Effects of Shielding Skirts on Salmon Lice Infestation in Cage Aquaculture: A Brief Literature Review
by Asbjørn Bergheim*, Nils Hovden, Svein B. Tveiten, Sara Rasouli, Martin Gausen
Bio Marine, 6650 Surnadal, Norway
*Corresponding authors: Asbjørn Bergheim, Bio Marine, 6650 Surnadal, Norway.
Received Date: 29 September 2025
Accepted Date: 07 October 2025
Published Date: 10 October 2025
Citation: Bergheim A, Hovden N, Tveiten SB, Rasouli S, Gausen M (2025) Effects of Shielding Skirts on Salmon Lice Infestation in Cage Aquaculture: A Brief Literature Review. Infect Dis Diag Treat 9: 281. https://doi.org/10.29011/2577-1515.100281
Abstract
The ectoparasite salmon lice is a major threat to farming of salmon and rainbow trout in sea cages generating large welfare and economic problems. Even moderate salmon lice infestations are stressful to the host and will increase the risk of secondary infections. A combination of treatment attempts at high lice occurrence and preventative measures to diminish the access of free-swimming lice larvae to the cages is commonly practised. Use of so-called lice skirts has demonstrated significantly reduced number of attached lice on salmon in most reported tests and contributed to less need for resourceintensive and occasionally harmful treatments. However, the effects of lice skirts highly fluctuate both within and between cages and farm localities. Several studies indicate between zero and more than 80% less lice number in shielded cages. Shielding skirts inevitably cause reduced water flow through the uppermost parts of the cages which can lead to lowered oxygen concentration and need of extra oxygen supply.
Keywords: Lepeophtheirus salmonis; Salmo salar; Cage aquaculture; Lice skirt
Introduction
Infestation of salmon lice (Lepeophtheirus salmonis) represents a serious challenge to the industry in the leading producer countries of Atlantic salmon (Salmo salar), such as Norway, Scotland and Canada. Another ectoparasite, Caligus elongatus, is less host-specific than salmon lice but can cause harmful effects to salmon and other fish species. Along with the increasing salmon production the occurrence of the ectoparasite salmon lice has expanded dramatically [1] and induces major costs and fish welfare problems for the industry [2] and, not least, poses a threat to stocks of wild salmon and sea trout [3,4]. Sea lice feeding may cause skin lesions of their hosts leading to secondary infestations and osmotic failures affecting growth, fertility, and survival [5].
Over the years, different approaches for controlling lice infestation in cage farming have been introduced, which can roughly be divided into three groups according to Jónsdóttir et al. [6]: immediate (chemotherapeutic treatment), continuous (mechanical, thermal and freshwater treatment, deployed cleaner fish), and preventative measures including physical barriers (lice skirts, submerged ‘snorkel’ cages).
Immediate treatment, use of medical drugs, has been the dominating measure to combat sea lice since the 1970s [7]; however, the growing concern about emerging treatment-resistant lice at cage farms [8] and demonstrated harmful effects on the local environment [9] has led to implantation of alternative treatment methods. Handling of fish during repeated mechanical – thermal – freshwater treatment causes stress and risk of injuries to the fish [10] and is considered to contribute to mortality and reduced growth during the cage-based grow-out cycle [e.g. 11].
Barriers, such as impermeable and semi-permeable skirts mounted floating cages, or use of submersed cages (‘snorkel’ cages) kept below the upper layers where infectious sea lice are most concentrated, have demonstrated substantially reduced lice infestation [7,12]. Preventative measures also include stocking of ‘cleaner fish’ which are feeding on lice attached to salmon often combined with physical barriers [13]. Of all sea lice management measures in Scotland, use of skirts was ranked the most costeffective measure with under £0.10 per fish per unit of effectiveness [14].
In several recent reviews, Jónsdóttir et al. [6], Oppedal et al. [15] and Barrett et al. [7] presented comprehensive surveys of efficiency and environmental challenges of lice shielding skirts and other lice-preventive barriers in salmon cages. This paper shortly describes use of lice skirts mounted floating cages emphasizing the lice reducing effect under different conditions based on literature.
Salmon lice
The ectoparasite has eight life stages: the first three are freeswimming (nauplii and copepodid stages), the next two are attached to the host (chalimus I-IV stages) and the final three are mobile (pre-adult and adult stages), Figure 1. Copepodids, the infective stage, are more actively moving than the preceding nauplii to detect and infest a host [16] and the swimming behaviour and infestation success are connected to environmental conditions, such as light, current velocity, salinity and temperature. Many factors, among others diurnal vertical movement pattern and the salinity profile, will affect the position of the lice larvae; however, it has been suggested that they mostly remain in the uppermost 4 m of the water surface [17]. The free-swimming larvae are small, between 0.4 and 0.7 mm long [5]. A detailed survey of sea lice ecology is presented by [16].
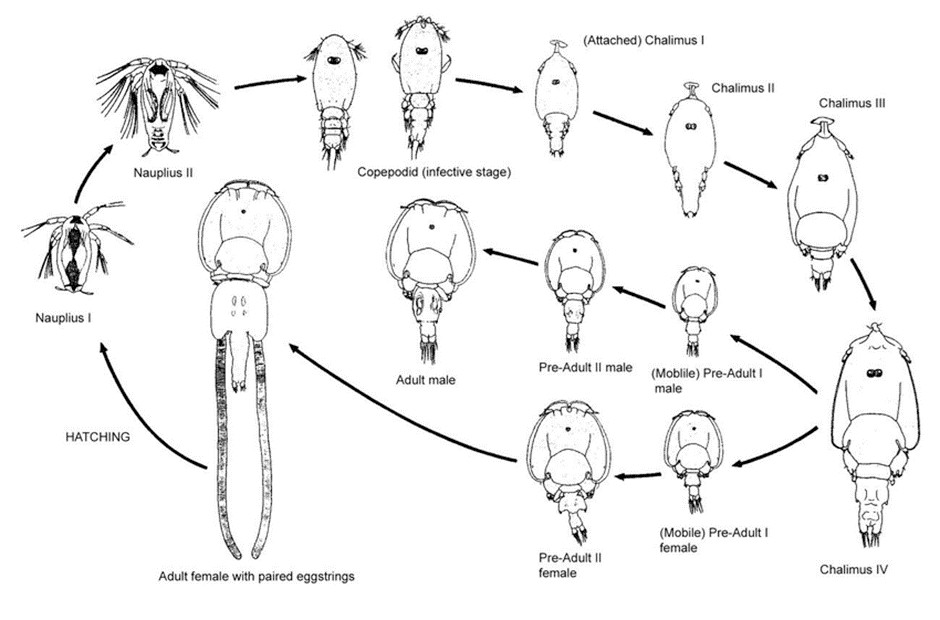
Figure 1: The eight life stages of salmon louse (Lepeophtheirus salmonis) [18].
Lice skirts
Lice skirts are either impermeable or semi-permeable plankton nets. The materials utilised as the barrier can be impermeable membranes (using canvas- or tarpaulin-like material) or semi-permeable to filtering out copepodids [15]. A standard WP-2 plankton net with 200 μm mesh size will collect free-swimming sea lice larvae satisfactorily [19]. As an example, skirts with a mesh size of 350 x 350 µm have been applied at Norwegian cage farms [6]. Skirts have wide application and were estimated used at more than 300 cages annually some years ago [15].

Figure 2: A 10 m deep lice-shielding skirt in a circular 120 m circumference cage (design: Svein B. Tveiten).
Over the years, deeper skirts of more than 6-8 m depth have become more common (Figure 2). The environmental conditions at the production site will largely affect choice of skirt depth and other practises, such as how to mount the skirts, period of use during the production cycle, etc. [6].
Several studies have demonstrated the strong impact of lice skirts on the current pattern and consequently reduced oxygen concentration in shielded cages [e.g. 20, 21]. To control the oxygen concentration in cages, direct injection of diffuser-based oxygen or lifting oxygen-rich deepwater by airlift are applied measures [22].
Prevention effects
The number of lice larvae, nauplii and copepodids, fluctuates strongly on salmon farm sites both seasonally and from one year to another, and direct measurements of densities of the salmon lice larvae in the water masses are difficult to implement [23]. Consequently, estimated effects of lice skirts are mostly based on comparison of lice number on fish, mobile or attached, in cages with and without skirt. Cage farmers often combine different preventive measures to combat sea lice [24], such as lice skirts, submerged lights, functional feed and stocking of ‘cleaner fish’ that further complicate calculation of the skirt effect.
Lice skirt only
Stien et al. [25] studied lice infestation rate and fish welfare at a commercial cage farm with and without 10 m deep skirts from May to September. At the end of the period, the number of lice on the fish was 80% reduced in the skirt cages compared to in standard cages though the reduced infestation rate varied through the period. No compromising effects on fish welfare scores between treatments were found.
Another study was conducted at five commercial farms in Northern Norway mounted 6 and 10 m deep permeable lice skirts [26]. Compared to open control cages, 30% less weekly infestation levels of pre-adult and adult male lice were estimated in the skirt shielded cages (Figure 3). Except for farm B, the effect of lice skirts was significant and the effect increased with elevated lice infestation, especially visible in cage A1 and D. The authors concluded that skirt barriers may reduce the need for direct treatment.
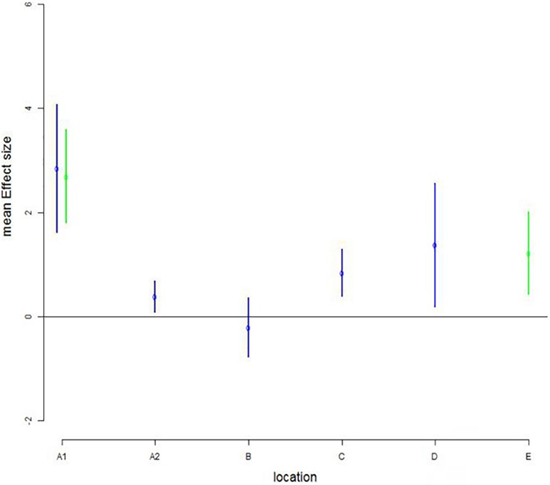
Figure 3: Estimated reduced number of PAAM (chalimus: preadults and adult males) per fish at six salmon cage farms in cages mounted 6 m (blue) and 10 m deep skirts (green) compared to open cages without skirt (horizontal line marked 0).
Grøntvedt et al. [26]. Consent: Randi M. Grøntvedt.
Combined prevention measures
A combined use of submerged feeding and lighting at 8-10 m depth, luring salmon away from the surface layer enriched with lice copepodids, and lice skirt reduced the lice infestation in cages by more than 50% in a fjord locality [26]. During periods with brackish surface water, the skirt barrier was removed.
Four different lice prevention strategies together with multiple treatments were conducted to assess the additive effects in a 12cage farm stocked salmon [1]:
1) Cleaner fish
2) Cleaner fish and functional feed
3) Cleaner fish, functional feed, deep lights and feeding
4) Cleaner fish and functional feed, deep lights/feeding and lice skirts
Though fluctuated seasonal infestation rates, the group with all strategies (4) demonstrated lower infestation rates than the groups with cleaner fish and functional feed (1 & 2). None of the prevention strategies affected the welfare status of salmon.
Other preventive measures, such as manipulation of swimming deep, functional feed, repellents, etc. can be additives to barriers technologies but lice skirts, submersed ‘snorkel’ cages and semiclosed cages provide the greatest protection against sea lice [7].
Conclusions
Use of barriers, especially shielding skirts mounted on floating cages stocked salmon and trout, is a widely applied preventive measure to reduce intrusion of sea lice. To control the lice infestation rate skirts are the most cost-efficient management attempt. In most cases, lice skirt use contributes to significantly less infestation rate and reduces the need for costly and potentially harmful direct treatments.
References
- Bui S, Stien LH, Nilsson J, Trengereid H, Oppedal F (2020) Efficiency and welfare impact of long-term simultaneous in situ management strategies for salmon louse reduction in commercial sea cages. Aquaculture 520:734934.
- Iversen A, Hermansen Ø, Nystøy R, Rolland KH, Garshol LD (2019) Konkurranseevne for norsk oppdrettslaks: Kostnader og kostnadsdrivere i konkurrentland (In English: Competitiveness of Norwegian farmed salmon: Costs and Cost Drivers, In: Competitor Countries). Report 28/2019, Nofima, Norway.
- Forseth T, Barlaup BT, Finstad B, Fiske P, Gjøsæter, H, et al. (2017) The major threats to Atlantic salmon in Norway. ICES J Mar Sci 74:1496-1513.
- Bøhn T, Nilsen R, Gjelland KØ, Biuw M, Sandvik AD, et al. (2022) Salmon louse infestation levels on sea trout can be predicted from a hydrodynamic lice dispersal model. J Appl Ecol 59:704-714.
- Boxaspen K (2006) A review of the biology and genetics of sea lice. ICES J Mar Sci 63:1304-1316.
- Jónsdóttir KE, Ugelvik Misund A, Sunde LM, Bjørgan Schrøder M, Volent Z (2023) Lice shielding skirts through the decade: Efficiency, environmental interactions, and rearing challenges. Rev Aquacult 562:738817.
- Barrett LT, Oppedal F, Robinson N, Dempster T (2020) Prevention not cure: a review of methods to avoid sea lice infestations in salmon aquaculture. Rev Aquacult 12: 2527-2543.
- Aaen SM, Helgesen KO, Bakke MJ, Kaur K, Horsberg TE (2015) Drug resistance in sea lice: a threat to salmonid aquaculture. Trends Parasitol 31:72-81.
- Bechmann RK, Arnberg M, Bamber S, Lyng E, Westerlund S, et al. (2020) Effects of exposing shrimp larvae (Pandalus borealis) to aquaculture pesticides at field relevant concentrations, with and without food limitation. Aquat Toxicol 222: 105453.
- Overton K, Dempster T, Oppedal F, Kristiansen TS,Gismervik K, et al. (2018) Salmon lice treatments and salmon mortality in Norwegian aquaculture: a review. Rev Aquacult 11: 1398-1417.
- Walde CS, Bang Jensen B, Stormoen M, Asche F, Misund B, et al., (2023) The economic impact of decreased mortality and increased growth associated with preventing, replacing or improving current methods for delousing farmed Atlantic salmon in Norway. Prev Vet Med 221:106062.
- Oppedal F, Bui S, Stien LH, Overton K, Demster T (2019) Snorkel technology to reduce sea lice infestations: efficacy depends on salinity at the farm site, but snorkels have minimal effects on salmon production and welfare. Aquac Environ Interact 11:445-457.
- Barrett LT, Overton K, Stien LH, Oppedal F, T. Dempster T (2020) Effect of cleaner fish on sea lice in Norwegian salmon aquaculture: a national scale data analysis. Int J Parasitol 50: 787-796.
- Boerlage AS, Shrestha S, Leinonen I, Dverdal Jansen M, Revie CW, et al. (2024) Sea lice management measures for farmed Atlantic salmon (Salmo salar) in Scotland: Costs and effectiveness. Aquaculture 580:740274.
- Oppedal F, Stien LH, Bui S, Oldham T, Barrett LT (2022) Physical prevention and control of sea lice. In: Ch. 22, Salmon Lice Biology and Control, pp. 493-528.
- Brooker AJ, Skern-Mauritzen R., Bron JE (2018) Production, mortality, and infectivity of planktonic larval sea lice, Lepeophtheirus salmonis (Krøyer, 1837): current knowledge and implications for epidemiological modelling. ICES J Mar Sci 75:1214-1234.
- Costello MJ (2006) Ecology of sea lice parasitic on farmed and wild fish. Trends Parasitol 22:475-483.
- Whelan K (2010) A Review of the Impacts of the Salmon Louse, Lepeophtheirus salmonis (Krøyer, 1837) on Wild Salmonids. Atlantic Salmon Trust.
- Schram TA (2004) Practical identification of pelagic sea lice larvae. J Mar Biol Assoc U.K. 84:103-110.
- Jónsdóttir KE, Volent Z, Alfredsen JA (2021) Current flow and dissolved oxygen in a full-scale stocked fish-cage with and without lice shielding skirts. App Ocean Res 108:102509.
- Stien LH, Nilsson J, Hevrøy EM, Oppedal F, Kristiansen TS, et al. (2012) Skirt around a salmon sea cage to reduce infestation of salmon lice resulted in low oxygen levels. Aquacult Eng 51:21-25.
- Berntsson EVC, Stevik TK, Bergheim A, Persson D, Stormoen M, et al. (2025) Managing the Dissolved Oxygen Balance of Open Atlantic Salmon Sea Cages: A Narrative Review. Rev Aquacult 17: e12992.
- Jevne L, Guttu M, Båtnes AS, Olsen Y, Reitan KI (2021) Planktonic and Parasitic Sea Lice Abundance on Three Commercial Salmon Farms in Norway Throughout a Production Cycle. Front Mar Sci 8:102509.
- Oldham T, Simensen B, Trengereid H, Oppedal F (2023) Environmentally responsive parasite prevention halves salmon louse burden in commercial marine cages. Aquaculture 563:738902.
- Stien LH, Bendiksen Lund M, Oppedal F, Wright DW, Seternes T (2018) Skirts on salmon production cages reduced salmon lice infestations without affecting fish welfare. Aquaculture 490:281-287.
- Grøntvedt RN, Kristoffersen AB, Jansen PA (2018) Reduced exposure of farmed salmon to salmon louse (Lepeophtheirus salmonis L.) infestation by use of plankton nets: Estimating the shielding effect. Aquaculture 495:865-872.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.