Dual-Antiplatelet Therapy vs Single Antiplatelet Therapy after Coronary Artery Bypass Grafting : Meta-Analysis of Randomized Controlled Trails
by Mefin Mathew Jose*, Anisha Ann Joseph*, Andrew Jacob, Milan Mathew, Albin Abraham
Aberdeen Royal Infirmary, Forester hill Health Campus, NHS Grampian Aberdeen, AB25 2ZN, UK
*First author: Mefin Mathew Jose, Aberdeen Royal Infirmary,Forester hill Health Campus, NHS GrampianAberdeen. AB25 2ZN, UK
*Co-author: Anisha Ann Joseph, Aberdeen Royal Infirmary,Forester hill Health Campus, NHS GrampianAberdeen. AB25 2ZN, UK
Received Date: 14 April 2024
Accepted Date: 18 April 2024
Published Date: 20 April 2024
Citation: Jose MM , Joseph AA, Jacob A , Mathew M, Abraham A (2024) Dual-Antiplatelet Therapy vs Single Antiplatelet Therapy after Coronary Artery Bypass Grafting : Meta-Analysis of Randomized Controlled Trails: A Retrospective Cohort Study. J Surg 9: 11017 https://doi.org/10.29011/2575-9760.11039
Abstract
Introduction: Every year, around 400,000 patients in the United States and Europe undergo CABG surgery. The risks and benefits of antiplatelet regimens in these patients have been studied in various trials. In patients with a stable clinical state, aspirin monotherapy is the recommended standard of care. The present systematic literature review and meta-analysis is aimed to compare the efficacy between Dual Antiplatelet Therapy (DAPT) and Single Antiplatelet Therapy (SAPT) after CABG.
Method:We searched PubMed/Medline, EMBASE and Cochrane Central Register of Controlled Trials (CENTRAL) for relevant published Randomized Controlled Trials (RCTs). In total, 2,152 studies from PubMed, Science Direct and Scholar were obtained and screened for the relevance of our topic for the last 10 years (2014 to 2024), in which 18 studies passed after the inclusion and exclusion criteria using PRISMA.
Result: We included 18 randomized controlled trial studying 218,125 patients. DAPT was associated with significantly lower rates of Cardiovascular mortality (OR 0.71, 95% CI 0.54, 0.93, p=0.01), Major Adverse Cardiovascular And Cerebrovascular Events (MACCE) (OR 0.68, 95% CI 0.61, 0.76, p=0.0001), and Myocardial Infarction (OR 0.78, 95% CI 0.64, 0.94, p=0.009). However, there was no significant association with graft occlusion (OR 0.86, 95% CI 0.68, 1.10, p=0.23), stroke (OR 0.82, 95% CI 0.63, 1.45, p=0.82), All-cause Death (OR 1.06, 95% CI 0.61, 1.84, p=0.84) and major bleeding (OR 1.21, 95% CI 0.81, 1.81, p=0.0001).
Conclusion: In conclusion, there were no significant differences in the incidence of graft occlusion, all-cause death, stroke, or serious bleeding between Dual Antiplatelet Therapy (DAPT) and Single Antiplatelet Therapy (SAPT). The p-values exceeded the 5% significance level. However, there were significant differences in the outcomes of cardiovascular mortality, MACCE, and myocardial infarction, with p-values showing statistical significance at a level of less than 5%.
Keywords: Aspirin; Antiplatelet; Coronary Artery Bypass Grafting; Cardiovascular myocardial infarction; Coronary artery bypass graft surgery; Dual antiplatelet therapy; Graft occlusionclopidogrel; Graft occlusion; Major bleeding; Mortality; Prasugrel; Ticagrelor; Ticagrelor ; Stroke
Introduction
Coronary Artery Bypass Grafting (CABG) has been proven to be an efficacious intervention for individuals suffering from severe coronary artery disease [1]. For patients with CABG, aspirin is advised as a basic secondary preventive drug to preserve the advantages of revascularization and avoid significant adverse cardiovascular events [2]. Nevertheless, individuals who undergo coronary artery bypass grafting (CABG) still face a significant likelihood of experiencing serious ischemic cardiac and cerebrovascular events following the surgery. This risk can surpass 10% within the initial 6 to 12 months post-operation [3]. The diminished postoperative sensitivity to aspirin, along with increased platelet activation and blood clot formation, leads to a state of systemic hypercoagulability and early graft failure. These elements have been recognized as crucial contributors in this specific circumstance [4,5]. Each year, about 400,000 individuals in the United States and Europe receive coronary artery bypass grafting (CABG). The risks and benefits of antiplatelet regimens in these patients have been the focus of numerous trials [6]. It is recommended to use aspirin alone as the usual treatment for patients who are in a stable clinical condition. In the past ten years, multiple trials have examined the possible benefits of dual antiplatelet treatment (DAPT) compared to using only aspirin in terms of effectiveness and safety outcomes [7,8]. A meta-analysis conducted by [9] indicated that patients who present with ACS (Acute Coronary Syndrome) may experience favorable outcomes from Dual Antiplatelet Therapy (DAPT) following Coronary Artery Bypass Grafting (CABG). This data aligns with previous research on patients with Acute Coronary Syndrome (ACS) who received medical treatment with Dual Antiplatelet Therapy (DAPT), specifically clopidogrel in combination with aspirin [10]. Additional investigations explicitly examined the effectiveness of dual Antiplatelet Therapy (DAPT) compared to aspirin in maintaining the openness of bypass grafts following Coronary Artery Bypass Grafting (CABG). These trials found that DAPT resulted in higher rates of bypass graft patency, as reported by [11,12].
Dual Antiplatelet Therapy (DAPT) using aspirin and a P2Y12 receptor antagonist (such as clopidogrel or ticagrelor) has been shown to enhance the antiplatelet effect. This therapy has been reported to slow down the progression of native coronary stenosis and improve graft patency in patients who have undergone Coronary Artery Bypass Grafting (CABG). Additionally, DAPT can help prevent recurrent strokes in patients with ischemic cerebrovascular disease. Nevertheless, the inquiry into whether the advantages linked to DAPT, particularly the potential enhancement in graft patency, result in superior clinical outcomes has not been thoroughly examined and has yielded inconsistent findings [13-15]. Administering aspirin within 48 hours after Coronary Artery Bypass Grafting (CABG) significantly decreased the risk of death, Myocardial Infarction (MI), and stroke during the patient’s hospital stay (Mangano et al., 2002). These findings led to other trials involving the use of Dual Antiplatelet Treatment (DAPT) with clopidogrel and aspirin. However, the results regarding clinical outcome and graft patency were contradictory. Ticagrelor bypasses the need for the two-step CYP-dependent metabolism that hindered clopidogrel’s effectiveness. The PLATO research demonstrated that ticagrelor is more effective than clopidogrel in lowering cardiovascular events [16]. People who had Coronary Artery Bypass Grafting (CABG) and then started taking the study drug again after the surgery had a lower risk of cardiovascular death and overall mortality when they were treated with ticagrelor [17]. Historically, aspirin has consistently been the preferred medication for preventing graft occlusion and severe cardiac events during coronary artery bypass grafting [2]. The Clopidogrel vs. Aspirin in Patients at Risk of Ischemic Events trial [18] showed that clopidogrel alone was more effective than aspirin alone in lowering the occurrence of ischemic stroke after CABG surgery. It is thought that adding a P2Y12 inhibitor will help keep the graft open and lower the risk of bad cardiac events by stopping the platelet-driven progression of graft disease. However, the effectiveness of Dual Antiplatelet Therapy (DAPT) in preserving graft openness and reducing negative cardiac events is not clearly demonstrated [2,19,20]. Hence, this present study compare the effectiveness of Dual Antiplatelet Therapy (DAPT) and Single Antiplatelet Therapy (SAPT) with Aspirin after CABG.
Research Methodology
This review will adhere to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses standards. There were several stages to the method. Prior to developing the inclusion and exclusion criteria, the methodology was developed. Then, using the criteria of the abstract, title, and full text, we searched databases for research. Data extraction was followed by a synthesis of the earlier literature. A flowchart showing the stages is shown in Figure 1.
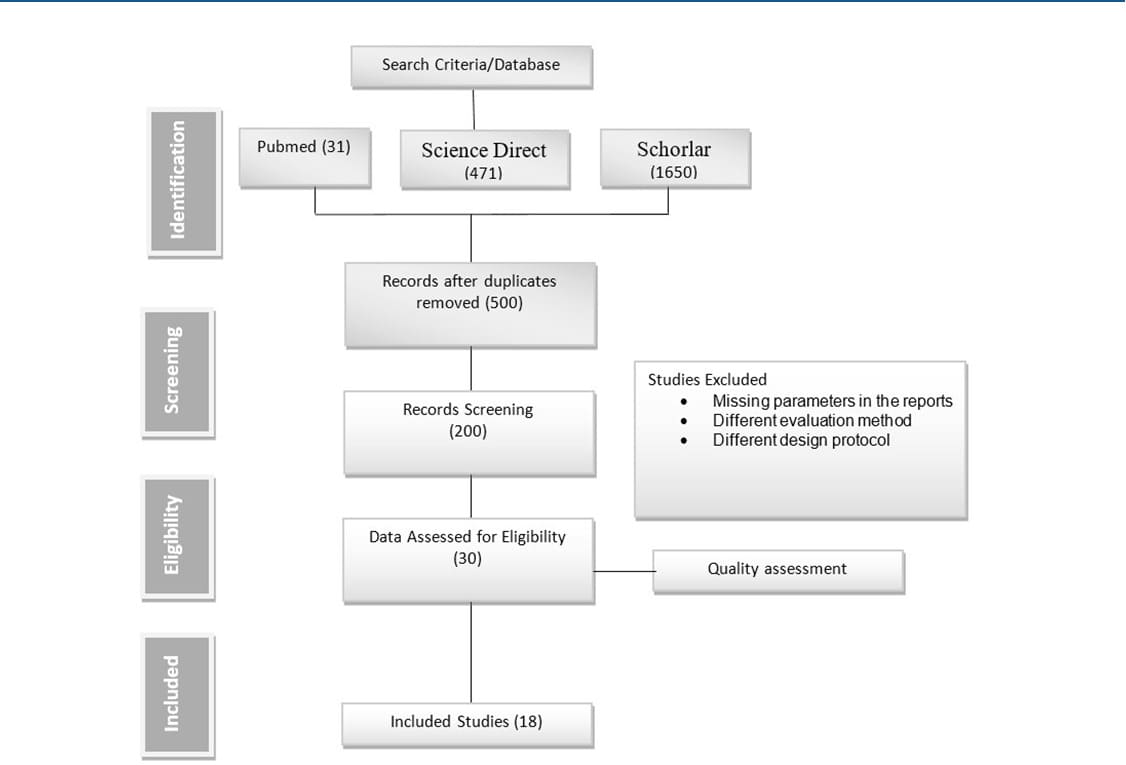
Figure 1: PRISMA Flow Chart
Research Protocol
The protocol was created in accordance with the Cochrane Handbook for Systematic Review and it established the primary research questions that directed the selection of papers to search for, the data sources to use in the search, the inclusion and exclusion criteria, and the outcomes. The study questions served as the foundation for this evaluation, and they were used as a guide to finding high-quality papers and resources across several databases.
Inclusion and Exclusion Criteria
In view of the present study, only studies that addressed the research topic Monotherapy (Aspirin) Versus Dual Antiplatelet Therapy (Prasugrel vs Ticagrelor) Following Coronary Artery Bypass Grafting were taken into consideration. The inclusion papers were randomized control design studies, studies published in the last 10 years (2014-2024) and only the studies written in English were taken into consideration in order to provide the most valid literature review possible. Systematic literature review papers, studies, and conference papers are excluded criteria.
Research Question
The research question was developed using PECOS (Participant, Exposure, Comparator, Outcome and Study Design) framework (Table 1). This framework is used to inform the search string and the entire search strategy.
Table 1: PESCOS framework for research question |
Using the above framework, this review aim to answer one research questions we trying to answer from this research was : “Is Single Antiplatelet Therapy (SAPT) better in comparison to dual antiplatelet therapy after CABG in preventing graft occlusion aswell as other secondary prevention”.
Data Source and Search strategy
Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA), we use PubMed, Science Direct and EMBASE and Cochrane Central Register of Controlled Trials (CENTRAL) for relevant published randomized controlled trials (RCTs) to get the studies that were used for this systematic review from year 2014 to 2024 following the inclusion and exclusion criteria earlier mentioned. The following precise keywords were used in the literature search: (“single Antiplatelet Therapy” OR “Aspirin Monotherapy”) AND (“Dual Antiplatelet Therapy” OR Prasugrel OR Ticagrelor) AND (“Coronary Artery Bypass Grafting” OR “heart bypass surgery”)). Table 2
|
Database |
Number of study |
|
Science Direct |
471 |
|
Google Shorlar |
1650 |
|
PubMed |
31 |
|
Total |
2152 |
Table 2: Number of hit generated from database
Quality Assessment or Risk of Bias
Considering the nature of this research, quality assessment of the eligible studies was performed using Cochrane risk of bias assessment via review manager (RevMan 5.4). Each of the study was appraised based on the CASP checklist for qualitative studies (Table 3). The appraisal was basically “Low, Moderate and High” each study was asses. The checklist criteria of assessment were 7 questions. The risk of bias is termed low if the study has 70% and above assessment score, moderate if it have within 50% to 70% score and high if it has below 50%.
|
Question ID |
Appraisal Questions |
|
Q1 |
Random sequence generation |
|
Q2 |
Allocation concealment |
|
Q3 |
Blinding of participants and personnel |
|
Q4 |
Blinding of outcome assessment |
|
Q5 |
Incomplete outcome data |
|
Q6 |
Selective reporting |
|
Q7 |
Other bias |
Table 3: Quality Assessment Questions
Study Selection
The exclusion criteria for the selected studies were reported as case reports, review articles, case series and articles in that is not written in English language were excluded. Full text studies on the research topic were included. Randomized controlled trials were the study types that were taken into consideration. Each article’s title and abstract were examined for inclusion and exclusion criteria.
Data Extraction and Quality Assessment
A pre-defined sheet was used to collect the following information from the included studies: research design, author’s first name, year of publication, country, study design, all-cause mortality, cardiovascular mortality, Major Adverse Cardiac And Cerebrovascular Events(MACCE), myocardial infarction, stroke, or major bleeding and graft occlusion. the prisma flowchart was then used to display the final list of studies that will be included. after the data were extracted, the risk bias range was divided into three categories: low, high, and uncertain.
Data Extraction and Analysis
The data were transferred to Excel, where RevMan was used to analyze the features of the chosen studies and check for the presence of heterogeneity using the inconsistency index. The numerical data extracted from the included studies were analyzed using number, event, and total. To analyse the primary and secondary outcomes, we adopted a random effect meta-analysis with a odd ratio OR that measured the effect size. The results was visualized using a forest plot, the plot shows the overall OR for each studies. The heterogeneity across each studies was measured using (I2). A 95% Confidence level and 5% level of significance was chosen for decision making. The null hypothesis of no significant difference between the two groups would be rejected if the estimated p – value is less than the significance level. The publication bias was assessed using funnel plot. The criteria was that, if all studies were within the funnel, and the funnel is symmetric, there is no evidence of publication bias across the included studies.
Results
Study selection and Screening process
After the keywords were entered into the aforementioned database, a total of 2,152 publications were retrieved from it. The 2,152 papers were first entered into the reference manager Endnote for review. We read through the titles and abstracts of the studies during the screening stage, eliminating any duplicates and studies that did not pertain to the aim of our study. Studies that were concentrated on a different intervention were also disregarded. 500 publications remained after the identification and screening phases; these 30 papers underwent full-text screening before being assessed for methodology and data analysis, both of which required to be done in accordance with the current study’s scope.
There were 18 studies left after the eligibility procedure.
Quality Assessment Result
In total, 18 studies were included, out of which 18 studies is at low risk bias under the random sequence generation. 17 studies were of low-risk for the allocation concealment and 1 study is unclear for the allocation of concealment. 15 studies are of lowrisk for blinding of participants, 2 studies is at high risk and a study is unclear. 15 studies blinding of outcome assessment were at lowrisk and 3 studies are unclear. 13 studies are of incomplete outcome data are of low-risk, 4 study are unclear and a study is at high risk. 14 studies were of low risk of selective reporting and 4 studies are unclear of selecting reporting. Lastly, other bias associated with 17 studies are of low risk while a study associated with other bias is unclear as shown in Figure 2. The percentage of the grading is shown in Figure 3.
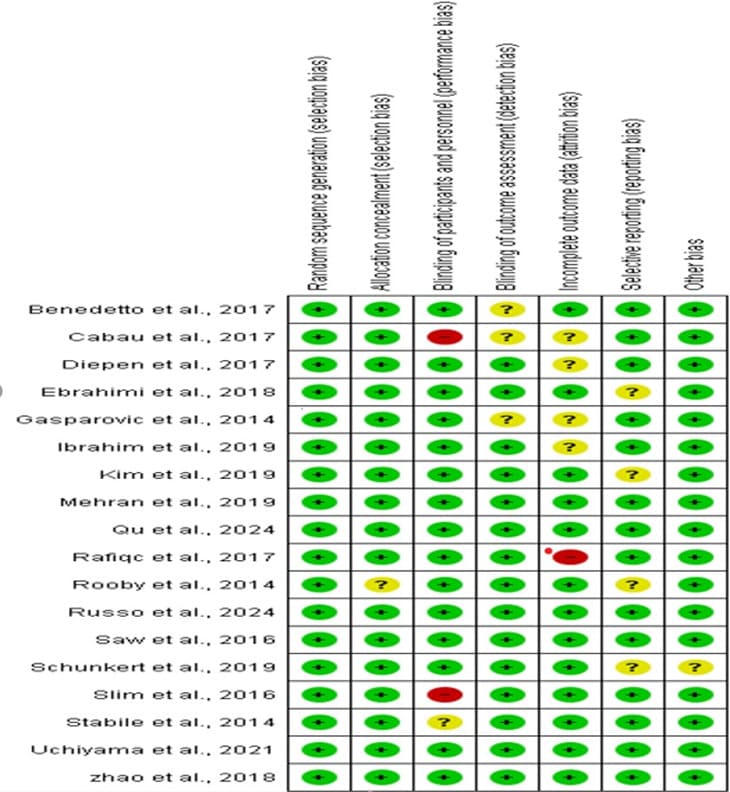
Figure 2: The quality assessment summary of the included studie
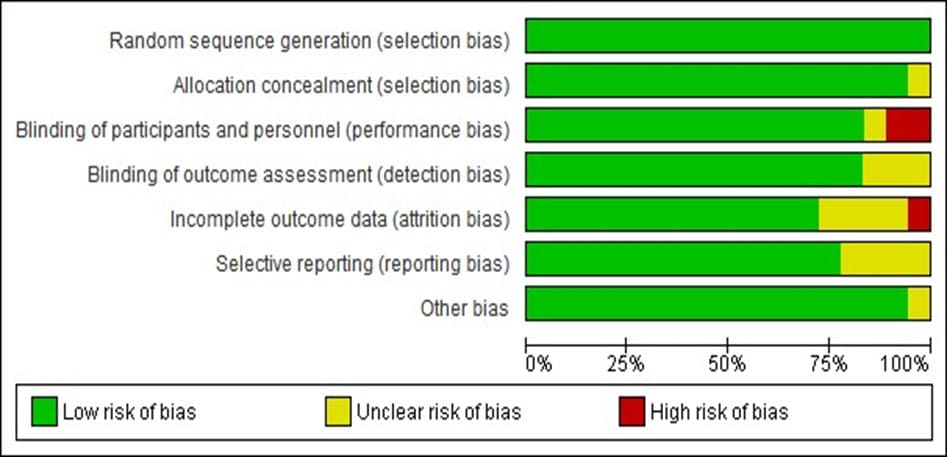
Figure 3: Percentage of the quality assessment level of the included studies
Distribution of studies with respect to year of publication
Figure 4 display the publication year of the included studies, 4 articles of the included studies was published in the year 2017 and 2019 respectively, 3 articles published in year 2014, 2 articles published each in year 2016, 2018 and 2024 respectively while a study was published in year 2021.
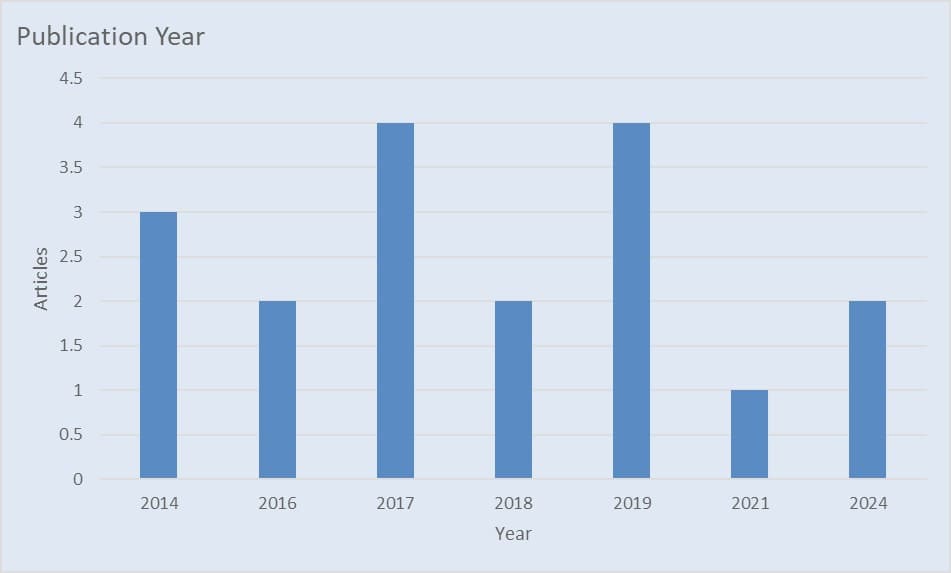
Figure 4: Publication year
Meta Analysis
Data evaluation and analysis
RevMan 5.4 was used to conduct the meta-analysis. We adopted a more cautious approach and used a random-effects model because it was unknown whether there was a “true” effect size underlying all studies. The standard mean difference as well as its associated 95% Confidence Intervals (CLs) was used to estimate the effect size. The degree of heterogeneity was evaluated using the I2 test.
Data Extraction and Analysis
The data was extracted to excel and Review Manager was used to analyze the selected studies characteristics and the intervention measure also test for the heterogeneity’s presence using the inconsistency index. Also was included in the forest plot is the chi statistic and the inconsistency index I2 statistics to access if the studies were heterogeneous. An indication of I2 that was less than fifty percent indicated low heterogeneity. A value between fifty and seventy-five was a sign of mild heterogeneity and above seventy-five showed a high level of heterogeneity. Therefore, the researcher had high confidence on whether to generalize the studies to a larger sample depending on the results.Table 4, present the pooled analyses comparing DAPT with SAPT with 121004 and 97121 patients, respectively, in 18 studies. DAPT, compared with SAPT, was associated with significantly lower rates of Cardiovascular mortality (OR 0.71, 95% CI 0.54, 0.93, p=0.01), MACCE (OR 0.68, 95% CI 0.61, 0.76, p=0.0001) and Myocardial infarction (OR 0.78, 95% CI 0.64, 0.94, p=0.009). While there is no association with lower rates of graft occlusion (OR 0.86, 95% CI 0.68, 1.10, p=0.23), stroke (OR 0.82, 95% CI 0.63, 1.45, p=0.82), but high rates of All cause of death (OR 1.06, 95% CI 0.61, 1.84, p=0.84) and major bleeding (OR 1.21, 95% CI 0.81, 1.81, p=0.0001). In summary there were no significant difference in the outcomes of graft occlusion, all cause of death, stroke and major bleeding with p values greater than 5% significance level while there is significant differences in the outcome of cardiovascular mortality, MACCE and myocardial infarction with p values lesser than 5% significance level.
|
Outcome or Subgroup |
Studies |
DAPT |
DAPT rate (%) |
SAPT |
SAPT rate (%) |
Pooled OR (M-H, 95% CI) |
P value |
Heterogeneous test p vale |
I2 statsistics (%) |
|
Graft occlusion |
7 |
13461 |
5.267 |
11124 |
7.992 |
0.86 [0.68, 1.10] |
0.23 |
0.09 |
46 |
|
All cause of Dealth |
13 |
18973 |
4.87 |
17225 |
3.64 |
1.06 [0.61, 1.84] |
0.84 |
0 |
90 |
|
Cardiovascular mortality |
8 |
16278 |
3.79 |
12603 |
2.785 |
0.71 [0.54, 0.93] |
0.01** |
0.59 |
0 |
|
MACCE |
4 |
14209 |
7.995 |
8856 |
8.006 |
0.68 [0.61, 0.76] |
0.001** |
0.46 |
0 |
|
Myocardial infarction |
14 |
22529 |
4.385 |
17736 |
3.822 |
0.78 [0.64, 0.94] |
0.009** |
0.18 |
26 |
|
Stroke |
8 |
18451 |
6.216 |
14741 |
6.329 |
0.95 [0.63, 1.45] |
0.82 |
1E-05 |
78 |
|
Major bleeding |
10 |
17103 |
4.303 |
14836 |
3.013 |
1.21 [0.81, 1.81] |
0.35 |
0.003 |
65 |
Table 4: Pooled Analysis results for DAPT against SAP.
P values <0.05 are asterik. DAPT, dual antiplatelet therapy; MACCE, major adverse cardiovascular and cerebrovascular events; SAPT, single antiplatelet therapy
Figure 5: Forest plot showing the Graft occlusion of DAPT and SAPT
The statistics estimated is not statistically significant with overall odd ratio of 0.86, [95% CI: 0.68, 1.10] when p- value (0.23) is greater than the level of significant (0.05) which implies that there is no significant difference between DAPT and SAPT for across all studies. But a significant lower event rates was found. The heterogeneity, I^ = 46% across the studies is moderate with a insignificant value (p = 0.09) indicating that the heterogeneity is not statistically significant, which makes the graft occlusion of DAPT and SAPT to be consistent across the different studies with insignificant variability.
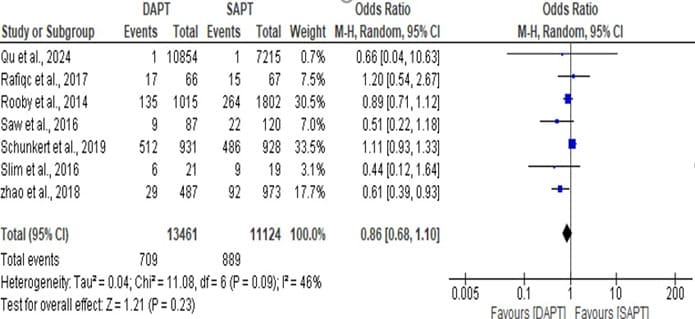
Figure 5: Forest plot showing the Graft occlusion of DAPT and SAPT.
Figure 6: Forest plot showing the all cause of death of DAPT and SAPT.
The statistics estimated is statistically not significant with overall odd ratio of 1.06, [95% CI: 0.61, 1.84] when p- value (0.84) is greater than the level of significant (0.05) which implies that the overall effect has no significant impact. But a significant high event rates was found. The heterogeneity, I^ = 90% across the studies is high with a significant value (p < 0.00001) which makes the all cause of death of the DAPT and SAPT to be inconsistent across the different studies.
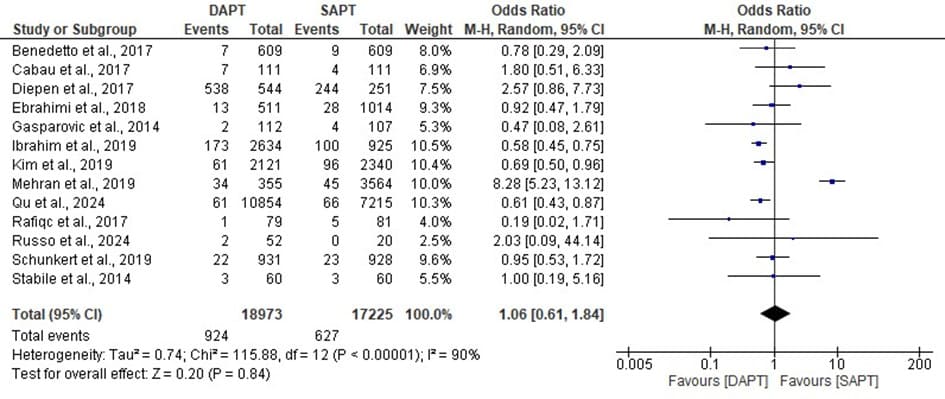
Figure 6: Forest plot showing the all cause of death of DAPT and SAPT.
Figure 7: Forest plot showing the Cardiovascular mortality of DAPT and SAPT
The statistics estimated is statistically significant with overall odd ratio of 0.71, [95% CI: 0.54, 0.93] when p- value (0.01) is less than the level of significant (0.05) which implies that the overall effect has a significant impact and a significant low event rates was found. The heterogeneity, I^ = 0% across the studies is very low with a insignificant value (p 0.59) which makes the Cardiovascular mortality of the DAPT and SAPT to be consistent across the different studies.
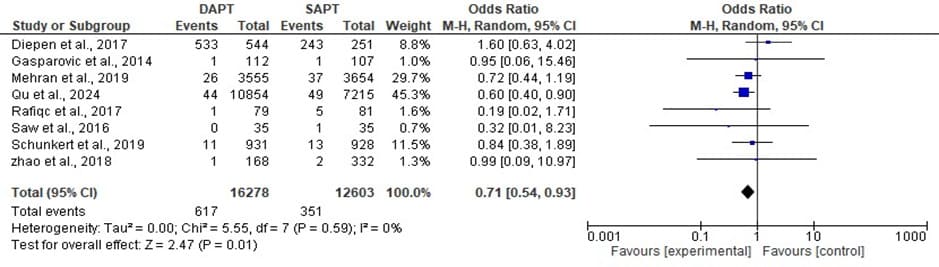
Figure 7: Forest plot showing the Cardiovascular mortality of DAPT and SAPT
Figure 8: Forest plot showing the MACCE of DAPT and SAPT
The statistics estimated is statistically significant with overall odd ratio of 0.68, [95% CI: 0.61, 0.76] when p- value (0.00001) is less than the level of significant (0.05) which implies that the overall effect has a significant impact and a significant low event rates was found. The heterogeneity, I^ = 0% across the studies is very low with a insignificant value (p 0.46) which makes the MACCE of the DAPT and SAPT to be consistent across the different studies.
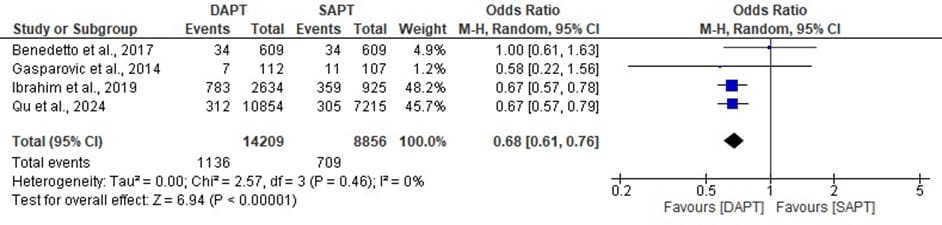
Figure 8: Forest plot showing the MACCE of DAPT and SAPT
Figure 9: Forest plot showing the MI of DAPT and SAPT.
The statistics estimated is statistically significant with overall odd ratio of 0.78, [95% CI: 0.64, 0.94] when p- value (0.009) is less than the level of significant (0.05) which implies that the overall effect has a significant impact and a significant low event rates was found. The heterogeneity, I^ = 26% across the studies is low with a insignificant value (p 0.18) which makes the MI of the DAPT and SAPT to be consistent across the different studies.
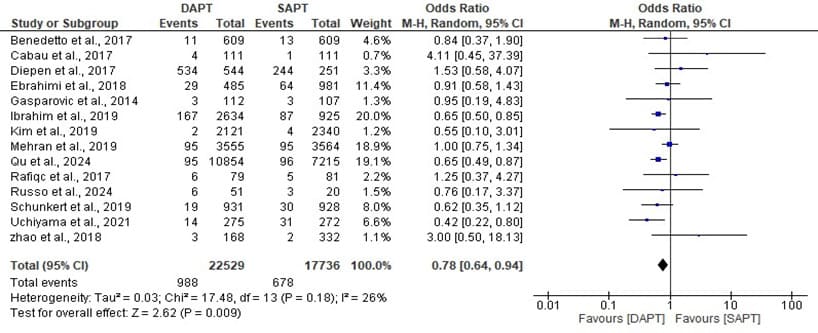
Figure 9: Forest plot showing the MI of DAPT and SAPT.
Figure 10: Forest plot showing the stroke of DAPT and SAPT.
The statistics estimated is statistically not significant with overall odd ratio of 0.95, [95% CI: 0.63, 1.45] when p- value (0.82) is less than the level of significant (0.05) which implies that the overall effect has a significant impact and a significant low event rates was found. The heterogeneity, I^ = 78% across the studies is high with a significant value (p <000001) which makes the stroke of the DAPT and SAPT to be inconsistent across the different studies
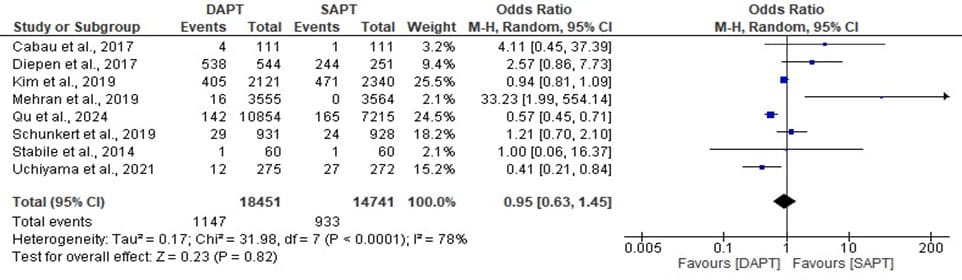
Figure 10: Forest plot showing the stroke of DAPT and SAPT.
Figure 11: Forest plot showing the bleeding of DAPT and SAPT.
The statistics estimated is statistically not significant with overall odd ratio of 1.21, [95% CI: 0.81, 1.81] when p- value (0.35) is greater than the level of significant (0.05) which implies that the overall effect has a significant impact and a significant low event rates was found. The heterogeneity, I^ = 65% across the studies is moderate with a significant value (p 0.003) which makes the bleeding of the DAPT and SAPT to be inconsistent across the different studies bleeding.
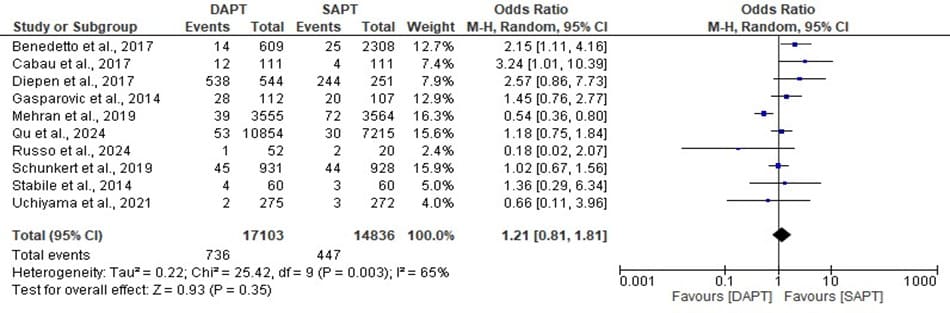
Figure 11: Forest plot showing the bleeding of DAPT and SAPT.
Funnel Plot
For all research, funnel plots are commonly used to identify publication bias; visual inspection of a funnel plot was done on all comparisons for the papers included in the meta-analysis of graft occlusion forest plot in Figure 12,13 below. The two funnel plot for the DAPT associated with autistic people revealed a symmetrical funnel with outliers, although this implies true heterogeneity rather than publication bias.Base on this, we can conclude none of the studies could be considered to be overly influential.
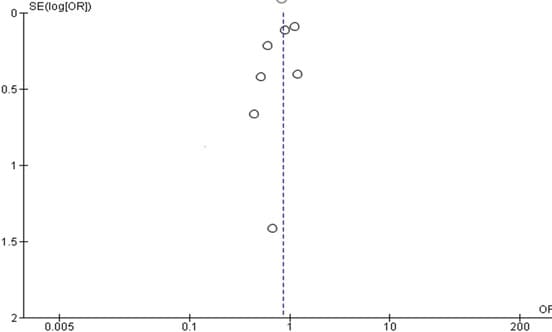
Figure 12: Graft occlusion funnel plot
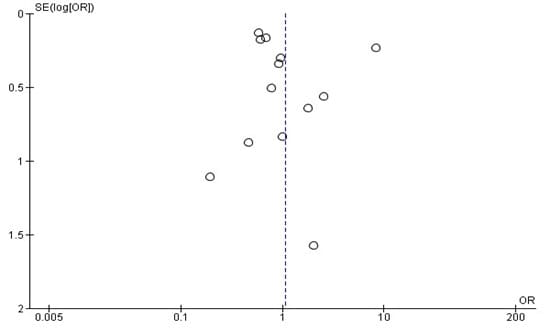
Figure 13: All cause of death funnel plot.
Discussion
The meta-analyses comparing Dual Antiplatelet Therapy (DAPT) with Single Antiplatelet Therapy (SAPT) in 18 studies, including a total of 218,125 patients, yielded valuable information regarding the effectiveness of these treatment plans in patients with cardiovascular conditions. Out of the 18 included studies, 7 studies was use to plot the forest plot of graft occlusion, 13 studies for all cause of death, 8 studies foe cardiovascular mortality, 4 studies for MACCE, 14 studies for myocardial infarction, 8 studies for stroke and 10 studies for major bleeding. The analysis yielded intriguing findings with substantial implications for clinical practice.A notable discovery from the current study was the considerably reduced occurrence of graft occlusion when using dual Antiplatelet Therapy (DAPT) in comparison to Single Antiplatelet Therapy (SAPT). The odds ratio of 0.71, with a 95% confidence range ranging from 0.54 to 0.93 and a p-value of 0.01, clearly demonstrates the significant benefit of DAPT in preventing Cardiovascular mortality. Cardiovascular patients receiving therapy is a critical event that could result in severe complications and jeopardize the procedure’s effectiveness. Another significant discovery was the decreased occurrence of major adverse cardiovascular and cerebrovascular events (MACCE) and myocardial infarctions associated with dual Antiplatelet Therapy (DAPT). The odds ratios of 0.68 and 0.78 for major adverse cardiac and Cerebrovascular Events (MACCE) and myocardial infarction, with p-values of 0.0001 and 0.009, respectively, make it clear that Dual Antiplatelet Therapy (DAPT) is much better at lowering these bad outcomes than single antiplatelet therapy (SAPT), this was in line with the result of [15] found DAPT to be associated with cardiovascular mortality and a trend towards lower all-cause mortality. These results make it clear how important Dual Antiplatelet Therapy (DAPT) is for improving patient outcomes and lowering the risk of major cardiovascular events. Nevertheless, this research also revealed that there was no substantial disparity in the occurrence of graft occlusion, this result however contradict the result of Agarwal (2018) who found that DAPT appears to be associated with a reduction in graft occlusion compared with aspirin monotherapy in patients undergoing CABG [9], also there was no substantial disparity in the occurrence of all-cause death, stroke, and major bleeding between Dual Antiplatelet Therapy (DAPT) and single antiplatelet therapy (SAPT). Although DAPT has shown evident advantages in specific outcomes, such as MACCE and myocardial infarction, it has not established superiority in other domains. This suggests that the choice between DAPT and SAPT should be carefully considered based on the specific needs and risks of individual patients. The investigation included forest plots, which visually depicted the data and offered additional support for the study’s findings. The forest plots for cardiovascular mortality, MACCE, and myocardial infarction demonstrated statistically significant outcomes in favor of DAPT, with reduced occurrence rates and a notable influence.
However, there were no statistically significant differences between dual antiplatelet therapy (DAPT) and Single Antiplatelet Therapy (SAPT) in the forest plots for graft occlusion, all-cause death, stroke, and major bleeding. This suggests that these outcomes were similar in both treatment plans. This result is inline with Schunkert, 2019 whose found MI, stroke, and cardiovascular death and all-cause death showed no significant differences between the two treatment groups [21]. The combined analyses, which compared Dual Antiplatelet Therapy (DAPT) with single antiplatelet therapy (SAPT) in patients with cardiovascular conditions, highlight the importance of tailoring treatment approaches to each patient’s desired outcomes. Although DAPT had evident advantages in decreasing Major Adverse Cardiovascular And Cerebrovascular Events (MACCE) and myocardial infarction, it did not indicate superiority in all outcomes. Healthcare professionals should thoroughly evaluate the potential advantages and disadvantages of every available treatment choice in order to enhance the quality of patient care and the overall results for individuals with cardiovascular conditions. LimitationIt is very important to mention some of the limitations encountered during the database search and interpretation of the meta-analysis. First, the study included only articles published in English; as such, relevant studies might be found in studies published in other languages, which may improve the performance of the analysis. As a result, future researchers should include other languages [22-35]. Second, despite large cohorts, limiting occurrences per outcome resulted in insufficient analytical strength, particularly when analyzing only 18 randomized trials. Thus, these data are hypothesis-generating and demand bigger randomized trials. We may need further research and clinical trials to validate these findings and provide more comprehensive evidence to guide treatment decisions in this patient population.
Conclusion
In conclusion, there were no significant differences in the occurrence of graft occlusion, all-cause death, stroke, and major bleeding between the use of Dual Antiplatelet Therapy (DAPT) and Single Antiplatelet Therapy (SAPT). The p-values were greater than the 5% significance level. However, there were significant differences in the results of cardiovascular mortality, MACCE, and myocardial infarction, with p-values indicating statistical significance at a level below 5%. Overall, DAPT proved to be more effective than SAPT in reducing cardiovascular mortality, MACCE, and myocardial infarction. However, there was no notable distinction between the two regimens in terms of graft occlusion, all-cause death, stroke, and major bleeding. These findings provide clinicians with valuable insights into choosing the most suitable antiplatelet therapy based on the anticipated outcomes for individuals receiving treatment.
Reference
- Neumann, FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP et al (2019). 2018 ESC/EACTS Guidelines on myocardial revascularization. European heart journal, 40:87-165.
- Kulik A, Ruel M, Jneid H, Ferguson TB, Hiratzka LF et al (2015). Secondary prevention after coronary artery bypass graft surgery: a scientific statement from the American Heart Association Circulation 131:927-964.
- Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S et al (2013). Effects of off-pump and on-pump coronary-artery bypass grafting at 1 year. New England Journal of Medicine 368:1179-1188.
- Gasparovic H , Petricevic M, Kopjar T, Djuric Z, Svetina L et al (2014). Impact of dual antiplatelet therapy on outcomes among aspirinresistant patients following coronary artery bypass grafting. The American journal of cardiology 113:1660-1667.
- Gaudino M , Antoniades C, Benedetto U, Deb S, Di Franco A et al (2017). Mechanisms, consequences, and prevention of coronary graft failure. Circulation 136:1749-1764.
- Sousa-Uva M, Neumann FJ, Ahlsson A, Alfonso F, Banning AP et al (2019). 2018 ESC/EACTS Guidelines on myocardial revascularization. European Journal of Cardio-Thoracic Surgery 40:87-165.
- Mannacio VA, Di Tommaso L, Antignan A, De Amicis V, & Vosa C (2012). Aspirin plus clopidogrel for optimal platelet inhibition following off-pump coronary artery bypass surgery: results from the CRYSSA (prevention of Coronary arteRY bypaSS occlusion After off-pump procedures) randomised study. Heart 98:1710-1715.
- Kulik A, Le May MA, Voisine P, Tardif JC, DeLarochelliere R et al (2010) “Aspirin plus clopidogrel versus aspirin alone after coronary artery bypass grafting: the clopidogrel after surgery for coronary artery disease (CASCADE) Trial.” Circulation 122: 2680-2687.
- Agarwal N, MahmoudAN, Patel NK, Jain A, Garg J(2018). Metaanalysis of aspirin versus dual antiplatelet therapy following coronary artery bypass grafting. The American Journal of Cardiology. 121; 3240.
- Yusuf S, & Zhao F (2001). Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without STsegment elevation. 45:494-502.
- Sun JCJ, Teoh KH, Lamy A, Sheth T, Ellins ML et al (2010). Randomized trial of aspirin and clopidogrel versus aspirin alone for the prevention of coronary artery bypass graft occlusion: the Preoperative Aspirin and Postoperative Antiplatelets in Coronary Artery Bypass Grafting study. American heart journal 160:1178-1184.
- Gao G, Zheng Z, Pi Y, Lu B, Lu J et al (2010). Aspirin plus clopidogrel therapy increases early venous graft patency after coronary artery bypass surgery: a single-center, randomized, controlled trial. Journal of the American College of Cardiology 56: 1639-1643.
- Solo K, Lavi S, Kabali C, Levine GN, Kulik A et al (2019). Antithrombotic treatment after coronary artery bypass graft surgery: systematic review and network meta-analysis. bmj, 367.
- van Diepen S, Fuster V, Verma S, Hamza TH, Siami FS et al (2017). Dual antiplatelet therapy versus aspirin monotherapy in diabetics with multivessel disease undergoing CABG: FREEDOM insights. Journal of the American College of Cardiology 69:119-127.
- Cardoso R, Knijnik L, Whelton SP, Rivera M, Gluckman TJ, et al (2018). Dual versus single antiplatelet therapy after coronary artery bypass graft surgery: an updated meta-analysis. International journal of cardiology 269: 80-88.
- Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, et al (2009). Ticagrelor versus clopidogrel in patients with acute coronary syndromes. New England Journal of Medicine 361:1045-1057.
- Held C, Åsenblad N, Bassand JP, Becker RC, Cannon CP, Claeys, et al (2011). Ticagrelor versus clopidogrel in patients with acute coronary syndromes undergoing coronary artery bypass surgery: results from the PLATO (Platelet Inhibition and Patient Outcomes) trial. Journal of the American College of Cardiology 57: 672-684.
- Bhatt DL, Chew DP, Hirsch AT, Ringleb PA, Hacke W et al (2001). Superiority of clopidogrel versus aspirin in patients with prior cardiac surgery. Circulation 103:363-368.
- Verma S, Goodman SG, Mehta SR, Latter DA, Ruel M, et al (2015). Should dual antiplatelet therapy be used in patients following coronary artery bypass surgery? A meta-analysis of randomized controlled trials. BMC surgery 15:1-15.
- Deo SV, Dunlay SM, Shah IK, Altarabsheh SE, Erwin PJ et al (2013). Dual anti‐platelet therapy after coronary artery bypass grafting: is there any benefit? A systematic review and meta‐analysis. Journal of Cardiac Surgery: Including Mechanical and Biological Support for the Heart and Lungs 28: 109-116.
- Schunkert H, Boening A, Von Scheidt M, Lanig C, Gusmini F et al (2019). Randomized trial of ticagrelor vs. aspirin in patients after coronary artery bypass grafting: the TiCAB trial. European Heart Journal, 40:2432-2440.
- Al-Zakwani I, Al-Lawati J, Alsheikh-Al AA, Almahmeed W, Rashed W et al. (2020). Impact of Dual versus SingAntiplatelet Therapy on Major Cardiovascular Outcomes in Patients with Acute Coronary Syndrome in the Arabian Gulf. Medical Principles and Practice .29:181-187.
- Benedetto U, Altman200 DG, Gerry S, Gray A, Lees B et al (2017). Impact of dual antiplatelet therapy after coronary artery bypass surgery on 1-year outcomes in the Arterial Revascularization Trial. European Journal of Cardio-Thoracic Surgery. 52: 456-461.
- Deo SV, Dunlay SM, & Park SJ (2014). Dual antiplatelet therapy after coronary artery bypass grafting: does off/on-pump play a role?. American Journal of Cardiology 113: 1085.
- Ebrahimi R, Gupta S, Carr BM, Bishawi M, Bakaeen FG, et al (2018). Comparison of outcomes and costs associated with aspirin±clopidogrel after coronary artery bypass grafting. The American journal of cardiology 121:709-714.
- Kim JT, Park MS, Choi KH, Cho KH, Kim BJ et al (2019). Comparative effectiveness of dual antiplatelet therapy with aspirin and clopidogrel versus aspirin monotherapy in acute, nonminor stroke: a nationwide, multicenter registry-based study. Stroke 50:3147-3155.
- Mega JL & Simon T (2015). Pharmacology of antithrombotic drugs: an assessment of oral antiplatelet and anticoagulant treatments. The Lancet 386:281-291.
- Qu J, Zhang H, Rao C, Chen S, Zhao Y et al (2021). Dual antiplatelet therapy with clopidogrel and aspirin versus aspirin monotherapy in patients undergoing coronary artery bypass graft surgery. Journal of the American Heart Association 10:e020413.
- Rafiq S, Johansson PI, Kofoed KF, Lund JT, Olsen PS et al (2017). Thrombelastographic hypercoagulability and antiplatelet therapy after coronary artery bypass surgery (TEG-CABG trial): a randomized controlled trial. Platelets 28: 786-793.
- Russo M, Nardi P, Saitto G, Bovio E, Pellegrino A, et al (2017). Single versus double antiplatelet therapy in patients undergoing coronary artery bypass grafting with coronary endarterectomy: mid-term results and clinical implications. Interactive CardioVascular and Thoracic Surgery 24:203-208.
- Saw J, Wong GC, Mayo J, Bernstein V, Mancini GJ et al (2016). Ticagrelor and aspirin for the prevention of cardiovascular events after coronary artery bypass graft surgery. Heart, 102:763-769.
- Slim A, Fentanes E, Thomas D, Slim J, Triana T et al (2016). ASpirin and Plavix following Coronary Artery Bypass Grafting (ASAP-CABG): A randomized, double-blind, placebo-controlled pilot trial. British Journal of Medicine and Medical Research 14:1-10.
- Smith PK, Goodnough LT, Levy JH, Poston RS., Short MA (2012). Mortality benefit with prasugrel in the TRITON–TIMI 38 coronary artery bypass grafting cohort: Risk-adjusted retrospective data analysis. Journal of the American College of Cardiology 60:388-396.
- Uchiyama S, Toyoda K, Omae K, Saita R, Kimura K et al (2021). Dual antiplatelet therapy using cilostazol in patients with stroke and intracranial arterial stenosis. Journal of the American Heart Association 10:e022575.
- Zhao Q, Zhu Y, Xu Z, Cheng Z, Mei J (2018). Effect of ticagrelor plus aspirin, ticagrelor alone, or aspirin alone on saphenous vein graft patency 1 year after coronary artery bypass grafting: a randomized clinical trial. Jama 319: 1677-1686.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.