Effectiveness and Safety of Onlife® Oral Administration in Chemotherapy-Induced Peripheral Neuropathy (CIPN)
by Juan Diego Rodríguez Castaño*
Hospital La Milagrosa, Madrid, Spain
*Corresponding Author: Juan Diego Rodríguez Castaño, Hospital La Milagrosa, Madrid, Spain
Received Date: 19 December, 2025
Accepted Date: 29 December, 2025
Published Date: 31 December, 2025
Citation: Castaño JDR (2025) Effectiveness and Safety of Onlife® Oral Administration in Chemotherapy-Induced Peripheral Neuropathy (CIPN). J Orthop Res Ther 10: 1412. https://doi.org/10.29011/2575-8241.001412
Abstract
Introduction: Chemotherapy-induced peripheral neuropathy (CIPN) is a common side effect persisting after the chemotherapies and it often results in dose limitation, switch to less efficacious agents or even therapy discontinuation. The present retrospective clinical survey aims to evaluate the clinical experience with Onlife® tablets in adult population with CIPN focusing on its efficacy, safety, and usability within its intended scope. Methods: Improvement of CIPN was evaluated in adult patients, previously treated with chemotherapy drugs, receiving OnLife® for 3 months after completion of chemotherapy. The primary endpoint was to evaluate the severity of peripheral sensory neuropathy (PSN), the peripheral motor neuropathy (PMN) and the autonomic neuropathy before and at the end of OnLife® treatment. The endpoints were evaluated with three different validated questionnaires, EORTC-QLQ-CIPN20 (MNSI-Q), Walking Impact Scale (Walk-12) and Visual Analogue Scale (VAS). Results: 20 patients were enrolled. PSN improved of 37%, Walking Impact Scale (Walk-12) improved of 37% and Visual Analogue Scale (VAS) improved of 42%. Conclusion: Onlife® tablets administration is safety and effective in the treatment of CIPN.
Keywords: OnLife®, Cancer, Peripheral sensory and motor neuropathy, Chemotherapy, Food supplement.
Introduction
CIPN is a common side effect of many antineoplastic drugs and often results in dose limitation, switch to less efficacious agents or even therapy discontinuation. CIPN mainly affects sensory nerves. Therefore, most patients with CIPN experience numbness, tingling, hyperesthesia, loss of vibratory perception, and burning pain. Due to the vulnerability of the long nerves [1].
Antineoplastic drugs that cause CIPN include platinum compounds (e.g., cisplatin and oxaliplatin), ant tubulins (vinca alkaloids [e.g., vincristine], taxanes [e.g., docetaxel and paclitaxel]), proteasome inhibitors (e.g., bortezomib), and immunomodulatory drugs (e.g., lenalidomide) [2,3]. CIPN symptoms occurs during or immediately after chemotherapy and persist from months to years after completion of chemotherapy, negatively impacting the quality of life (QoL) [4]. The extent of CIPN and the degree of recovery depend on how severe the damage to peripheral nerves had been. The symptoms disappear it the damage are not yet relevant, while they become permanent and irreversible in case of extensive damage.
Currently, we have very poor solution to prevent and treat CIPN. In a previous study, was described the efficacy and the safety of an innovative food supplements, Onlife® tablets, in reducing CIPN severity and in limiting the progression of neuropathy, more markedly in paclitaxel-treated patients and also in patients with oxaliplatin-induced CIPN [5]. OnLife® tablet contains F.A.G, magnesium pidolate and lycopene. F.A.G. (Fatty Acid Group) is a specific quali-quantitative mixture of fatty acids developed by Again Life S.r.l.; it is covered by a patent registered in different offices worldwide and it is marketed in Europe and in some nonEuropean countries. The present retrospective clinical survey aims to evaluate the clinical experience with Onlife® tablets in adult population with CIPN focusing on its efficacy, safety, and usability within its intended scope.
Materials and Methods
Settings
The clinical survey has been conducted by a Spanish oncology specialist and it is based on its clinical experience in patients treated with OnLife® tablets. The retrospective observational survey was conducted in accordance with the Standards of Good Clinical Practice of the European Union and the ethical principles expressed in the Declaration of Helsinki. Data were retrospectively collected in the period February 2025 – July 2025 evaluating their clinical manifestations during medical examination. Ethical approval was not necessary according to National Code on Clinical Trials declaration because this data derives from a real-life retrospective study [6].
Patients
The target population was composed by 20 patients, 19 female and 1 male, with a mean age of 57 years. 9 patients had breast cancer, 4 patients had ovary cancer, 3 patients had endometrium cancer, 2 patients had colon-rectal cancer, 2 patients had cervix cancer. The inclusion criteria were: a) diagnosis of cancer, specifically colorectal, breast, ovarian, uterine, and endometrial cancer b) chemotherapy cycle completed by the enrolment date, c) presence of peripheral sensory neuropathy (PSN) or/and the peripheral motor neuropathy (PMN) or/and the autonomic neuropathy. The exclusion criteria were: a) presence of neuropathies before the start of the chemotherapy, b) presence of intolerance to the ingredients of OnLife®, c) pregnant or lactating women.
Treatment and Evaluated Outcomes
Each patient was treated for 3 months with OnLife® Tablets. The oral administration was daily according to the method of use and posology indicated on the package leaflet. The patient’s clinical condition was assessed with three different validated questionnaires: EORTC-QLQ-CIPN20 (MNSI-Q), Walking Impact Scale (Walk-12) and Visual Analogue Scale (VAS). The questionnaires were assessed at the date of enrolment and at the follow-up visit after 3 months. The patient did not take any other drugs.
Results
Between February 2025- July 2025, 20 patients were assessed. All the patients completed the 3-month treatment period.
EORTC-QLQ-CIPN20
EORTC-QLQ-CIPN20 is a questioner developed to assess the impact of chemotherapy-induced peripheral neuropathy on the QoL (quality of life) of cancer patients. This questionnaire contains 20 items on which patients rate their experience for each symptom during the previous week using scores from 1 (not at all) to 4 (very much). The sum score was obtained by adding the scores of items 1 to 19 resulting in a sum score. The 20 items are divided into three subscales that encompass specific sensory, motor, and autonomic symptoms associated with chemotherapy-induced peripheral neuropathy [7]. As reported in figure 1, at T0 patients showed a mean score for EORTC-QLQ-CIPN20 questionnaire of about 50, after three months of administration of Onlife® tablet a great reduction of about 37% was obtained with an improvement of the symptomatology such as difficulty distinguishing hot from cold water, difficulty walking because his feet couldn’t support him, headache when he stood up, numb hands and feet etc.
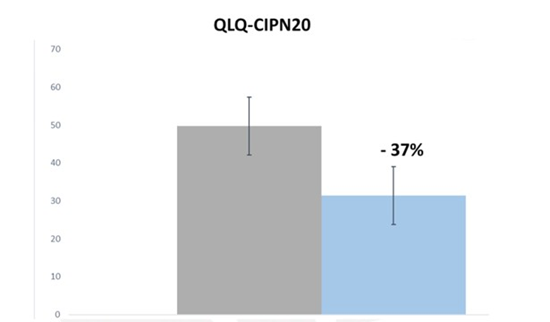
Figure 1: QLQ-CIPN20 scale score at T0 (Grey) and after three months of treatment with online® tablet (Light Blue). Data are expressed as mean +/- standard deviation.
Walking Impact Scale (Walk-12)
The Walk-12 comprises 12 items that ask about a person’s selfperceived walking limitations in activities related to walking, running, climbing stairs, standing, walking distance and effort, need for support indoors and outdoors, gait quality aspects, and concentration when walking. The scale has five response options: 1 = not at all, 2 = a little, 3 = moderately, 4 = quite a bit, and 5 = extremely. In clinical practice, the response options from each item are summed into a total score ranging from 12 to 60 points [8,9]. As reported in figure 2, at T0 patients showed a mean score for The Walk-12 of about 40, after three months of administration of Onlife® tablet a great reduction of about 37% was obtained, also considering specific activity related to motion such as limited ability to run, walk, go up or down stairs etc.
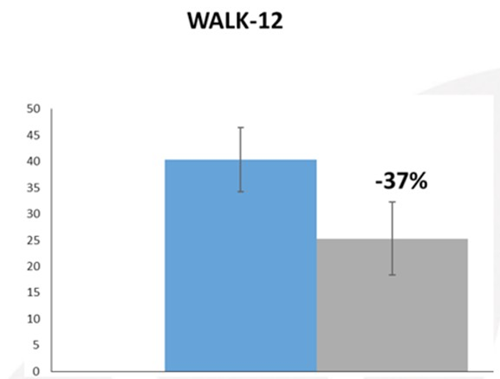
Figure 2: Walk-12 scale at T0 (Grey) and after three months of Treatment with Onlife® tablet (light blue).
Data expressed as mean of +/- standard deviation.
VAS Scale
The VAS scale is a 10-item questionnaire, with scores ranging from 1 to 5 depending on severity; 1 is the lowest score, 5 is the highest. The range score is 10-50. The items evaluate also autonomic parameters, like appetite, vomiting, constipation, abdominal swelling, urinary incontinence. As reported in figure 3, at T0 patients showed a mean score for the VAS SCALE of about 35, after three months of administration of Onlife® tablet a great reduction of about 42% was obtained.
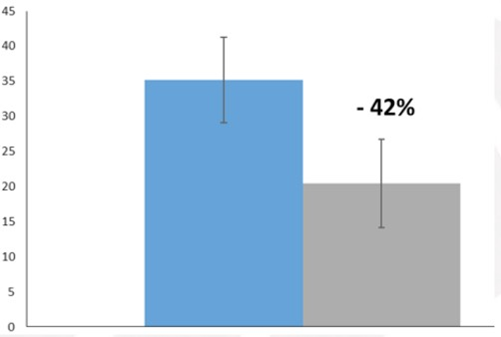
Figure 3: VAS Scale score at T0 (grey) and after three months of treatment with Onlife® tablet (light blue).
Data are expressed as mean +/- standard deviation.
Discussion
CIPN is a common side effect of many antineoplastic agents and may last from months to years after chemotherapy completion, substantially affecting patients’ QoL [10]. Many typologies of treatments, pharmaceutical therapies and nonpharmaceutical treatments, have been used to manage CIPN, but evidence of their clinical benefit has not been clearly determined, except for duloxetine. The observational study STEFANO assessed the effectiveness of OnLife® in reducing the severity of CIPN in breast cancer patients with paclitaxel-induced PN and in colon cancer patients with oxaliplatin-induced PN [10].
The results of this clinical survey indicate that the treatment with OnLife® tablets for 3 months, starting soon after completion of the chemotherapy, can be of help in reducing or stabilizing CIPN symptoms.
PSN, PMN and Autonomic Neuropathy were significantly improved. PSN was evaluated with the EORTC-QLQ-CIPN20, PMN was evaluated with the Walking Impact Scale (WALK-12), Autonomic Neuropathy was evaluated with the VAS SCALE. PSN was reduced of about 37%, PMN was reduced of about 37% and Autonomic Neuropathy was reduced of about 42%. No side effects were collected, and all the patients concluded the clinical survey.
The findings of this clinical survey suggest that OnLife®, which consists F.A.G, magnesium pidolate and lycopene is an interesting product to treat the manifestation of CIPN. Furthermore, our data indicate a very high tolerability of the product, which is an appreciable aspect to consider in patients who have just completed chemotherapy, which is usually not free from heavy side effects.
Conclusion
Onlife® tablets administration is safety and effective in the treatment of CIPN. More randomized and placebo-controlled clinical trials are necessary to confirm OnLife® tablets as a novel therapeutic tool for the control of the symptomatology related to peripheral, motor and autonomic neuropathy in patients treated with chemotherapy.
Conflict of Interest
We declare that Juan Diego Rodríguez Castaño received an honorarium from Neilos.
References
- Zajączkowska R, Kocot-Kępska M, Leppert W, Wrzosek A, Mika J, et al. (2019) Mechanisms of chemotherapy-induced peripheral neuropathy. Int J Mol Sci 20(6): E1451.
- Han Y, Smith MT (2013) Pathobiology of cancer chemotherapyinduced peripheral neuropa thy (CIPN). Front Pharmacol 4: 156.
- Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, et al. (2013) Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin 63(6): 419-437.
- Da Costa R, Passos GF, Quintão NLM, Fer nandes ES, Maia JR, et al. (2020) Taxane-induced neurotoxicity: pathophysiology and therapeutic perspectives. Br J Pharmacol 177(14): 3127-3134.
- Zaiss M, Uhlig J, Zahn MO, Decker T, Lehmann HC, et al. (2021) Improving Chemotherapy-Induced Peripheral Neuropathy in Patients with Breast or Colon Cancer after End of (Neo) adjuvant Therapy: Results from the Observational Study STEFANO. Oncol Res Treat 44(11): 613-621.
- Kıraç FS (2013) Is ethics approval necessary for all trials? A clear but not certain process. Mol Imaging Radionucl Ther 22(2013): 73-75.
- Jennifer Le-Rademacher (2017) Patient-Reported (EORTC QLQCIPN20) Versus Physician-Reported (CTCAE) Quantification of Oxaliplatin- and Paclitaxel/Carboplatin-Induced Peripheral Neuropathy in NCCTG/Alliance Clinical Trials. 25(11): 3537-3544.
- Brogardh C, Flansbjer UB, Espelund C, Lexell J (2013) Relationshipbetween self-reported walking ability and objectively assessed gaitperformance in persons with late effects of polio. Neuro Rehabil 33(1): 127-132.
- Brogardh C, Lexell J (2015) How various self-reported impairments influ-ence walking ability in persons with late effects of polio. NeuroRehabil 37(2): 291-298.
- Kerckhove N, Collin A, Condé S, Chaleteix C, Pezet D, et al. (2017) Long-term effects, pathophysiological mechanisms, and risk fac tors of chemotherapy-induced peripheral neuropathies: a comprehensive literature re view. Front Pharmacol 8: 86.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.