Design and Evaluation Novel Gene-Specific, Cell-Permeable Antisense Peptide Nucleic Acids to Prevent Staphylococcus Aureus Biofilm Formation
by Nihan Akguc Col1*, Jayasimha Rao2, Govindarajan Rajagopalan3, Nammalwar Sriranganathan4
1Department of Laboratory Animals, Division of Basic Science, Veterinary Faculty, Muğla Sıtkı Koçman University, Milas, Muğla, Türkiye
2University Department of Medicine, Division of Infectious Diseases, Carilion Clinic, Virginia Tech Carilion School of Medicine, Roanoke, Virginia, USA
3Section of Pulmonary Critical Care and Sleep Medicine, Department of Internal Medicine, Yale School of Medicine, Yale University, New Haven, CT, USA
4Department of Biomedical Sciences and Pathobiology, Virginia-Maryland College of Veterinary Medicine, Virginia Tech, Blacksburg, Virginia, USA
*Corresponding authors: Nihan Akguc Col, Department of Laboratory Animals, Division of Basic Science, Veterinary Faculty, Muğla Sıtkı Koçman University, Milas, Muğla, Türkiye.
Received Date: 18 December 2024
Accepted Date: 23 December 2024
Published Date: 27 December 2024
Citation: Col NA, Rao J, Rajagopalan G, Sriranganathan N (2024) Design and Evaluation Novel Gene-Specific, CellPermeable Antisense Peptide Nucleic Acids to Prevent Staphylococcus Aureus Biofilm Formation. Infect Dis Diag Treat 8: 270. https://doi.org/10.29011/2577-1515.100270.
Abstract
Staphylococcus aureus, a Gram-positive bacterium, is a leading cause of various biofilm-associated infections in humans and animals, posing significant economic and healthcare challenges. Biofilms exhibit heightened resistance to antimicrobial agents as well as to immune-mediated clearance, thus persisting for long periods of time. Hence, novel therapeutic approaches are needed to eradicate S. aureus biofilms. Peptide nucleic acids (PNAs), synthetic DNA analogs with a peptide backbone instead of sugar backbone, offer a promising approach. In this study, we designed, synthesized and tested the efficacy of several synthetic antisense PNAs coupled with cell-penetrating peptides (CPPs), targeting essential and biofilm related S. aureus genes to inhibit staphylococcal biofilm growth using standard microtiter plate and tygon catheter biofilm assays. P-PNAs targeting the genes for intercellular adhesion locus, ica, cell wall/membrane/envelope biogenesis, fmhb, accessory regulator, sarA, sensor histidine kinase, saeS, repressor of toxins, rot, response regulator, yycF and histidine kinase, yycG genes were tested. Two scrambled PNAs and CPP alone were used as controls. Only one P-PNA, targeting sarA, showed the strongest biofilm inhibitory activity (up to 40 %) at a concentration of 50 μM or higher. This novel P-PNA could be a useful adjunct for the treatment S. aureus biofilm infections.
Keywords: Peptide nucleic acids (PNAs); Staphylococcus aureus; Biofilm; sarA; Antisense technology
Introduction
The Gram-positive opportunistic pathogen, Staphylococcus aureus, is the major causative agent of diverse biofilm-associated infections in humans and animals. Being a part of the normal microbiota of the skin and upper airways, S. aureus can readily establish various kinds of biofilm infections of the living tissue (biotic surfaces) such as the skin, soft tissue, bones and cystic fibrosis airways among others [1-6]. Staphylococcus aureus can also easily reach implanted devices such as catheters and prosthetic joints to establish biofilm infections on these abiotic surfaces [7]. In most cases, the S. aureus infection remains localized on these infected surfaces. However, the infection can spread to other parts of the body via the bloodstream causing serious complications [8].
The unique structural organization of the biofilms, with complex extracellular matrix, prevent the deeper penetration of antimicrobial agents into the biofilms and reaching the organisms at sufficient concentration to mediate antibacterial effects. This confers bacterial antibiotic resistance without the presence of resistance genes [9-11]. It has been demonstrated that the staphylococcal biofilms are also resistant to immune-mediated clearance [12, 13]. Overall, the biofilms can persist on biotic and abiotic surfaces for prolonged periods of time by multiple mechanisms [14-19]. Unfortunately, there are currently no antimicrobial therapies available that specifically target bacteria in biofilms, resulting in significant health as well as economic impact [20, 21]. For example, in patients with persistent biofilm infection of prosthetic joints, either replacement of the infected joint or even amputation of the limb may be the only viable options [22, 23]. Hence, novel anti-biofilm therapies are urgently needed.
Antisense technology has been widely applied in the medical field because of their ability to limit, and in some cases eliminate, the expression of targeted genes through sequence-specific recognition without much off-target effects. While antisense technology has been used to block the expression of human genes in the biomedical field, in microbiology they are used as an antimicrobial agent for targeting the expression of specific gene(s) in pathogens. Peptide nucleic acids (PNAs) are synthetic DNA mimics that have target DNA or RNA sequences with a higher affinity than natural nucleic acids and have been used in a variety of applications, including inhibition of bacterial growth [24, 25]. Several studies have tested the ability of PNAs to block the expression of specific genes in S. aureus [26-30]. However, the effectiveness of PNA to inhibit biofilm formation in S. aureus has not been studied until recently. In this study, we have identified a novel PNA that effectively inhibited S. aureus biofilm formation in vitro.
Materials and Methods
Strain and growth conditions
Throughout this study, a pathogenic S. aureus isolate that expresses the luciferase gene and can also form biofilms (S. aureus P231) and a non-biofilm forming S. epidermidis isolate were used. Expression of the lux operon in S. aureus facilitates rapid screening of the effect of PNAs on bacterial growth/viability using in vivo imaging system “IVIS” [31, 32]. All bacterial strains were stored as 15–25 % (v/v) glycerol stocks at −70 °C as cryogenic stocks and plated on tryptic soy agar (TSA) when needed. Cultures were revived by streaking the stock onto TSA agar followed by overnight incubation at 37 °C. Single colonies were picked to inoculate precultures in tryptic soy broth (TSB) with-1% glucose and incubated at 37 °C and 220 rpm for 16–18 h in a shaking incubator.
Design and Synthesis of P-PNAs
The genome was searched for genes that are known or theorized to be necessary in biofilm formation. The candidate bacterial genes were selected by scanning among those relevant to biofilm formation of S. aureus. Commonly targeted seven bacterial gene functions and pathways are those essential for biofilm formation such as adherence of cells to a surface and several global regulators. Suitable antisense PNAs for target genes were obtained from NCBI Database and designed to bind their translation initiation regions within the coding strands of each mRNA, which covered the AUG start codon [33, 34] (Table 1). Another two sequences (scrambled PNAs) mismatching the base pair with the target genes were designed as controls. All PNAs linked to a cell penetrating peptide (CPP) were designed and submitted to a commercial company (Table 1). All the P-PNAs were synthesized, purified, and conjugated with either (RXR)4XB, or (KFF)3K bacterial penetration peptide by PNA Bio (Newberry Park, CA).
Genbank Gene Reference Number and/or Name | Function | Target DNA Sequence (5’-3’) | PNA Sequence (5’-3’) with Attached CPP |
AAD52055.1 The intercellular adhesion(ica) locus | It is involved in the synthesis of poly N-acetylglucosamine for intercellular adhesion in S. aureus. | TGTATTTATGTCT | (RXR)4XB- TGACATATTTTCT |
NBR005743 Cell wall/membrane/envelope biogenesis (fmhb) | The essential gene that encodes the factor that catalyzes the first step in the synthesis of the characteristic pentaglycine interpeptide in the S. aureus peptidoglycan. | ATAAATCATGGA | (RXR)4XB-TCCTTGATTTAT |
AF515775.1 Staphylococcus aureus variant staphylococcal accessory regulator region (SarA) | An important transcriptional regulator that activates the agr operon and modulates the expression of many virulence genes and is a positive regulator of S. aureus PNAG-dependent biofilm formation. | TTTAAACATGGCA | (RXR)4XB- TGCCATGTTTATA |
AJ556795.1 Staphylococcus aureus Sensor histidine kinase (SaeS) | Coordinate environmental signals with the internal regulatory circuitry governing virulence and other adaptive processes | GAGCCGATAATG | (RXR)4XB- CATTTTCGGCTC |
AF189239.2 Staphylococcus aureus repressor of toxins Rot (rot) | Plays a key role in regulating S. aureus virulence through activation or repression of promoters that control expression of many critical virulence factors. | AGAATAATGCA | (KFF)3K-O-TGCATTATTCT |
AF136709.1 Two-component system YycFG Staphylococcus aureus response regulator YycF (yycF) | YycF binds to the upstream promoter regions of the target genes to positively and negatively regulate their expression. | AAGAGGTTAATG | (KFF)3K-O-CATTAACCTCTT |
AF136709.1 Two-component system YycFG Staphylococcus aureus histidine kinase YycG (yycG) | YycG is a sensor protein with histidine kinase activity | GAAACGAATGA | (KFF)3K-O-TCATTCGTTTC |
Scramble PNA | Random (non-specific) | NONE | RXR)4XB- TTTTGCCAT |
Scramble PNA | Random (non-specific) | NONE | (RXR)4XB- CCCTGATATA |
Cell Penetrating Peptide | Short peptides that can deliver PNAs across cellular membranes | N/A | (RXR)4XB |
Table 1: Sequences of designed P-PNAs and CPP for Staphylococcus aureus.
Inhibitory activities of synthesized P-PNAS against Staphylococcus aureus biofilm formation
Briefly, frozen stock of S. aureus P231 was streaked on TSA (Tryptic Soy Agar) plates to obtain single, well-isolated colonies. Plates were incubated overnight at 37 °C with 5 % CO2. One colony was inoculated to 10 ml of TSB (trypticase soya broth) supplemented with 1 % glucose and incubated for 20-24 h at 37 °C in shaker at 200 rpm. The overnight cultures grown in TSB-1 % glucose was diluted 180 folds (approximately 105 CFU/ml) with 2x TSB-1 % glucose. Fifty microliters of the diluted cultures were incubated with indicated concentrations of PNA in a volume of 50 μl, in duplicates, in a 96-well, low adhesion, flat-bottomed microtiter plates (cat. # 3474; Corning Inc., Corning, NY), making the total volume per well to 100 μl. The plates were incubated in a humidified incubator at 37 °C with 5 % CO2. To reduce edge effects and evaporation, 200 µl TSB was added to circumferential wells on the plate. For determining biofilm formation by crystal violet (CV) staining, the plates were incubated for 48 hours, and the liquid culture medium was removed by gently tapping the plates on paper towel to remove planktonic bacteria. Subsequently, 200 µl 0.1 % aqueous CV was added to each well and incubated for 10-15 minutes at room temperature. The plates were washed by submerging the plates in a tray of water, filling the wells and tapping the plates gently upside down on a stack of paper towels. After 2 washes, 200 µl of 30 % glacial Acetic acid was added to each well, incubated at room temperature for 10-15 mins to dissolve the biofilm-bound CV and the absorbance was determined using a microtiter plate reader at 570 nm. The average and standard deviation of the OD570 values obtained for each strain were calculated [35]. For experiments with the bioluminescent feature of S. aureus isolate, the plates were incubated for 24 h before determining bioluminescence using IVIS (R) imaging system.
Inhibitory activities of anti-sarA P-PNA against S. aureus biofilm formation on tygon tubing
The tygon catheter tubes mimics with an inner diameter of 1/16 in. and a wall thickness of 1/16 in, giving a total diameter of 3/16 in (Part No.5894K31, McMaster Carr, Santa Fe Springs, CA, USA) [36, 37] were cut into 3.5 cm to 4 cm long segments and sterilized by soaking in 10 % bleach for 15 minutes. Silicon stoppers were also sterilized similarly. After sterilization, catheter fragments and stoppers were washed with sterile distilled water (diH2O) several times in a laminar flow hood and allowed to dry prior to inoculation with S. aureus P231. For tygon biofilm assay, 50 µl of the bacteria (105 CFU/ml) was transferred into a low-binding 1.5 ml microfuge tube and centrifuged at 13,300 rpm for 3 minutes. After aspirating the supernatant, the bacterial pellets were resuspended in 50 µl anti-sarA P-PNA (50 or 100 µM) in TSB-1 % glucose and the mixes were applied into the lumen of the catheter fragments. After incubation for 48 hours at 37 °C with 5 % CO2, the liquid culture medium was removed from the catheter lumen by gentle aspiration to remove planktonic bacteria. Then, 50 µl of 0.1 % aqueous CV was added to each lumen of the catheter and incubated for 10-15 minutes at room temperature. The CV-stained bacterial biofilm on the catheter pieces was washed several times by pipetting with water. After washing, they were carefully dried with a tissue and the catheter pieces were transferred to 96 well flat bottom microtiter plates. To dissolve the biofilm bound CV, 50 µl of 30 % glacial acetic acid was added to the lumen of each the catheter piece, incubated at room temperature for 10-15 minutes and the absorbance was determined at 570 nm using a microtiter plate reader.
Statistics
All statistical analyses were performed with Student’s two-tailed t-test using Microsoft Excel (Microsoft Corp., Redmond, WA) or using GraphPad Prism software (Version 10.4). P-values of ≤0.05 were considered significant.
Results and Discussion
Biofilm formation by S. aureus is a complex process which is not fully understood. However, several genes and pathways have been shown to be critical in this process. Therefore, we hypothesized that targeting important genes using cell-penetrating antisense PNAs will be a viable approach to treat S. aureus biofilm infections. For this, we first systematically reviewed the literature and identified several candidate bacterial genes that have been shown to regulate staphylococcal biofilm formation or critical for staphylococcal growth and virulence. The icaA gene, which plays a role in the synthesis of poly-N-acetylglucosamine for intercellular adhesion, has been reported to play an important role in biofilm formation [38, 39]. The fmhB gene, encodes for the factor that catalyzes the first step in the synthesis of the characteristic pentaglycine interpeptide in the S. aureus peptidoglycan, which is involved in wall biosynthesis [40]. SarA has been shown to be an important transcriptional regulator that activates the agr operon and modulates the expression of many virulence genes and is a positive regulator of PNAG-dependent biofilm formation of S. aureus [41-44]. Another global autoinducer regulator, sae regulon, includes both biofilm formation factors (i.e., Coa, Emp, Eap, FnBPA, FnBPB, Hla, Hlb) and biofilm degradation factors [45-50].
The DNA-binding protein Rot, which belongs to the sarA family of S. aureus regulators, controls S. aureus virulence by activating or repressing promoters that regulate the expression of several important virulence factors [51-52]. Several studies have demonstrated a relationship between the presence of the Rot gene and the formation of S. aureus biofilms [53]. One of the major properties of S. aureus is that it can adapt to the environment by coordinating gene expression thereby allowing it to withstand a variety of environmental stresses. The two-component systems (TCS), plays a central role in this process [54]. The YycFG TCS regulates cellular physiology, structure and biofilm organization [55]. The YycG TCS controls genes related to cell wall metabolism, virulence regulation, biofilm formation, oxidation stress resistance and antibiotic resistance through a direct or indirect regulation of autolysins. It acts as a sensor protein kinase that automatically phosphorylates a histidine residue in the dimerization domain and then shifts the phosphate group to the aspartic acid residue conserved in the regulatory domain of YycF. The upstream promoter regions of the target genes can control the expression of these genes either positively or negatively [56, 57]. Anti-sense PNAs targeting these genes were synthesized and tested in in vitro assays.
First, we tested whether the PNAs had direct bactericidal activity in 96-well microtiter plate bioluminescence assay. The extent of bioluminescence was comparable in all wells, when treated with test PNAs, scrambled PNAs, cell-permeable peptide or medium alone at concentrations ranging from 15 to 50 μM (Fig. 1). This indicated that our PNAs are not bactericidal. Next, we tested the ability of the compounds to specifically inhibit biofilm formation in CV assay. In this assay, only anti-sarA P-PNA exhibited the strongest biofilm inhibitory effect at concentration of 50 μM or higher. Biofilm formation was reduced by 95 % after 48 h of treatment with anti-sarA P-PNA at concentration 50 μM (Fig 2). As expected, neither of the scrambled PNAs nor cell-permeable peptide alone had any inhibitory effect. Interestingly, certain PNAs, such as icaA, fmhb and lower concentrations of sarA appeared to promote biofilm formation. While we do not understand the mechanisms, it is possible that the PNAs may interact with the extracellular DNA that is known to be present in the biofilm matrix and promote biofilm formation.
Since only anti-sarA P-PNA showed strong inhibitory activity in microtiter plate assay, we next tested the ability of anti-sarA P-PNA to inhibit S. aureus biofilm formation on tygon tubes. In this assay, anti-sarA P-PNA caused approximately 50 % inhibition of biofilm formation of S. aureus strain P231 on the surface of tygon catheter tubes at a concentration of 50 μM and over 95 % inhibition at a concentration of 100 μM compared to the control (Fig 3). The CPP alone had no inhibitory effect on biofilm formation (Fig 3). In conclusion, we have designed a novel PNA that significantly inhibited biofilm formation by pathogenic S. aureus. However, there are some limitations associated with our study. While anti-sarA PNA clearly inhibited biofilm formation, the mechanisms need to be worked out. Fully understanding the molecular pathways regulated by sarA that are important for biofilm formation may lead to novel therapies to inhibit biofilm formation. Once the PNA is tested in in vivo models, this could be as an alternative and probably as a complementary therapeutic strategy with broad spectrum antimicrobials.
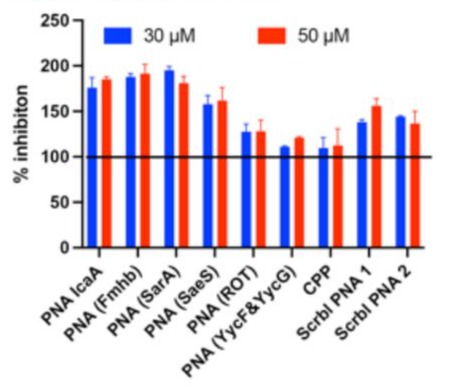
Figure 1: Determination of direct antibacterial activity of PNAs.To investigate whether the PNAs have direct antibacterial activity, the S. aureus P231 bioluminescent strain was grown in microtiters plates in the presence of various gene-specific PNAs, scrambled PNAs or CPP ((RXR)4XB) alone for 24 hours. The luminescence intensities were calculated using IVIS system as described in methods. The percent of inhibition of bioluminescence by the test compounds was calculated by comparing the luminescence with medium alone. The bars represent Mean ± SE of two to three independent experiments.
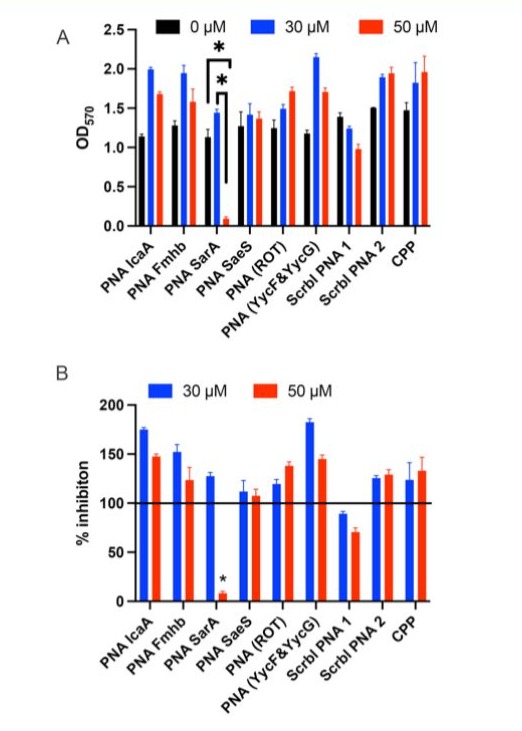
Figure 2: Inhibition of S. aureus biofilm formation by PNAs. S. aureus P231 strain biofilms were grown in 96-well low binding microtiter plate. Indicated concentrations of PNAs were added in duplicates and the extent of biofilm formation was determined by crystal violet staining assay at 48 hours. (A) OD570 values by crystal violet staining assay. (B) Percentage inhibition by PNAs compared to medium alone. The bars represent Mean ± SE of two to three independent experiments. * p<0.05
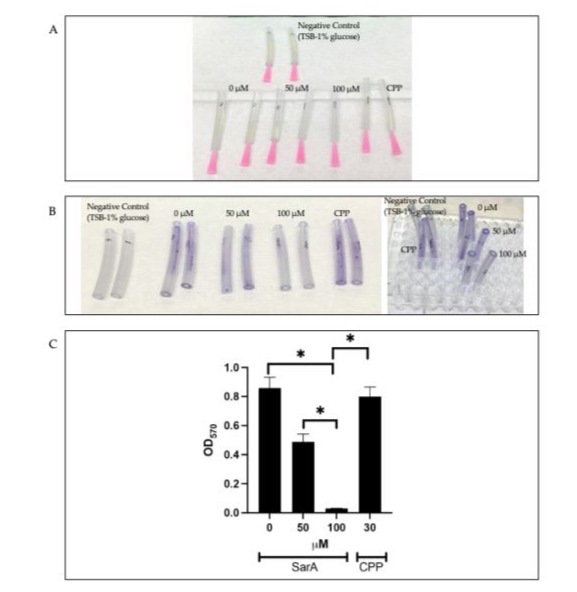
Figure 3: Inhibition of biofilm formation by S. aureus P231 strain on tygon catheters by (RXR)4XB-linked anti-sarA P-PNA. (A) Biofilms of S. aureus P231 strain were grown for 48 h on the tygon catheter segments. (B) Crystal violet-stained biofilms of S. aureus
P321 on tygon catheter tube segments. (C) OD570 values by Crystal violet staining to determine the extent of biofilm inhibition at 48 hours. Anti-sarA P-PNA was tested at 50 and 100 μM and the control CPP was used at 30 μM. * p<0.05.
Studies with antisense peptide-PNAs targeting essential genes have shown growth inhibitory effects in S. aureus [27]. PNAs’ potential as antibacterial agents has been thoroughly evaluated, and it has been found that design parameters like the base sequence, the location of the membrane penetrating peptide, and the presence of a linker can affect the activity of P-PNAs. As a result, the most effective PNAs for Gram-positive bacteria had a base pare length of 10 nucleotides, the most effective PNAs for mRNA regions that bound to the ribosomal binding sequence of the mRNA (RBS region), and the PNA activity of different carrier agents produced distinct antibacterial effects on both Gram-positive and Gram-negative bacteria [57].
Conclusion
In conclusion, we have designed a novel PNA that significantly inhibited biofilm formation by pathogenic S. aureus. Once the PNA is tested in in vivo models, this could be as an alternative and probably as a complementary therapeutic strategy with broad spectrum antimicrobials.
Author Contributions: Design and planning of experiment – NAC, JR, GR and NS. Execution of experiments – NAC. Analysis and interpretation of data and manuscript preparation - NAC, JR, GR and NS.
Funding: This research was supported by the Scientific and Technological Research Council of Turkey (TUBITAK) under the BIDEB 2219-International Postdoctoral Research Scholarship Program [1059B191801593], The IRC Grant project (Departmental) and from The Center for One Health Research (COHR).
Acknowledgments: We thank Nancy M. Tenpenny for her help with the experiments.
Conflicts of Interest: The authors declare no conflict of interest.
References
- Darboe S, Dobreniecki S, Jarju S, Jallow M, Mohammed NI, et al. (2019) Prevalence of Panton-Valentine leukocidin (PVL) and antimicrobial resistance in community-acquired clinical Staphylococcus aureus in an urban Gambian hospital: A 11-year period retrospective pilot study. Front Cell Infect Microbiol. 9:170.
- Tsuji R, Fujii T, Nakamura Y, Yazawa K, Kanauchi O (2019) Staphylococcus aureus Epicutaneous Infection is Suppressed by Lactococcus lactis Strain Plasma via Interleukin 17A Elicitation. J Infect Dis220:892-901.
- Cheng M, Zhang L, Zhang H, Li X, Wang Y, et al. (2018) An ointment consisting of the phage lysin LysGH15 and apigenin for decolonization of methicillin-resistant Staphylococcus aureus from skin wounds. Viruses 10:244.
- Del Pozo JL, Patel R (2009) Clinical practice. Infection associated with prosthetic joints. N Engl J Med 361:787-794.
- Kaye KS, Petty LA, Shorr AF, Zilberberg MD (2019) Current epidemiology, etiology, and burden of acute skin infections in the United States. Clin Infect Dis 68: S193-S199.
- Vestby LK, Grønseth T, Simm R, Nesse LL (2020) Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics (Basel) 9:59.
- Purrello SM, Garau J, Giamarellos E, Mazzei T, Pea F, et al. (2016) Methicillin-resistant Staphylococcus aureus infections: A review of the currently available treatment options. J Glob Antimicrob Resist 7:178-186.
- Lowy FD (1998) Staphylococcus aureus infections. N Engl J Med 339:520-532.
- Gautam A, Lear G, Lewis GD (2022) Time after time: detecting annual patterns in stream bacterial biofilm communities. Environ Microbiol 24:2502-2515.
- Uruén C, Chopo-Escuin G, Tommassen J, Mainar-Jaime RC, Arenas J (2020) Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics (Basel) 10:3.
- Kaplan JB, Mlynek KD, Hettiarachchi H, Alamneh YA, Biggemann L, et al. (2018) Extracellular polymeric substance (EPS)-degrading enzymes reduce staphylococcal surface attachment and biocide resistance on pig skin in vivo. PLoS One 13: e0205526.
- Høiby N, Krogh Johansen H, Moser C, Song Z, Ciofu O, et al. (2001) Pseudomonas aeruginosa and the in vitro and in vivo biofilm mode of growth. Microbes Infect 3:23-35.
- Bhattacharya M, Berends ETM, Chan R, Schwab E, Roy S, et al. (2018) Staphylococcus aureus biofilms release leukocidins to elicit extracellular trap formation and evade neutrophil-mediated killing. Proc Natl Acad Sci 115:7416-7421.
- Foster TJ, Geoghegan JA, Ganesh VK, Hook M (2014) Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 12:49-62.
- Foster TJ (2019) The MSCRAMM Family of Cell-Wall-Anchored Surface Proteins of Gram-Positive Cocci. Trends Microbiol 27:927-941.
- Gross M, Cramton SE, Gotz F, Peschel A (2001) Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect Immun 69:3423-3426.
- Holland LM, Conlon B, O’Gara JP (2011) Mutation of tagO reveals an essential role for wall teichoic acids in Staphylococcus epidermidis biofilm development. Microbiology 157:408–418.
- Otto M (2014) Physical stress and bacterial colonization. FEMS Microbiol Rev 38:1250-1270.
- Qin Z, Ou Y, Yang L, Zhu Y, Tolker-Nielsen T, et al. (2007) Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermidis. Microbiology 153:2083-2092.
- Wolcott RD, Rhoads DD, Bennett ME, Wolcott BM, Gogokhia L, et al. (2010) Chronic wounds and the medical biofilm paradigm. J Wound Care 19:45-46, 48-50, 52-53.
- Tuon FF, Suss PH, Telles JP, Dantas LR, Borges NH, et al. (2023) Antimicrobial Treatment of Staphylococcus aureus Biofilms. Antibiotics (Basel) 12:87.
- Zaborowska M, Tillander J, Brånemark R, Hagberg L, Thomsen P, et al. (2016) Biofilm formation and antimicrobial susceptibility of staphylococci and enterococci from osteomyelitis associated with percutaneous orthopaedic implants. J Biomed Mater Res B Appl Biomater 105:2630-2640.
- Visperas A, Santana D, Klika AK, Higuera-Rueda CA, Piuzzi NS (2022) Current treatments for biofilm-associated periprosthetic joint infection and new potential strategies. J Orthop Res 40:1477-1491.
- Good L, Nielsen PE (1998) Antisense inhibition of gene expression in bacteria by PNA targeted to mRNA. Nat Biotechnol 16:355-358.
- Good L, Nielsen PE (1999) Peptide nucleic acid (PNA) antisense effects in Escherichia coli. Curr Issues Mol Biol 1:111-116.
- Ji Y, Yin D, Fox B, Holmes DJ, Payne D, et al. (2004) Validation of antibacterial mechanism of action using regulated antisense RNA expression in Staphylococcus aureus. FEMS Microbiol Lett 231:177-184.
- Nekhotiaeva N, Awasthi SK, Nielsen PE, Good L (2004) Inhibition of Staphylococcus aureus gene expression and growth using antisense peptide nucleic acids. Mol Ther 10:652-659.
- Bai H, Sang G, You Y, Xue X, Zhou Y, et al. (2012) Targeting RNA polymerase primary σ70 as a therapeutic strategy against methicillin-resistant Staphylococcus aureus by antisense peptide nucleic acid. PLoS One 7: e29886.
- Meng J, Wang H, Hou Z, Chen T, Fu J, et al. (2009) Novel anion liposome-encapsulated antisense oligonucleotide restores susceptibility of methicillin-resistant Staphylococcus aureus and rescues mice from lethal sepsis by targeting mecA. Antimicrob Agents Chemother 53:2871-2878.
- Lee HT, Kim SK, Lee JB, Yoon JW (2019) A novel peptide nucleic acid against the cytidine monophosphate kinase of S. aureus inhibits staphylococcal infection in vivo. Mol Ther Nucleic Acids 18:245-252.
- Kadurugamuwa JL, Sin L, Albert E, Yu J, Francis K, et al. (2003) Direct continuous method for monitoring biofilm infection in a mouse model. Infect Immun 71:882-890.
- Guo Y, Ramos RI, Cho JS, Donegan NP, Cheung AL, et al. (2013) In vivo bioluminescence imaging to evaluate systemic and topical antibiotics against community-acquired methicillin-resistant Staphylococcus aureus-infected skin wounds in mice. Antimicrob Agents Chemother 57:855-863.
- Good L, Awasthi SK, Dryselius R, Larsson O, Nielsen PE (2001) Bactericidal antisense effects of peptide-PNA conjugates. Nat Biotechnol 19:360-364.
- Dryselius R, Aswasti SK, Rajarao GK, Nielsen PE, Good L (2003) The translation start codon region is sensitive to antisense PNA inhibition in Escherichia coli. Oligonucleotides 13:427-433.
- Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, et al. (1985) Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol 22:996-1006.
- Childers C, Edsall C, Mehochko I, Mustafa W, Durmaz YY, et al. (2022) Particle-mediated histotripsy for the targeted treatment of intraluminal biofilms in catheter-based medical devices. BME Front 2022:9826279.
- Morse R, Childers C, Nowak E, Rao J, Vlaisavljevich E (2023) Catheter-based medical device biofilm ablation using histotripsy: A parameter study. Ultrasound Med Biol 49:2152-2159.
- Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, et al. (1996) The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: purification and structural analysis. J Bacteriol 178:175-183.
- Maira-Litrán T, Kropec A, Abeygunawardana C, Joyce J, Mark G, et al. (2002) Immunochemical properties of the staphylococcal poly-N-acetylglucosamine surface polysaccharide. Infect Immun 70:4433-4440.
- Schneewind O, Fowler A, Faull KF (1995) Structure of the cell wall anchor of surface proteins in Staphylococcus aureus. Science 268:103-106.
- Dunman PM, Murphy E, Haney S, Tucker-Kellogg, Wu S, et al. (2001) Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. J Bacteriol 183:7341-7353.
- Balamurugan P, Praveen Krishna V, Bharath D, Lavanya R, Vairaprakash P, et al. (2017) Staphylococcus aureus Quorum Regulator SarA Targeted Compound, 2-[(Methylamino) methyl]phenol Inhibits Biofilm and Down-Regulates Virulence Genes. Front Microbiol 8:1290.
- Beenken KE, Blevins JS, Smeltzer MS (2003) Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect Immun 71:4206-4211.
- Valle J, Toledo-Arana A, Berasain C, Ghigo JM, Amorena B, et al. (2003) SarA and not sigmaB is essential for biofilm development by Staphylococcus aureus. Mol Microbiol 48:1075-1087.
- Johnson M, Cockayne A, Morrissey JA (2008) Iron-regulated biofilm formation in Staphylococcus aureus Newman requires ica and the secreted protein Emp. Infect Immun 76:1756-1765.
- Zapotoczna M, McCarthy H, Rudkin JK, O'Gara JP, O'Neill E (2015) An Essential Role for Coagulase in Staphylococcus aureus Biofilm Development Reveals New Therapeutic Possibilities for Device-Related Infections. J Infect Dis 212:1883-1893.
- O'Neill E, Pozzi C, Houston P, Humphreys H, Robinson DA, et al. (2008) A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. J Bacteriol 190:3835-3850.
- McCourt J, O'Halloran DP, McCarthy H, O'Gara JP, Geoghegan JA (2014) Fibronectin-binding proteins are required for biofilm formation by community-associated methicillin-resistant Staphylococcus aureus strain LAC. FEMS Microbiol Lett 353:157-164.
- Caiazza NC, O'Toole GA (2003) Alpha-toxin is required for biofilm formation by Staphylococcus aureus. J Bacteriol 185:3214-3217.
- Huseby MJ, Kruse AC, Digre J, Kohler PL, Vocke JA, et al. (2010) Beta toxin catalyzes formation of nucleoprotein matrix in staphylococcal biofilms. Proc Natl Acad Sci U S A 107:14407-14412.
- Saïd-Salim B, Dunman PM, McAleese FM, Macapagal D, Murphy E, et al. (2003) Global regulation of Staphylococcus aureus genes by Rot. J Bacteriol 185:610-619.
- McNamara PJ, Milligan-Monroe KC, Khalili S, Proctor RA (2000) Identification, cloning, and initial characterization of rot, a locus encoding a regulator of virulence factor expression in Staphylococcus aureus. J Bacteriol 182:3197-3203.
- Mootz JM, Benson MA, Heim CE, Crosby HA, Kavanaugh JS, et al. (2015) Rot is a key regulator of Staphylococcus aureus biofilm formation. Mol Microbiol 96:388-404.
- Dubrac S, Boneca IG, Poupel O, Msadek T (2007) New insights into the WalK/WalR (YycG/YycF) essential signal transduction pathway reveal a major role in controlling cell wall metabolism and biofilm formation in Staphylococcus aureus. J Bacteriol 189:8257-8269.
- Dubrac S, Msadek T (2008) Tearing down the wall: peptidoglycan metabolism and the WalK/WalR (YycG/YycF) essential two-component system. Adv Exp Med Biol 631:214-228.
- Monk IR, Shaikh N, Begg SL, Gajdiss M, Sharkey LK, et al. (2019) Zinc-binding to the cytoplasmic PAS domain regulates the essential WalK histidine kinase of Staphylococcus aureus. Nat Commun 10:3067.
- El-Fateh M, Chatterjee A, Zhao X (2024) A systematic review of peptide nucleic acids (PNAs) with antibacterial activities: Efficacy, potential and challenges. Int J Antimicrob Agents 63:107083.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.