Blume-Peytavi U, Scarci F, Jansat JM, Falqués M, et al. (2022) Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial. J Eur Acad Dermatol Venereol 36: 286-294.
- EY Chin (2013) Androgenetic alopecia (male pattern hair loss) in the United States: what treatments should primary care provid-ers recommend. J Am Assoc Nurse Pract 25: 395-401.
- Severi G, Sinclair R, Hopper JL, English DR, McCredie MRE, et al. (2003) Androgenetic alopecia in men aged 40-69 years: prevalence and risk factors. Br J Dermatol 149:1207-1213.
- Paik JH, Yoon JB, Sim WY, Kim B, Kim N (2001) The prevalence and types of androgenetic alopecia in Korean men and women. Br J Dermatol 145: 95-99.
- Randall VA (2008) Androgens and hair growth. Dermatol Ther 21: 314328.
- Sawaya ME, Price VH (1997) Different levels of 5α-reductase type I and II, aromatase, and androgen receptor in hair follicles of men and women with androgenetic alopecia. J Invest Dermatol 109: 296-300.
- Itami S, Kurata S, Takayasu S (1995) Androgen induction of follicular epithelial cell growth is mediated via the androgen receptor in dermal papilla cells. J Invest Dermatol 105: 330-334.
- Yun SI, Lee SK, Goh EA, Kwon OS, Choi W, et al. (2022) Weekly treatment with SAMiRNA targeting the androgen receptor ameliorates androgenetic alopecia. Sci Rep 12: 5675.
- Sergio V, Park YJ (2024) Current Landscape and Emerging Therapies in Hair Loss Treatment for Androgenetic Alopecia. EMJ Dermatol 12: 95-102.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.