Efficacy and Tolerability of a Topical Gel Containing Mimicking Growth Factors Oligopeptides, Caffeine, Taurine and an Iron-Chelating Complex in Subjects with Androgenetic Alopecia Treated with Topical Minoxidil or Oral Finasteride: a Prospective, 6-Month, Randomized, Assessor-Blinded, 4-Arm, Parallel Group Study
Michela Starace 1 , Massimo Milani 3* , Bianca Maria Piraccini 1,2
1Dermatology Unit- IRCCS Azienda Ospedaliero-Universitaria di Bologna, Italy
2Department of Experimental, Diagnostic and Specialty Medicine Alma Mater Studiorum University of Bologna, Italy
3Medical Direction Cantabria Labs Difa Cooper, Caronno P. Italy
*Corresponding author: Massimo Milani, Cantabria Labs Difa Cooper Medical Direction , Via Milano 160; Caronno Pertusella Italy
Received Date: 01 December 2022
Accepted Date: 14 December 2022
Published Date: 19 December 2022
Citation: Starace M, Milani M, Piraccini BM (2022) Efficacy and Tolerability of a Topical Gel Containing Mimicking Growth Factors Oligopeptides, Caffeine, Taurine and an Iron-Chelating Complex in Subjects with Androgenetic Alopecia Treated with Topical Minoxidil or Oral Finasteride: a Prospective, 6-Month, Randomized, Assessor-Blinded, 4-Arm, Parallel Group Study. Clin Exp Dermatol Ther 7: 199. DOI:https://doi.org/10.29011/2575-8268.100199
Abstract
Introduction: Topical minoxidil and oral finasteride are the first-line pharmacological treatments for Androgenic Alopecia (AGA). However, adjuvant strategies with non-pharmacological products could increase their clinical efficacy. A topical gel containing oligopeptides with growth-factor mimicking activity, caffeine, taurine, and a lactoferrin-based iron-chelating complex has been recently available (GFM-DA gel). Study Aim: We evaluated in a prospective, randomized, 4-arm, assessor-blinded, 6-month trial the efficacy of the GFM-DA as add-on treatment (one 12.5 ml application once a week) in subjects with AGA using topical 5% minoxidil (T-MNX), 1 ml twice daily, or oral 1 mg daily finasteride (O-F) in comparison with drugs treatment alone (T-MNX or O-F). Subjects and Methods: Fifty-eight subjects, 27 men and 31 women (mean age 38 years, range: 19-61 years) with AGA (Grade III-IV-V of Norwood-Hamilton scale) or FAGA (Ludwig Grade I/II) participated in the trial, after their written informed consent. The subjects were randomized to one of the following four treatment groups: Group1: GFM-DA+TMNX (n=17); Group 2: GFM-DA-O-F (n= 15); Group 3: T-MNX alone (n=14) and Group4: O-F alone (n=12). For female subjects randomization to F groups was allowed only for menopausal women. The primary endpoint was the evolution of a 7-point Global Photographic Assessment score (GPAS; from -3 to +3) from baseline to week 24, evaluated in a blinded fashion.
GPAS was evaluated also at week 12. Secondary endpoints were: 1)the comparison of GPAS scores at week 24 in all GFM-DA treated subjects (GFM-DA-T-MNX and GFM-DA-OF; n=32) vs. drugs treatments alone groups (T-MNX and O-F; n=26); 2)the evolution of a global physician 5-point (from -2 to +2) efficacy score (EPS); and 3)the percentage of subjects with a GPAS of +3 at week 24. Results: All subjects but one concluded the trial. After 24 weeks, a significant increase of GPAS in comparison with baseline was observed in all groups: 2.2 in T-MTX; 1.9 in O-F; 2.4 in GFM-DA+T-MNX and 2.7 in GFM-DA+O-F groups. At week 12 in comparison with baseline GPAS scores where 1.6 in T-MNX, 1.6 in GFM-DA+T-MNX, 1.1 in OF and 1.8 in GFMDA+OF. At week 24, in the group of all GFM-DA-treated subjects the GPAS score was significantly greater in comparison with the drug-only treated group (2.6±0.5 vs. 2.2±0.6; p=0.023). No difference in clinical efficacy was observed between T-MNX and GFM-DA+T-MNX groups, whereas GFM-DA significantly (P=0.01) increase the clinical efficacy in O-F treated subjects in comparison with O-F alone (2.7 vs. 1.9). At week 24, subjects with a GPA score of +3 (very good improvement) were 43% in T-MNX group, 56% in the GFM-DA+T-MNX group, 16% in OF group and 73% in GFM-DA+OF group. In GFM-DA gel treated subjects this percentage was 62% in comparison with 27% in the drugs-only treated subjects (P=0.003). Similar results were obtained for the EPS scores. The GFM-DA gel was very well tolerated. Conclusion: Adding a once-week application gel containing mimicking growth factors peptides, caffeine, taurine and an iron-chelating complex to standard AGA drugs treatments increases significantly the clinical efficacy. The greatest effect was observed when the gel was added to oral finasteride treatment.
(Trial Registration Number: ISRCTN10404684)
Keywords: Androgenic alopecia; Randomised study;Minoxidil; Finasteride; Mimicking growth factors; Caffeine; Taurine
Introduction
Androgenic Alopecia (AGA) is a common hair disorder affecting both men and women, with a great impact on quality of life and self-esteem [1]. Topical minoxidil and oral finasteride are the only drugs approved as first-line pharmacological treatment for AGA and female AGA (FAGA) [2]. However, adjuvant strategies with non-pharmacological products could increase the clinical efficacy of anti-AGA drugs [3]. A topical gel containing oligopeptides with growth-factor mimicking activities, caffeine, taurine, and a lactoferrin-based iron-chelating complex has been recently available (GFM-DA gel). This product has shown to improve the clinical efficacy of Platelet-rich-plasma treatment in AGA subjects [4]. So far, no data are available regarding the effect of this product on the clinical efficacy in AGA when used in combination with topical minoxidil or oral finasteride.
Study Aim
We evaluated the efficacy of the GFM-DA gel as add-on treatment (one 12.5 ml application per week) in subjects with AGA/FAGA using topical 5% minoxidil (T-MNX) 1 ml twice daily or oral 1 mg daily finasteride (O-F) in comparison with drugs treatment alone (T-MNX or O-F).
Subjects and Methods
Study Design
We performed a single centre, prospective, controlled, randomized, 4-arm, assessor-blinded, 6-month trial.
Subjects
Fifty-eight subjects (27 men and 31 women) (mean age 38±12 years) with AGA/FAGA (Grade III-IV-V of Norwood-Hamilton scale or Ludwig scale Grade I or II) participated in the trial, after their written informed consent. The main inclusion criteria were adult men or women with mild forms of AGA or FAGA candidates for drug treatment (topical minoxidil 5% in men or women; or oral finasteride 1 mg/daily for men or post-menopausal women). Exclusion criteria were inflammatory scalp skin conditions, iron deficient anaemia, positive history of minoxidil or finasteride treatments in the previous 6 months before enrolment in the present study. The subjects were randomly assigned to one of these four treatment groups: Group 1: GFM-DA+T-MNX (n=17); Group 2: GFM-DA+O-F (n= 15); Group 3: T-MNX alone (n=14) and Group4 O-F alone (n=12). Only menopausal women could be randomised in finasteride groups (group 2 and 4). Women in reproductive age could be randomised only in groups 1 and 3.
Ethical Issues
The study protocol, the subject information sheet, and informed consent form were reviewed and approved by the Ethics Committees of the Centre (approval October 2020, Study Code SBCLAB01/19). The study has been conducted in accordance with the GCP, the ethical principles deriving from the Helsinki Declaration16 and the current legislation on interventional studies. All subjects were informed regarding the nature of the study. The written informed consent was collected immediately after the due information about the objectives and methods of the study. Trial registration number was: ISRCTN10404684.
Outcomes of the study
The primary trial endpoint was the evolution of a 7-point Global Photographic Assessment score, according to Iorizzo et al [5] (GPAS; from -3 to +3) from baseline to week 24, evaluated in a blinded fashion comparing the scores at week 24 in all GFM-DA treated subjects (GFM-DA+MNX and GFM-DA+OF; N=32) vs. drugs treatments alone (T-MNX and O-F; N=26). GPAS score was calculated for each participating subject using standardized highdefinition colour pictures of the scalp by an assessor unaware of the treatment allocation but knowing the temporal sequence (baseline, week 12 and week 24). In more details, the dermatologist compared photographs taken at baseline and at week 12 and 24, then rated the paired photographs separately based on 7-point scale: -3 = greatly decreased, -2 = moderately decreased, -1 = slightly decreased, 0 = no change, 1 = slightly increased, 2 = moderately increased, 3 = greatly increased. Secondary endpoints were: 1)the comparison of GPAS scores evolution at week 24 in the T-MNX alone group vs. the GFM-DA+T-MNX group; 2) the comparison of GPAS scores evolution at week 24 in the OF alone group vs. the GFM-DA+OF group; and 3) the evolution of a global physician 5-point (from -2 to +2) efficacy score (EPS) in each group and finally, 4) The percentage of subjects with a GPAS Score of +3 at week 24.
Statistical Analysis
Graph Pad Prism statistical software (version 9) was utilized for data analysis. Continuous variables were expressed as mean ± standard deviation (SD). The primary endpoint of the trial was the evolution of GPA score in comparison with baseline and between the groups. The paired t-test, the Wilcoxon test and the Mann-Whitney test were used, when appropriate, for the analysis of the study outcomes. According to the primary outcome of the trial (difference in GPA score at week 24 between GFMDA treated subjects and drugs-only treated patients), the sample size calculation was performed assessing a test hypothesis of superiority of GFM-DA treatment in comparison with drugs only treatment. With an effect size (Cohen’s d value) of 0.65, an alpha value of 0.05, and a power of 80%, with an allocation ratio of 1:1, a total of at least 60 subjects (30 in group GFM and 30 in drugs only group) should be enrolled to detect this difference. This effect size represents a clinical improvement of 10% in the 7-point GPAS scale. Therefore, we decided to enrol 15 subjects per group, planning to have a total of 4 groups (Minoxidil alone, minoxidil plus GFM-DA, oral finasteride alone and oral finasteride plus GFM-DA). The sample size was calculated using G-Power statistical software version 3.9 (Kiel, Germany). A p value of <0.05 was considered significant. Analysis was performed based on “intention-to-treat” principle. Randomization list was generated using a dedicated statistical software (Randomization-it!).
Results
All but one subjects concluded the trial. Figure 1 shows the study flow. The Table reports subjects’ characteristics at baseline. In comparison with baseline, after 24 weeks, a significant increase of GPAS was observed in all groups, mean±SD: 2.2±0.7 in T-MNX; 1.9±0.9 in O-F; 2.4±0.7 in GFM-DA+T-MNX and 2.7±0.4 in GFM-Da+O-F groups. Regarding the primary endpoint of the trial, at week 24, in the group of all GFM-DA-treated subjects (N=32) the GPAS score was significantly greater in comparison with the drug-only treated group (N=26) (2.6±0.5 vs. 2.2±0.6; absolute difference 0.4; 95% CI of the difference: from 0.05 to 0.8; p=0.023) (Figure 2). No difference in clinical efficacy was observed between T-MNX and GFM-DA+T-MNX groups, even if the GPAS score was a little be higher in the combination group vs monotherapy (2.4 vs. 2.2), whereas GFM-DA significantly increase the clinical efficacy in O-F treated subjects in comparison with O-F alone (2.7 vs. 1.9; P=0.001). Figure 3 shows the evolution of GPAS in the four study groups. Similar results were obtained for the EPS scores. EPS score was 2.3±0.4 in T-MNX, 2.4±0.6 in GFM-DA+TMNX, 2±0.7 in OF and 2.7±0.4 in GFM-DA+OF. EPS score in GFM-DA treated group was 2.5 and in drug-only treated subjects 2.1 (mean difference 0.4; 95% CI of the difference: from 0.05 to 0.7; p=0.023). The efficacy of GFM-DA gel was similar in men and women (GPAS 2.5 in both groups). At week 24, the percentage of subjects with a GPA score of +3 (very good improvement) were 43% in MNX group, 56% in the GFM+MNX group, 16% in OF group and 73% in GFM+OF group. In GFM-DA gel treated subjects (N=32) this percentage was 62% in comparison with 27% in the drugs-only treated subjects (N=26). This difference was statistically significant (P=0.003, Chi-square test) (Figure 4). Figure 5 reports pictures of the scalp of four subjects, one for each treatment group. The gel was very well tolerated. No local side effects were reported.
|
Group |
N |
Age, mean |
Sex |
AGA/FAGA Grade (%) |
|
Group 1 GFM-T-MNX |
17 |
36 |
8 women 7 men |
III (60%) II (40%) |
|
Group 2 GFM-OF |
15 |
39 |
7 women 8 men |
IV (5%) III (55%) II (40%) |
|
Group 3 T-MNX |
14 |
38 |
8 women 7 men |
III (65%) II (35%) |
|
Group 4 OF |
12 |
37 |
7 women 8 men |
III (50%) II (50%) |
Table 1: Subjects’ characteristics at baseline.
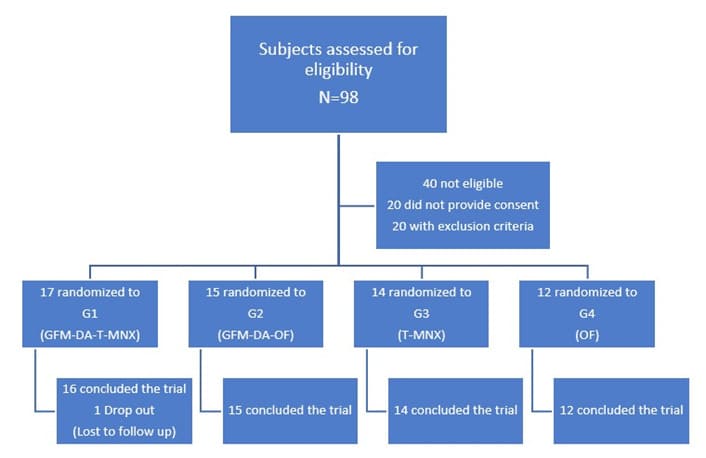
Figure 1: Trial Flow Diagram.
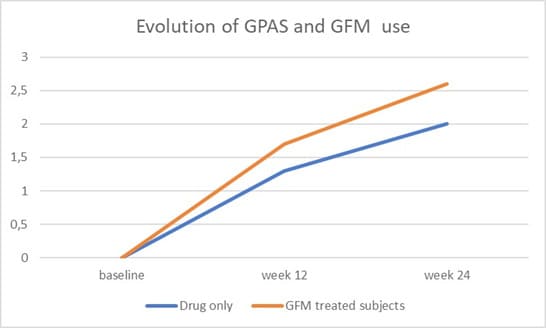
Figure 2: Primary Outcome: Evolution of GPA Score (GPAS) in GFM-DA gel treated subjects (N=29) and drug-only subjects (N=29). At week 24 the difference is statistically significant (P=0.023).
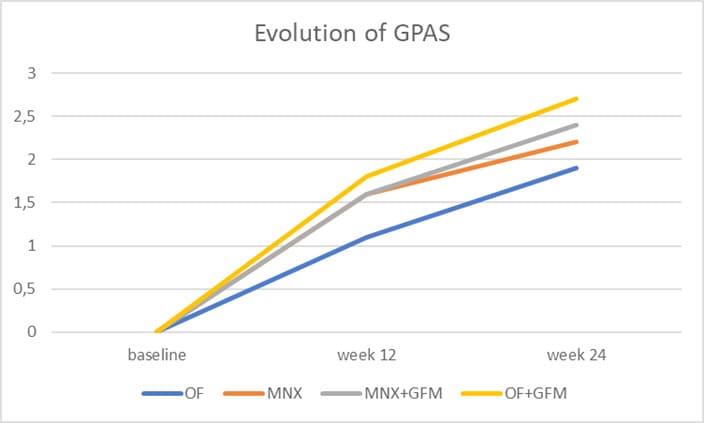
Figure 3: Secondary outcome: Evolution of GPA Score (GPAS) in the four groups (OF: oral finasteride, T-MNX: topical minoxidil; T-MNX+GFM-DA: topical minoxidil plus GFM-DA gel once a week; OF+GFM-DA: oral finasteride plus GFM-DA gel once a week). At week 24 the difference between OF+GFM-DA group and OF group is statistically significant (P=0.01).
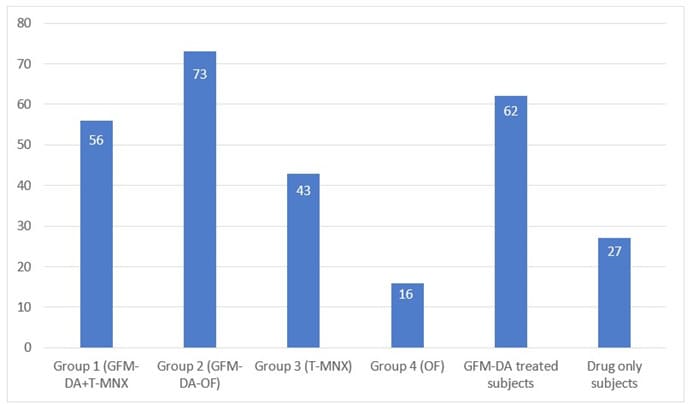
Figure 4: Percentage of subjects with GPA Score of +3 (very good improvement) at week 24 (OF: oral finasteride, T-MNX: topical minoxidil; T-MNX+GFM-DA: topical minoxidil plus GFM-DA gel once a week; OF+GFM-DA: oral finasteride plus GFM-DA gel once a week).
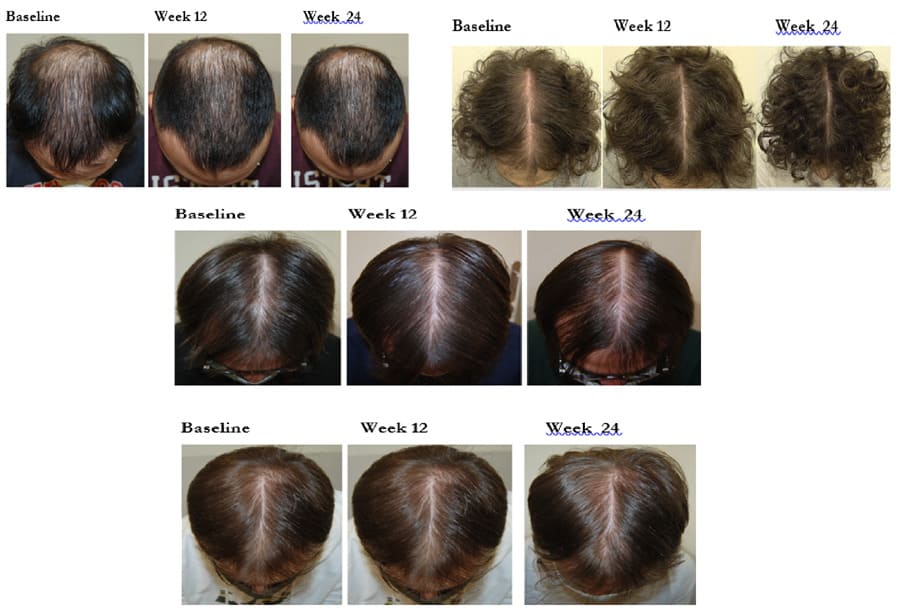
Figure 5: Picture of 4 subjects (baseline, week 12 and 24) for each allocation group (G1,G2,G3 and G4).
Discussion
Our trial supports the concept that adjuvant topical treatment with a gel containing caffeine, taurine, iron-chelating complex and a mix of growth factors mimicking oligopeptides, applied one weekly, increase the clinical efficacy in subjects with AGA/FAGA treated with specific anti hair loss drugs such as topical minoxidil or oral finasteride. The greater additive effect was observed in subjects treated with oral finasteride whereas the beneficial additive effect in minoxidil treated subjects was marginal or moderate. The tested gel contains caffeine. Caffeine used topically can increase intracellular cAMP concentrations [6], resulting in stimulatory effects on cell metabolism and proliferation [7]. In vitro studies have shown that caffeine prolonged the duration of the anagen phase and counteracted testosterone-induced TGF-β2 protein expression in hair follicles [8]. In both males and females, IGF-1 protein expression was potentiated by caffeine, promoting anagen phase [9]. A caffeine containing lotion has demonstrated in a randomised double blind trial similar efficacy of topical minoxidil in AGA subjects [10]. This gel contains also taurine. Taurine is a beta-amino acid produced by methionine and cysteine metabolism [11]. It is involved in a variety of physiological functions, including immunomodulatory and antifibrotic [12,13]. Taurine has a specific tropism for the hair bulb [14]. In vitro studies have shown that taurine can counteract the negative effect of TGF-beta on hair growth [15]. In vitro, taurine prolongs the survival rate of human hair follicles [16]. The test gel also contains three components with iron chelating activity (lactoferrin [17], EDTA and sodium gluconate). The hypoxia-inducible factor- 1a (HIF-1α) may counteract hair loss [18]. Its activity could be blocked by iron molecules [19]. When iron at scalp level is chelated the HIF-1α activity is enhanced [20]. This could represent a rational for the topical use of chelating substances in the strategic therapeutic approach of hair loss. Finally, this gel contains a mix of four high-purified, growth factors-like polypeptides (octapeptide 2, acetyl decapeptide 3, oligopeptide 20 and copper tripeptide). Copper tripeptide has anti-inflammatory, antioxidant and blood vessel growth promoting action [21]. In addition, it can increase the activity of FGF (Fibroblast Growth Factor) and VEGF [22]. Interestingly, copper tripeptide decreases the secretion of dermal fibroblasts TGF-β, a pro catagen factor [23]. Finally, copper tripeptide can also interfere with the activity of 5-α-reductase, therefore reducing the production of DHT [24]. This peptide is also able to stimulate the production of decorin [25]. Decorin, at scalp level, improves the anagen phase and consequently hair growth [26]. The acetyl decapeptide is a synthetic peptide that mimics the action of two growth factors: the keratinocyte growth factor (KGF) and the epidermal growth factor (EGF), which acts on the follicle, promoting the anagen growth phase, through an antiapoptotic effect, maintaining the biochemical activity of the bulge stem cells [27]. Octapeptide 2, can promote hair growth, to reduce apoptosis and to increase keratinocyte proliferation [28]. Androgenic alopecia is a common disorder affecting both men and women altering hair growth [29]. The only pharmacological treatments approved for AGA/FAGA are topical minoxidil at 2 or 5% concentration applied twice daily and oral finasteride 1 mg daily [30]. More recently a topical formulation of finasteride 0.25% was approved for the treatment of AGA in adult men [31]. Recent data also support the use of oral minoxidil in AGA treatment [32]. However, both drugs, used topically or orally possess side effects and are effective in less than 50% of treated patients [33]. Controlled trials report clinical success (moderate or good hair growth) in up to 30-39% of subject with both topical minoxidil and oral finasteride after 48 weeks of treatment [34-36]. Therefore, there is a margin for clinical improvement in the pharmacological strategic treatment approach of AGA/FAGA and the present study has addressed this specific question. Some limitations should be taken in account in evaluating our results. First, this trial was not a double-blind study. To increase the internal validity of the trial results, we decided however to adopt an assessor-blinded approach in evaluating the main clinical efficacy outcomes of the study (evolution of GPA score). Another limit of the present trial is the relatively small sample size (58 patients in total). However, taking in account previous trials, we have performed a specific sample size calculation which justifies the number of subjects we have enrolled in the trial. Finally, the study duration of our trial (6 months) could be considered too short for obtaining the full therapeutic potential of drug treatments, especially for the finasteride treated subjects. However, the main objective of our study was to evaluate if the efficacy of the combination strategy could offer in the medium term (i.e., 6 months) a better clinical outcome, which could help both the dermatologist and the patient to have a good compliance/ adherence to the therapy, further improving the results in the long term.Conclusion
Adding a once-week application gel containing mimicking growth factors peptides, caffeine, taurine and an iron-chelating complex to standard AGA drugs treatments increases significantly the clinical efficacy. The greatest effect was observed when the gel was added to oral finasteride treatment.
Author Contributions
BMP and MS performed all the research activities (subjects’ selection, trial visits and clinical evaluation, and clinical score evaluation). In addition, BMP designed the research study. MM and MS analyzed the data. MM and BMP wrote the paper. All authors have read and approved the final manuscript.
Ethical Approval
The study protocol, the Subject Information Sheet (SIS), and Informed Consent Form (ICF) were reviewed and approved by the Ethics Committees of the Centre (approval October 15, 2020 Study Code SBCLAB01).
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- Lolli F, Pallotti F, Rossi A, Fortuna MC, Caro G, et al. (2017) Androgenetic alopecia: a review. Endocrine. 57: 9-17.
- Adil A, Godwin M (2017) The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol 77: 136-141.
- Rajput RJ (2010) Controversy: is there a role for adjuvants in the management of male pattern hair loss? J Cutan Aesthet Surg 3: 82-86.
- Lazzari T, Milani M, (2019) Efficacy of Autologous Platelet-Rich Plasma alone or in Combination with a Lotion Containing GrowthFactor like Polypeptides and Taurine in the Treatment of Androgenic Alopecia:A Randomized, Prospective, Assessor-Blinded Trial. J Clin Exp Dermatol Res 10: 506.
- Iorizzo M, Vincenzi C, Voudouris S, Piraccini BM, Tosti A (2006) Finasteride treatment of female pattern hair loss. Arch Dermatol 142: 298-302.
- Belibi FA, Wallace DP, Yamaguchi T, Christensen M, Reif G, et al. (2002) The effect of caffeine on renal epithelial cells from patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol 13: 2723-2729.
- Ojeh N, Stojadinovic O, Pastar I, Sawaya A, Yin N, Tomic‐Canic M (2016) The effects of caffeine on wound healing. Int Wound J 13: 605
- Fischer TW, Herczeg‐Lisztes E, Funk W, Zillikens D, Bíró T, et al. (2014) Differential effects of caffeine on hair shaft elongation, matrix and outer root sheath keratinocyte proliferation, and transforming growth factor‐β2/insulin‐like growth factor‐1‐mediated regulation of the hair cycle in male and female human hair follicles in vitro. Br J Dermatol 171: 1031-1043.
- Völker JM, Koch N, Becker M, Klenk A (2020) Caffeine and its pharmacological benefits in the management of androgenetic alopecia: a review. Skin Pharmacol Physiol 33: 93-109.
- Dhurat R, Chitallia J, May TW, Jayaraaman AM, Madhukara J, et al. (2017) An open-label randomized multicenter study assessing the noninferiority of a caffeine-based topical liquid 0.2% versus minoxidil 5% solution in male androgenetic alopecia. Skin Pharmacol Physiol 30: 298-305.
- Birdsall TC (1998) Therapeutic applications of taurine. Altern Med Rev 3: 128-136.
- Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 72: 101-163.
- Bouckenooghe T, Remacle C, Reusens B (2006) Is taurine a functional nutrient?. Curr Opin Clin Nutr Metab Care 9: 728-733.
- Da Silva DLP, Thiago SB, Pessôa FA, Mrestani Y, Rüttinger HH, et (2008) Penetration profile of taurine in the human skin and its distribution in skin layers. Pharm Res 25: 1846-1850.
- Collin C, Gautier B, Gaillard O, Hallegot P, Chabane S, et al. (2006) Protective effects of taurine on human hair follicle grown in vitro 1. Int J Cosmet Sci 28: 289-298.
- Labrozzi A (2020) Nutrients in hair supplements: evaluation of their function in hair loss treatment. Hair Ther Transplant 10:1-6.
- Oram JD, Reiter B, (1968) Inhibition of bacteria by lactoferrin and other iron-chelating agents. Biochem Biophys Act 170: 351-365.
- Zheng M, Jang Y, Choi N, Kim DY, Han TW et al. (2019) Hypoxia improves hair inductivity of dermal papilla cells via nuclear NADPH oxidase 4‐mediated reactive oxygen species generation’. Br J Dermatol 181: 523-534.
- Park BS, Kim WS, Choi JS, Kim HK, Won JH, et al. (2010) Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: evidence of increased growth factor secretion. Biomed Res 31: 27-34.
- Gleadle JM, Ebert BL, Firth JD, Ratcliffe PJ (1995) Regulation of angiogenic growth factor expression by hypoxia, transition metals, and chelating agents. Am J Physiol 268: C1362-C1368.
- DeHaven C (2014) Copper Tripeptide-1. Science of Skincare.
- Pollard JD, Quan S, Kang T, Koch RJ (2005) Effects of copper tripeptide on the growth and expression of growth factors by normal and irradiated fibroblasts. Arch Facial Plastic Surg 7: 27-31.
- Gruchlik A, Chodurek E, Dzierzewicz Z (2014) Effect of gly-his-lys and its copper complex on TGF-β1 secretion in normal human dermal Acta Pol Pharm 71: 954-958.
- Leite AMDS, Fiorese MS, Thema FT, Costa EMAB (2021) Study on Antioxidants and Growth Factors in the Treatment of Alopecia with Recent Developments in Medicine and Medical Research. 8: 160-165.
- Pickart L, Margolina A (2018) Regenerative and protective actions of the GHK-Cu peptide in the light of the new gene data. Int J Mol Sci 19: 1987.
- Jing J, Wu XJ, Li YL, Cai SQ, Zheng M, et al. (2014) Expression of decorin throughout the murine hair follicle cycle: hair cycle dependence and anagen phase prolongation. Exp Dermatol 23: 486-491.
- Rinaldi F, Trink A, Pinto D (2020) Efficacy of postbiotics in a PRPlike cosmetic product for the treatment of alopecia area Celsi: a randomized double-blinded parallel-group study. Dermatol Ther 10: 483-493.
- Ji CY, Deug KY, Ryul KC (2012) Peptides for promoting hair growth and improving wrinkle and cosmetic compositions comprising the same. US Patent. 8: 106.
- Piraccini BM, Alessandrini A (2014) Androgenetic alopecia. G Ital Dermatol Venereol 149: 15-24.
- McElwee KJ, Shapiro JS (2012) Promising therapies for treating and/ or preventing androgenic alopecia. Skin Therapy Lett 17: 1-4.
- Piraccini BM, Blume‐Peytavi U, Scarci F, Jansat JM, Falqués M, Otero R, Topical Finasteride Study Group. (2022) Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: A phase III, randomized, controlled clinical trial. J Eur Acad Dermatol Venereol 36: 286-294.
- Randolph M, Tosti A (2021) Oral minoxidil treatment for hair loss: A review of efficacy and safety. J Am Acad Dermatol 84: 737-746.
- DeVillez RL (1987) Topical minoxidil for androgenetic alopecia: optimizing the chance for success by appropriate patient selection. 175: 50-53.
- Sung CT, Juhasz ML, Choi FD, Mesinkovska NA (2019) The efficacy of topical minoxidil for non-scarring alopecia: a systematic review. J Drugs Dermatol 18: 155-160.
- Libecco JF, Bergfeld WF, (2004) Finasteride in the treatment of Expert Opin Pharmacother 5: 933-940.
- Hajheydari Z, Akbari J, Saeedi M, Shokoohi L, (2009) Comparing the therapeutic effects of finasteride gel and tablet in treatment of the androgenetic alopecia. Indian J Dermatol Venereol Leprol 75: 47-51.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.