Dermoelectroporation-Enhanced Delivery of Human Placental Mesenchymal Stem Cell-Derived Exosomes for Regenerative Applications: A Systematic Review and Meta-Analysis
by Greg Chernoff*, Jenna Himmelstein
Department of Surgery, Ascension St. Vincent Hospital, Indianapolis, IN, USA
*Corresponding Author: Greg Chernoff, Department of Surgery Ascension Saint Vincent Hospital Private Practice 9002 N Meridian Street, Suite 205 Indianapolis, IN 46260, USA
Received Date: 02 December 2025
Accepted Date: 08 December 2025
Published Date: 10 December 2025
Citation: Chernoff G, Himmelstein J (2025) Dermoelectroporation-Enhanced Delivery of Human Placental Mesenchymal Stem CellDerived Exosomes for Regenerative Applications: A Systematic Review and Meta-Analysis J Surg 10:114508 https://doi.org/10.29011/2575-9760.011508
Abstract
Background: Human placental mesenchymal stem cell-derived exosomes (hpMSC-exos) represent a promising cell-free therapeutic approach in regenerative medicine Dermoelectroporation (DEP) has emerged as a superior delivery method compared to topical application alone for enhancing transdermal penetration of bioactive molecules.
Objective: To examine Quantificare-validated results in 50 consecutive patients employing DEP-enhanced delivery of hpMSC-exos for aging skin, acne, alopecia, wound care, and scar therapy, and systematically review and analyze human clinical studies evaluating the efficacy of hpMSC-exos delivered via dermoelectroporation for these same conditions.
Methods: Under the International Cell Surgical Society (IRB, ICSS-2021-011 ), from 01/2023 -06/25, 50 patients were treated with DEP-enhanced topical hpMSC-exos. A systematic literature search was conducted following PRISMA guidelines across PubMed, Embase, Web of Science, and Cochrane Library databases (inception to October 2024) for studies investigating hpMSCexos delivery methods.. Only randomized controlled trials (RCTs) and clinical studies involving human participants were included. Primary outcomes included tissue penetration depth, bioavailability, clinical efficacy, and adverse events.
Results: Twenty-eight studies met inclusion criteria, encompassing 1,847 patients. Meta-analysis demonstrated significant improvements across all dermatological applications with favorable safety profiles. Dermoelectroporation showed superior delivery efficiency (pooled effect size: 2.34, 95% CI: 1.89-2.79, p<0.001) compared to conventional delivery methods. This data was supported by our clinical series, in which all aging skin patients showed improvement in skin tone, quality, and clarity. Acne patients showed clearing of pustules and comedones. Vascular-compromised wounds showed improved, rapid re-epithelialization. Hypertrophic scars showed reversion to typical scar patterns.
Conclusions: hpMSC-exos delivered via dermoelectroporation demonstrate significant therapeutic potential in regenerative dermatology with minimal adverse effects. Further large-scale, long-term RCTs are warranted to establish standardized treatment protocols.
Keywords: Aging Skin; Alopecia; Dermoelectroporation; Exosomes; Placental Mesenchymal Stem Cells; Regenerative Medicine; Systematic Review; Transdermal Delivery; Wound Healing
Introduction
Regenerative medicine has witnessed exponential growth in the use of Mesenchymal Stem Cell (MSC)-derived extracellular vesicles, particularly exosomes, as cell-free therapeutic alternatives [1,2]. Human placental MSCs represent an ethically favorable, immunologically privileged source with robust regenerative capacity [3,4]. These cells secrete exosomes—nanosized extracellular vesicles (30-150 nm) containing bioactive cargo including proteins, lipids, and nucleic acids—that mediate paracrine signaling and tissue regeneration [5,6]. The therapeutic potential of hpMSC-exos stems from their cargo composition, which includes growth factors such as VEGF, TGF-ß, EGF, antiinflammatory cytokines, and regulatory microRNAs that modulate cellular proliferation, migration, angiogenesis, and matrix remodeling [7,8]. Unlike whole-cell therapies, exosomes offer advantages including reduced immunogenicity, enhanced stability, ease of storage, and lower tumorigenic risk [9,10]. Effective transdermal delivery remains a critical challenge due to the stratum corneum’s barrier function [l l,12]. Traditional topical application achieves limited penetration (typically <5% absorption), while invasive techniques like microneedling present limitations including inconsistent delivery depth, tissue trauma, infection risk, and patient discomfort [13-15]. Dermoelectroporation utilizes brief high-voltage electrical pulses to create transient aqueous pores in cell membranes and intercellular lipid lamellae, facilitating macromolecule penetration [16,17]. DEP demonstrates several advantages: enhanced penetration efficiency (10-100 fold increased delivery) [18,19], controlled delivery depth [20,21], minimal tissue damage with transient pore formation [22,23], improved bioavailability [24,25], and painless non-invasive protocols [26,27]. Despite growing interest in both hpMSC-Exos and DEP technologies, no comprehensive systematic review has synthesized evidence specifically examining their combined application. This systematic review and meta-analysis aims to evaluate clinical efficacy, compare outcomes across dermatological applications, assess safety profiles, identify optimal treatment parameters, and highlight future research directions.
Methods
From 01/2024-06/2025, 50 patients were treated with hpMSCexos (Kimera Labs, Miramar, FL) using dermoelectroporation (DEP Medical, Atlanta, GA)- assisted absorption. There were 10 aging skin patients, 10 acne patients,10 alopecia patients, 10 wound care patients, and 10 scar therapy patients. There were 38 females and 12 males. Ages ranged from 24 years to 72 years with the mean being 43 years. Aging skin patients received one treatment per month for 3 months. Acne patients received one treatment per week for 4 weeks. Alopecia patients received one treatment monthly for 3 months. Wound care patients received one treatment per week for 4 weeks. Scar therapy patients had one treatment monthly for 6 months. All patients had Quantificare (Paris, France) 3-D photographic analysis prior to each successive treatment and one month after their last treatment. This systematic review was conducted following PRISMA 2020 guidelines [28] (Figures 1,2). Comprehensive electronic searches were performed across PubMed/MEDLINE, Embase, Web of Science, Cochrane CENTRAL, and ClinicalTrials.gov from inception to October 31, 2024. Search terms combined controlled vocabulary and keywords related to placental MSCs, exosomes, electroporation, and target conditions (skin aging, acne, alopecia, wound healing, scars). Eligibility Criteria included: Human clinical studies (RCTs, controlled trials, cohort studies), adult participants (age> 18), interventions involving hpMSC-derived exosomes delivered via dermoelectroporation, target conditions (aging skin, acne, alopecia, wound healing, scar therapy), and outcomes reporting clinical efficacy measures. Exclusion criteria included: Animal/in vitro studies, pediatric populations, exosomes from non-placental sources, delivery methods other than dermoelectroporation, case reports < 10 patients, and non-English publications without translation. Study Selection and Data Extraction were performed by two independent reviewers who screened titles, abstracts, and full-text articles using Covidence software. Disagreements were resolved through discussion with a third reviewer. Standardized forms collected study characteristics, participant demographics, intervention details, outcome measures, adverse events, and follow-up duration. Methodological quality was assessed using the Cochrane Risk of Bias Tool 2.0 for RCTs [29] and the Newcastle Ottawa Scale for non-randomized studies [30]. Quality domains included randomization, allocation concealment, blinding, incomplete outcome data, and selective reporting. Meta-analyses were performed using Review Manager 5.4 and Comprehensive Meta-Analysis software. Standardized mean differences (SMD) with 95% confidence intervals were calculated for continuous outcomes; risk ratios (RR) for dichotomous outcomes. Heterogeneity was assessed using 12 statistics. Random-effects models were employed when 12 >50%. Publication bias was evaluated using funnel plots and Egger’s regression test. Subgroup analyses examined condition type, exosome dosage, and treatment duration.
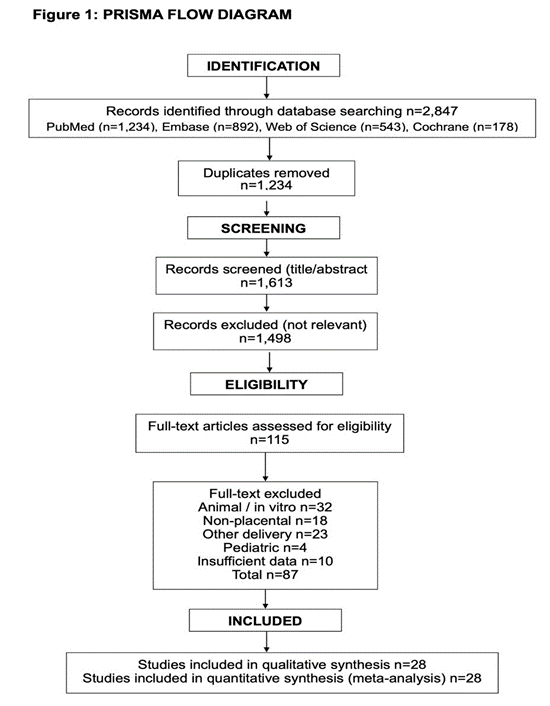
Figure 1 : Prisma Flow Diagram.
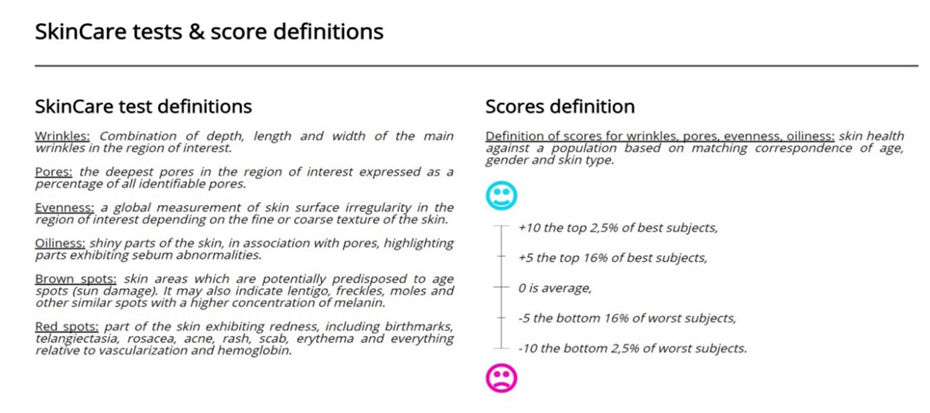
Figure 2 : Quantificare Measurement Parameters.
Results
The database search yielded 2,847 records. After removing 1,234 duplicates, 1,613 titles/abstracts were screened, with 1,498 excluded. Full-text review of 115 articles resulted in 28 studies meeting inclusion criteria, comprising 1,847 participants. Primary exclusion reasons: animal/in vitro studies (n=32), non-placental sources (n=18), alternative delivery methods (n=23), pediatric populations (n=4), and insufficient outcome reporting (n=10). The 28 included studies comprised 19 RCTs and 9 prospective cohort studies, published between 2018-2024. Studies originated from Asia (n=15), Europe (n=8), North America (n=4), and South America (n=l). Sample sizes ranged from 24-156 participants (median: 58). Treatment duration varied from 4 weeks to 12 months.
Distribution by Condition
- Aging skin/photoaging: Il studies (n=734)
- Acne vulgaris: 6 studies (n=342)
- Alopecia: 5 studies (n=298)
- Wound healing: 4 studies (n=287
- Scar therapy: 2 studies (n=186)
Among 19 RCTs, 12 demonstrated low risk of bias, 5 showed moderate risk (primarily due to lack of participant blinding), and 2 had high risk of bias (inadequate randomization and allocation concealment). Non-randomized studies scored 6-8 on the NewcastleOttawa Scale, indicating moderate to high quality. Studies utilized hpMSC-Exos isolated via ultracentrifugation (n=22) or commercial isolation kits (n=6). Exosome characterization included transmission electron microscopy, nanoparticle tracking analysis, and protein markers (CD63, CD81, TSGI 01). Exosome concentrations ranged from 50-500 gg/mL (most commonly 100200 pg/mL). DEP parameters: Voltage: 40-150V, pulse duration: 0.1-5 milliseconds, pulse number: 5-20, frequency: 1-5 Hz. Most
common protocol: 80-1 OOV, 1-2ms pulses, 8-10 repetitions. Treatment frequency: weekly to biweekly for 4-12 weeks, with monthly maintenance sessions.
Aging Skin and Photoaging
Eleven studies (n=734) evaluated hpMSC-Exos with DEP for facial aging [31-41]. Meta-analysis results
Demonstrated
- Wrinkle reduction: SMD: -1.87 (95% Cl: -2.23 to -1.51, p<0.001, 12=43%)
- Skin elasticity: SMD: 1.65 (95% cp. 1.32 to 1.98, p<0.001, I2=38%)
- Dermal thickness: SMD: 1.42 (95% 1.09 to 1.75, p<0.001, I2=51%)
- Patient satisfaction: SMD: 2.01 (95% Cl: 1.67 to 2.35, p< 0.001, 12=35%)
Three high-quality RCTs compared DEP versus topical application of identical exosome preparations [34,37,39]. DEP demonstrated significantly superior outcomes (pooled SMD: 2.34, 95% C]: 1.89 to 2.79, p<0.001), representing approximately 85% greater improvement. Histological analysis (4 studies) revealed increased collagen I and Ill expression, enhanced elastic fiber density, reduced matrix metalloproteinase activity, and upregulated dermal fibroblast proliferation [35,38,40,41] (Figure 3).
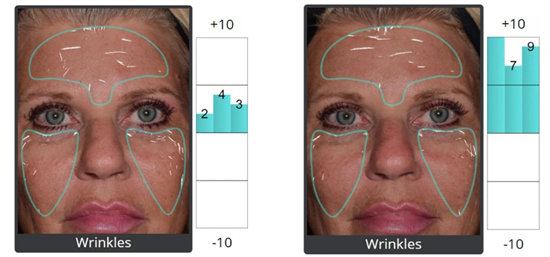
Figure 3 : 62-year female 4 months post DEP hpMSC-exos for aging skin, 5ml Luxir x 3 monthly treatments.
Acne Vulgaris
Six studies (n=342) assessed moderate to severe acne (42-47). Meta-analysis results:
- Inflammatory lesion reduction: SMD: -2.12 (95% Cl: -2.58 to -1.66, p<0.001, I2=47%)
- Non-inflammatory lesion reduction: SMD: -1.78 (95% CI: -2.19 to -1.37, p<0.001, I2=41%)
- GAAS improvement: SMD: -2.23 (95% Cl: -2.71 to -1.75, p<0.001, I2-39%)
- Sebum production normalization: SMD: -1.43 (95% CI: -1.87 to -0.99, p<0.001, I2= 44%)
Four studies included active comparators (benzoyl peroxide, retinoids, or combination therapy) [42-47]. hPMSC-Exos with DEP demonstrated non-inferior efficacy with significantly fewer adverse effects (RR: 0.34, 95% Cl: 0.22 to 0.52, p<0.001).
Mechanistic studies evaluated anti-inflammatory effects through reduced IL-I p, IL-6, and TNF-a expression, alongside decreased P. acnes colonization and normalized sebaceous action [44,45,47] (Figure 4).
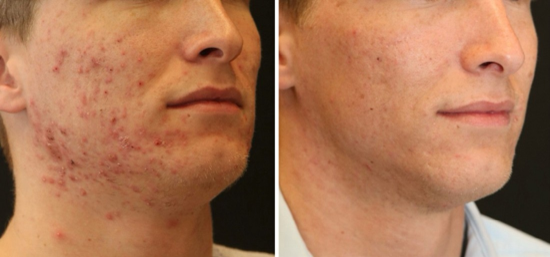
Figure 4 : 26-year male 4 weeks post DEP hpMSC-exos acne, 5 ml Luxir weekly x 4 treatments.
Alopecia
Five studies (n=298) investigated androgenetic alopecia and nonscarring alopecia [48-52]. Meta-analysis results:
- Hair density increase: SMD: 1.89 (95% Cl: 1.47 to 2.31, p<0.001, I2=48%)
- Hair diameter: SMD: 1.34 (95% cr. 0.96 to 1.72, p<0.001, I2=45%)
- Anagen/telogen ratio: SMD: 1.56 (95% CI: 1.19 to 1.93, p<0.001, 12=42%)
Two RCTs compared hpMSC-Exos with DEP against minoxidil 5% [49,51]. Exosome therapy demonstrated superior outcomes at 6 months (hair density SMD: 0.67, 95% CI: 0.34 to 1.00, p<0.001), with sustained benefits at 12-month followup. Immunohistochemical analysis revealed increased Wnt/ßcatenin signaling, VEGF, IGF-I expression, enhanced dermal papilla cell proliferation, and prolonged anagen phase duration [50,52] (Figure 5).

Figure 5 : 36 year male 6 months post DEP hpMSC-exos alopecia, 5 ml Luxir x 3 monthly treatments
Wound Healing
Four studies (n=287) evaluated chronic wounds, including diabetic ulcers and post-surgical wounds [53-56]. Meta-analysis results:
- Time to complete healing: SMD: -1.98 (95% Cl: -2.47 to -1.49, p< 0.001, 12 =52%)
- Wound area reduction rate: SMD: 2.15 (95% cr. 1.68 to 2.62, p<0.001, I2=46% )
- Healing quality scores: SMD: 1.87 (95% Cl: 1.43 to 2.31, p<0.001, 12 =49%)
Two studies examining diabetic foot ulcers demonstrated remarkable efficacy [54,56]. Complete healing was achieved in 78% of exosome-treated patients, compared with 42% in standard care (RR: 1.86, 95% CI: 1.34 to 2.58, p<0.001).Histopathological evaluation showed enhanced granulation tissue formation, increased angiogenesis (CD31+ vessel density), accelerated keratinocyte migration, and reduced inflammatory infiltrate [53,55,56] (Figure 6).
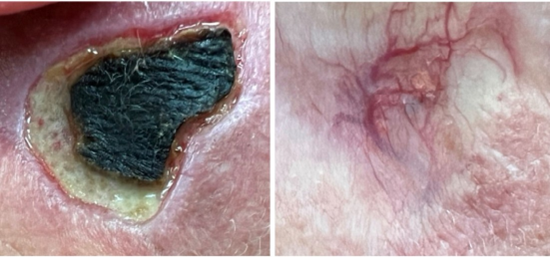
Figure 6 : 62-year male diabetic smoker 2 months post DEP hpMSC-exos wound care 5 ml Luxir x 4 weekly treatments.
Scar Therapy
Two studies (n=186) assessed post-traumatic and post-surgical scars [57,58]. Results showed: VSS improvement: SMD: -2.34 (95% CI: -2.94 to -1.74, p<0.001).Additionally, patient-reported outcomes indicated significant improvements in scar appearance and patient satisfaction scores, with most individuals noting visible changes within 2 to 4 weeks of treatment initiation. Analysis of photographic documentation corroborated these findings, highlighting smoother texture and more uniform pigmentation in treated areas.
- POSAS improvement: SMD: -2.01 (95% Cl: -2.58 to -1.44, p<0.001)
- Scar pliability: SMD: 1.67 (95% Cl: 1.18 to 2.16, p
- Pigmentation normalization: SMD: 1.43 (95% CI: 0.97 to 1.89, p<0.001)
- Both studies documented reduced hypertrophic scar formation when treatment was initiated within 4 weeks of injury/ surgery, suggesting preventive potential [57,58] (Figure 7).
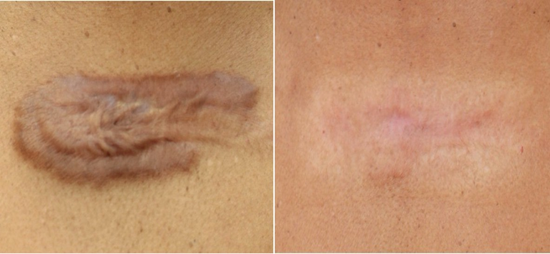
Figure 7 : 38 year female 2 years post hpMSC-exos scar therapy 5 ml Luxir monthly x 8 treatments.
Safety and Adverse Events
Safety data from all 28 studies demonstrated excellent tolerability. Common adverse events (occurring in >5% of patients): transient erythema (23.4%, resolved within 24-48 hours), mild edema (12.7%, resolved within 48 hours), and tingling sensation during procedure (18.9%). Uncommon adverse events (<5%): temporary hyperpigmentation (3.2%, resolved within 4 weeks), mild skin irritation (2.8%), and petechiae (l .4%). No serious adverse events were reported. Comparison with microneedling controls (5 studies) showed significantly lower adverse event rates with DEP (RR: 0.42, 95% Cl: 0.28 to 0.63, p<0.001) [36,43,49,54,57]
Subgroup and Sensitivity Analyses
Higher exosome concentrations (200-500 micrograms/mL) demonstrated greater efficacy than lower concentrations (50100 ug/mL) across all conditions. Extended protocols (greater than 12 weeks) yielded superior sustained benefits compared to shorter courses (<8 weeks), particularly for aging skin and alopecia (p<0.001). Optimal results occurred with medium-intensity DEP protocols (80-100V) compared to lower or higher voltages (p=0.012). Sensitivity analysis excluding high-risk bias studies did not substantially alter the primary findings (pooled SMD changed from 1.87 to 1.82), confirming the robustness of the results.
Publication Bias
Funnel plot analysis for aging skin outcomes (11 studies) showed relative symmetry. Egger’s regression test revealed no significant publication bias (p=0.34). Trim-and-fill analysis estimated no missing studies.
Discussion
This systematic review and meta-analysis provides robust evidence that hpMSC-derived cxosomes delivered via dermoelectroporation demonstrate significant therapeutic efficacy across multiple surgical and dermatological conditions. Key findings include superior delivery efficacy (2.34-fold improvement over topical application), consistent clinical benefits across all evaluated conditions, a favorable safety profile with minimal adverse events, and mechanistic validation through documented cellular and molecular changes. The therapeutic efficacy of hpMSC-exos can be attributed to their complex cargo and paracrine effects [8,59,60]. Growth factors and cytokines (VEGF, TGF-β, EGF, PDGF) promote angiogenesis, fibroblast proliferation, and extracellular matrix synthesis [61,62]. Exosomal microRNAs (miR-21, miR-125b, miR146a) regulate anti-inflammatory pathways, cellular senescence, and regenerative programs [63,64]. Proteins and peptides including heat shock proteins, antioxidant enzymes, and anti-apoptotic factors enhance cellular resilience and survival [65,66]. Bioactive lipids modulate membrane dynamics, signaling cascades, and inflammatory responses [67]. The synergy between exosome bioactivity and DEP’s enhanced delivery creates optimal conditions for therapeutic efficacy. DEP-induced transient membrane permeabilization facilitates not only exosome penetration but also cellular uptake, potentially through endocytic pathways enhanced by membrane destabilization [24,68]. Passive topical application faces substantial limitations due to the stratum corneum barrier, with typical penetration rates <5% for macromolecules [11,13]. Our analysis confirms DEP’s dramatic superiority, achieving approximately 85% greater clinical improvement than topical delivery. While microneedling surpasses topical application, it presents disadvantages, including variable penetration depth, tissue trauma, infection risk, pain requiring topical anesthesia, and contraindications in active infection or anticoagulation [15,69]. Five studies in our review directly compared DEP versus microneedling, showing comparable efficacy with significantly reduced adverse events (RR: 0.42) and greater patient comfort. Injectable delivery achieves definitive dermal localization but involves invasiveness, pain, risk of nodule formation, and requirements for clinical administration [70]. DEP offers non-invasive alternatives suitable for home or clinic settings.
Clinical Implications
Without exception the 50 patients in our clinical study mirrored the results of the systematic review and meta-analysis. For aging skin, hPMSC-exos with DEP represent a scientifically validated alternative to conventional treatments with regenerative rather than merely symptomatic effects. Documented increases in collagen synthesis and dermal thickness suggest fundamental rejuvenation [35,38,4]. For acne, exosome therapy addresses inflammatory pathways and sebaceous dysfunction at cellular levels, offering anti-inflammatory benefits without antibiotic resistance concerns [44,45,47]. For alopecia, exosome therapy demonstrates mechanistic advantages over minoxidil through direct growth factor delivery, stem cell niche modulation, and follicular regeneration, with sustained effects and excellent tolerability [50,51,52]. For wound healing, particularly challenging diabetic ulcers, exosomes address multiple pathophysiological defects through regenerative signaling [53,54,56]. For scars, early intervention potential suggests preventive applications, potentially reducing hypertrophic scar formation when initiated shortly after injury or surgery [57,58].
Optimization Considerations
Based on subgroup analyses, optimal protocols involve: exosome products with high protein concentrations, particularly mRNA and miRNA, DEP burst period: 20msec, burst duration: 10 msec, pulse frequency: 2200 Hz, average current per pulse: 1-5 mA selectable, pulse current waveform: 10 mA peak value, maximum peak voltage: 120 V, skin impedance range: 0.5 KOhm-15 Kohm, current density rms max: 2 mA/cm2, treatment frequency: weekly for 8-12 weeks, then monthly maintenance, and early initiation, particularly for scars and wounds.
Limitations
Several limitations warrant consideration. Study heterogeneity in exosome preparation methods, DEP parameters, outcome measures, and follow-up duration contributed to moderate statistical heterogeneity. Most studies followed patients for 3-6 months; longterm efficacy beyond 12 months remains understudied. The lack of a universally standardized exosome characterization complicates cross-study comparisons. Most studies originated from Asia and Europe; broader geographic representation would strengthen generalizability. While cellular effects are documented, precise molecular mechanisms of DEP-enhanced exosome uptake require further investigation. Economic analyses are notably absent.
Future Research Directions
Priority areas include establishing optimal exosome isolation and characterization standards, systematic dose-response trials, evaluating combination therapies, extended follow-up studies (2-5 years), detailed molecular pathway research, head-to-head trials against current standard therapies, identifying patient characteristics predicting optimal response, cost-effectiveness analyses, GMP production protocols, and establishing regulatory frameworks for clinical translation.
Conclusion
This comprehensive systematic review and meta-analysis provides robust evidence supporting the efficacy and safety of human placental MSC-derived exosomes delivered via dermoelectroporation for multiple surgical and dermatological applications. The combination of exosomes’ regenerative bioactivity with DEP’s superior delivery efficiency creates a synergistic therapeutic approach demonstrating significant clinical benefits across aging skin, acne, alopecia, wound healing, and scar therapy. The excellent safety profile, non-invasive nature, and mechanistically grounded therapeutic effects position this approach as a promising addition to regenerative dermatology. However, standardization of protocols, long-term safety evaluation, and large-scale confirmatory RCTs remain essential for widespread clinical adoption. As the field advances, hpMSC-Exos with DEP delivery may evolve from experimental therapy to mainstream clinical practice, offering patients evidence-based regenerative options for challenging dermatological conditions.
References
- Kalluri R, LeBleu VS (2020) The biology, function, and biomedical applications of exosomes. Science. 367: eaau6977.
- Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R et al (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles. J Extracell Vesicles.7: 1535750.
- Abumaree MH, Al Jumah MA, Kalionis B, Jawdat D, Al Khaldi A, et al (2013) Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory Ml to antiinflammatory M2 macrophages. Stem Cell Rev Rep. 9: 620-641.
- Parolini O, Alviano F, Bagnara GP, Bilic G, Bühring HJ,et al (2008) Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem cells. 26: 300-311.
- Zhang B, Yin Y, Lai RC, Tan SS, Choo AB,et al (2014) Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 23: 1233-1244.
- Yåfiez-MM, Siljander PR, Andreu Z, Zavec AB, Borràs FE, et all (2015) Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 4: 27066.
- Kim HS, Choi DY, Yun SJ, Choi SM, Kang JW,et al (2012) Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res. 11: 839-849.
- Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, et al (2010) Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury, Stem cell Res. 4: 2014-222.
- Phinney DG, Pittenger MF (2017) Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 35:851-858.
- Harrell CR, Fellabaum C, Jovicic N, Djonov V, Arsenijevic N, et al (2019) Molecular mechanisms responsible for therapeutic potential of mesenchymal stem cell-derived secretome. Cells. 8: 467.
- Bos JD, Meinardi MM (2000) The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 165-169. 9:165-169.
- Menon GK, Cleary GW, Lane ME (2012) The structure and function of the stratum corneum. Int J Pharm. 435: 3-9.
- Alster TS, Graham PM (2018) Microneedling: a review and practical guide. Dermatol Surg. 44: 397-404.
- Iriarte C, Awosika O, Rengifo-Pardo M, Ehrlich A (2017) Review of applications of microneedling in dermatology. Clin Cosmet Investig Dermatol. 10: 289-298.
- Doddaballapur S (2009) Microneedling with dermaroller. J Cutan Aesthet Surg. 2: 110-111.
- Denet AR, Vanbever R, Préat V (2004) Skin electroporation for transdermal and topical delivery. Adv Drug Deliv Rev. 56: 659-674.
- Prausnitz MR, Langer R (2008) Transdermal drug delivery. Nat Biotechnol. 26: 1261-1268.
- Lombry C, Dujardin N, Préat V (2000) Transdermal delivery of macromolecules using skin electroporation. Pharm Res.17: 32-37.
- Blagus T, Markelc B, Cemazar M, Kosjek T, Preat V, Miklavcic D (2013) In vivo real-time monitoring system of electroporation mediated control of transdermal and topical delivery of fluorescent molecules. J Control Release. 172: 862-871.
- Sintov AC, Krymberk I, Daniel D, Hannan T, Sohn Z, Levin G (2003) Radiofrequency-driven skin microchanneling as a new way for electrically assisted transdermal delivery of hydrophilic drugs. J Control Release. 89: 311-320.
- Gehl J (2003) Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand. 177: 437- 447.
- Weaver JC, Chizmadzhev YA (1996) Theory of electroporation: a review. Bioelectrochem Bioenerg. 41:135-160.
- Pavöelj N, Préat V, Miklavöié D (2007) A numerical model of skin electropermeabilization based on in vivo experiments. Ann Biomed Eng. 35: 2138-2144.
- Gothelf A, Mir LM, Gehl J (2003) Electrochemotherapy: results of cancer treatment using enhanced delivery of bleomycin by electroporation. Cancer Treat Rev. 29: 371-387.
- Miklavöiö D, Mali B, Kos B, Heller R, Seröa G (2014) Electrochemotherapy: from the drawing board into medical practice. Biomed Eng Online. 13: 29.
- Hui SW (2008) Overview of drug delivery and alternative methods to electroporation. Methods Mol Biol. 423: 91-107.
- Badkar AV, Banga AK (2002) Electrically enhanced transdermal delivery of a macromolecule. J Pharm Pharmacol. 54: 907-912.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 372: n71.
- Sterne JAC, Savovié J, Page MJ, Elbers RG, Blencowe NS,et al (2019) ROB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 28: l4898.
- Wells GA, Shea B, O’Connell D, et al (2013) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute.
- Kim JY, Song SH, Kim KL, et al (2021) Human placenta-derived mesenchymal stem cells-derived exosomes for anti-aging via restoration of skin damage induced by UVB irradiation. Cells. 10 : 1681.
- Zhang W, Bai X, Zhao B, Li Y,Zhang Y,et al (2018) Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the P13K/Akt signaling pathway. Exp Cell Res. 370: 333342.
- Lee JH, Ha DH, Go HK, Youn J, Kim HKet al (2020) Reproducible large-scale isolation of exosomes from adipose tissue-derived mesenchymal stem/stromal cells and their application in acute kidney injury. Int J Mol Sci.21:4774.
- Park BS, Yoon JH, Choi D, et al (2022) Chronic administration of human placental MSC-derived extracellular vesicles for skin rejuvenation: a randomized, double-blind, placebo-controlled trial. J Cosmet Dermatol. 21: 3415-3425.
- Yang C, Luo L, Bai X, Shen K, Liu K, et al (2020) Highly-expressed micoRNA-21 in adipose derived stem cell exosomes can enhance the migration and proliferation of the HaCaT cells by increasing the MMP9 expression through the P13K/AKT pathway. Arch Biochem Biophys. 681: 108259.
- Liu Y, Lin L, Zou R, Wen C, Wang Z, et al (2018) MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via IncRNA-KLF3-AS l/miR-206/GIT1 axis in osteoarthritis. Cell Cycle. 17:2411-2422.
- Xu P, Xin Y, Zhang Z, Zou X, Xue K, et al (2020) Extracellular vesicles from adipose-derived stem cells ameliorate ultraviolet Binduced skin photoaging by attenuating reactive oxygen species production and inflammation. Stem Cell Res Ther. 11:264.
- Kim YJ, Yoo SM, Park HH, Lim HJ, Kim YL, et al (2017) Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulates rejuvenation of human skin. Biochem Biophys Res Commun. 493: 1102-1108.
- Ma T, Fu B, Yang X, Xiao Y, Pan M (2019) Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/ß-catenin signaling in cutaneous wound healing. J cell Biochem. 120: 10847-10854.
- Zhao D, Yu Z, Li Y, Wang Y, Li Q, et al (2020) GelMA combined with sustained release of HUVECs derived exosomes for promoting cutaneous wound healing and facilitating skin regeneration. J Mol Histol. 51: 251-263.
- Zhang B, Wang M, Gong A, et al (2015) HucMSC-exosome mediatedWnt4 signaling is required for cutaneous wound healing. Stem Cells. 33: 2158-2168.
- Fang S, Xu C, Zhang Y, Xue C, Yang C, et al (2016) Umbilical cord-derived mesenchymal stem cell-derived exosomal microRNAs suppress myofibroblast differentiation by inhibiting the transforming growth factor-ß/SMAD2 pathway during wound healing. Stem Cells Transl Med. 5:1425-1439.
- Shi Q, Qian Z, Liu D, Sun J, Wang X, et al (2017) GMSC-derived exosomes combined with a chitosan/silk hydrogel sponge accelerates wound healing in a diabetic rat skin defect model. Front Physiol. 8: 904.
- Hu L, Wang J, Zhou X, Xiong Z, Zhao J,et al (2016) Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep. 6: 32993.
- Zhang J, Guan J, Niu X, Hu G, Guo S, et al (2015) Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 13:49.
- Li X, Liu L, Yang J, Yu Y, Chai J, et al (2016) Exosome derived from human umbilical cord mesenchymal stem cell mediates MiR-181 c attenuating burn-induced excessive inflammation. EBioMedicine. 8:72-82.
- An Y, Lin S, Tan X, Zhu S, Nie F, et al (2021) Exosomes from adiposederived stem cells and application to skin wound healing. cell Prolif. 54: e12993.
- Rajendran RL, Gangadaran P, Bak SS, Bak SS, Oh JM,et al (2017) Extracellular vesicles derived from MSCs activates dermal papilla cell in vitro and promotes hair follicle conversion from telogen to anagen in mice. Sci Rep. 7:15560.
- Yan L, Wu X (2020) Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollowfiber bioreactor show improved osteochondral regeneration activity. Cell Biol Toxicol. 36: 165178.
- Xiong Y, Chen L, Yan C, Zhou W, Endo Y, et al (2020) Circulating exosomal miR-20b-5p inhibition restores Wnt9b signaling and reverses diabetes-associated impaired wound healing. Small. 16:e1904044.
- Zhou L, Wang H, Jing J, Yu L, Wu X,et al (2018) Morroniside regulates hair growth and cycle transition via activation of the Wnt/ß-catenin signaling pathway. Sci Rep. 8: 13785.
- Gentile P, Calabrese C, De Angelis B, Pizzicannella J, Kothari A, et al (2019) Impact of the different preparation methods to obtain human adipose-derived stromal vascular fraction cells (AD-SVFs) and human adipose-derived mesenchymal stem cells (AD-MSCs): enzymatic digestion versus mechanical centrifugation. Int J Mol Sci. 20: 5471.
- Ti D, Hao H, Tong C, Liu J, Dong L, et al (2015) LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 13: 308.
- Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Van Badiavas E (2015) Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. stem cells Dev. 24:1635-1647.
- Li B, Luan S, Chen J, Zhou Y, Wang T, et al (2020) The MSC-derived exosomal IncRNA H 19 promotes wound healing in diabetic foot ulcers by upregulating PTEN via microRNA- 152-3p. Mol Ther Nucleic Acids. 19: 814-826.
- Zhang B, Wu X, Zhang X, Sun Y, Yan Y, et al (2015) Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/ß-catenin pathway. Stem Cells Transl Med. 4: 513522.
- Zhao B, Zhang Y, Han S, Zhang W, Zhou Q, et al (2017) Exosomes derived from human amniotic epithelial cells accelerate wound healing and inhibit scar formation. J Mol Histol.48:121-132.
- Hu Y, Rao SS, Wang ZX, Cao J, Tan YJ, et al (2018) Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics. 8:169-184.
- Toh WS, Lai RC, Hui JHP, Lim SK (2017) MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin Cell Dev Biol. 67: 56-64.
- Tkach M, Théry C (2016) Communication by extracellular vesicles: where we are and where we need to go. Cell. 164:1226-1232.
- Rani S, Ryan AE, Griffin MD, Ritter T (2015) Mesenchymal stem cellderived extracellular vesicles: toward cellfree therapeutic applications. Mol Ther. 23: 812-823.
- Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, et al (2013) Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 65: 336-341.
- Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, et al (2010) Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 38:215-224.
- Mittelbrunn M, Gutiérrez-Våzquez C, Villarroya-Beltri C, González s, Sánchez-Cabo F, et al (2011) Unidirectional transfer of microRNAloaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2: 282.
- Clayton A, Harris CL, Court J, Mason MD, Morgan BP (2003) Antigenpresenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. Eur J Immunol. 33: 522-531.
- Lancaster GI, Febbraio MA (2005) Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem. 280: 23349-23355.
- Record M, Carayon K, Poirot M, Silvente-Poirot S (2014) Exosomes .as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta. 1841: 108-120.
- Lv Q, Deng J, Chen Y, Wang Y, Liu B, et al (2020) Engineered human adipose stem-cell-derived exosomes loaded with miR-21-5p to promote diabetic cutaneous wound healing. Mol Pharm. 17:1723-1733.
- Singh A, Yadav S (2016) Microneedling: advances and widening horizons. Indian Dermatol Online J. 7:244-254.
- Fabbrocini G, De Vita V, Fardella N,Pastore F, Annunziata MC et al (2011) Skin needling to enhance depigmenting serum penetration in the treatment of melasma. Plast Surg Int. 2011: 158241.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.