Delayed Activation of Sirt1 Induced Autophagy Signaling Promotes Astrogliosis, Infarct in Experimental Stroke
Sireesh Kumar Teertam1, Varalakshmi Manchana2, Prakash Babu Phanithi3*
1Department of Biotechnology & Bioinformatics, school of Life sciences, University of Hyderabad, Hyderabafd-500046 (T.S), India.
2School of Medical Sciences, University of Hyderabad, Hyderabad (T.S), India
3Department of Biotechnology and Bioinformatics, School of Life Sciences, University of Hyderabad, Hyderabad (T.S), India
*Corresponding author: Prakash Babu Phanithi, Department of Biotechnology & Bioinformatics, School of Life Sciences, University of Hyderabad, Prof. C. R. Rao Road, Gachibowli, Hyderabad -500 046 (T.S), India
Received Date: 10 April, 2023
Accepted Date: 24 April, 2023
Published Date: 27 April, 2023
Citation: Teertam SK, Manchana V, Phanithi PB (2023) Delayed Activation of Sirt1 Induced Autophagy Signaling Promotes Astrogliosis, Infarct in Experimental Stroke. Int J Cerebrovasc Dis Stroke 6:148. DOI: https://doi.org/10.29011/2688-8734.100048
Abstract
Curcumin, a polyphenol, is an active constituent derived from Curcuma longa. The protective nature of curcumin against cerebral ischemia-reperfusion (CIR) is well documented. This study aims evaluate the changes in Sirtuin-1 (Sirt1)induced autophagy signaling. We also investigated the neuroprotective effect of curcumin on Sirt1-mediated autophagy and neuronal survival on day7 post-stroke in a rat model of middle cerebral artery occlusion (MCAO). MCAO was performed for 60 min in male Sprague Dawley rats. Immunoblotting and immunohistochemistry were used to detect alteration in Sirt1 and autophagy-related proteins Beclin-1, autophagy-related genes-3, 5, and 7 (Atg), microtubule-associated protein-1A light chain3 (Lc3-I/II), mammalian target of rapamycin (mTOR), p-mTOR, and cleaved caspase-3. CIR, on day1 post-stroke, resulted in significant alteration of Lc3-II/I and p-mTOR. Further, on day7 post-stroke, Sirt1-induced autophagy decreased, while mTOR phosphorylation and cleaved caspase-3 immunoreactivity increased. Curcumin treatment promoted astrogliosis and infarct. Sirt1-mediated autophagy signaling is involved in the promotion of astrogliosis and infarct. EX-527 blocked the increase in Sirt1/ autophagy signaling evoked by curcumin and reduced astrogliosis and infarct. These findings show that curcumin may promote infarct and reactive astrogliosis via activation of Sirt1/autophagy signaling.
Keywords: Curcumin; Sirtuin-1; Autophagy; Astrogliosis; Caspase-3; MCAO
Introduction
Cerebral ischemia (CI) is the leading cause of human mortality and morbidity. Recent studies have demonstrated that some mediators of acute neuronal injury have a protective role in the delayed phase following CI [1]. Failure in neuroprotection may partly be because many neuroprotectants inhibit both the injury mechanisms and those molecular mechanisms required for repair.
In this study, we questioned whether the same phenomenon might also be manifested in neuronal survival pathways.
Curcumin, a polyphenolic compound extracted from Curcuma longa’s rhizomes [2], exhibits protective activity as proven in cardiomyocytes, liver, and lung against ischemiareperfusion injury [3]. Curcumin exhibits anti-inflammatory and anti-apoptotic properties against CIR [4]. It promotes Sirt1 expression and modulates autophagy by regulating several cellular mechanisms [5,6].
Sirt1 is a class-III histone and non-histone deacetylase that depends on NAD+ for its enzymatic activity [7-9]. Sirt1 activates neuronal survival against numerous neurological disorders, like Alzheimer’s and Parkinson’s [10]. Further, Sirt1 activation is shown to reduce neuronal injury in experimental stroke models. Sirt1 protects against CI by regulating p53-mediated neuronal apoptosis and mediates hyperbaric oxygen preconditioning-induced CI tolerance [11-13]. Recent research has linked an increase in Sirt1 expression in the brains of stroke patients [14]. Further, sirt1 can directly or indirectly influence autophagy induction under nutrient starvation/cellular stress [15,16].
Recent studies have shown that some mediators of acute neuronal injury have a protective role in the delayed phase of CIR. Stress-responsive JNK is shown to promote neurovascular remodeling and recovery during delayed stroke [1]. Activation of matrix metalloproteinases (MMP) degrades extracellular matrix during acute ischemia, but inhibition of MMPs in chronic phase prevents neurovascular remodeling and functional recovery [17]. Similarly, delayed inhibition of excitotoxic N-methyl D-aspartate (NMDA) receptors prevent synaptic plasticity and stroke recovery [18]. In the current study looked at the role of Sirt1/autophagy signaling in neuronal death in rats following MCAO. We also examined the neuroprotective effect of curcumin on Sirt1/ autophagy signaling and neuronal survival on day7 post-stroke to understand the effect of curcumin on cellular mechanisms required for neuronal repair during delayed stroke.
Materials and Methods
Reagents and Chemicals
All the antibodies used in this study were purchased from Cell Signaling Technology (CST, US) and drugs were purchased from Sigma (US). Sirt1 (CST; #8649), Autophagy Antibody Sampler Kit (CST; #4445), beta-actin (CST; #8457), EX-527 (Sigma; #E7034-5MG), Curcumin (Sigma; #C1386), GFAP (CST; #12389), Anti-rabbit Ig-G-Alexa fluor-488 (CST; #4412), Antimouse Ig-G Alexaflour-555 (CST; # 4409), Prolong Gold Antifade Reagent with DAPI (CST; #8961), MCAo suture (Ethicon 3-0 non-absorbable; #NW3321), Immunohistochemistry kit (Bio SB, US; #BSB0016), TTC stain (Sigma; #T8877-5G), Ac-DEVDAFC substrate (Sigma; #A0466-1MG).
Transient Middle Cerebral Artery Occlusion (MCAO)
Animal experiments were performed following Institutional and National Animal Ethical Committees (IAEC) guidelines. The National Centre for Laboratory Animal Sciences (NCLAS) in India provided young (3-4 month) male Sprague-Dawley (SD) rats. Animals were housed in a controlled environment with free access to water and food. To avoid sex and age differences, adult male rats were used exclusively. Focal CI was induced by MCAO as per Longa et al. [19]. Rats were anesthetized with ketamine (60mg/Kg) and xylazine (10mg/Kg) administration via the IP route. A middle neck incision was made to identify the left common carotid artery (CCA), internal carotid artery (ICA), and external carotid artery (ECA). CCA and ICA were clipped with microvascular clips, and the ECA was ligated distally. To block the origin of MCA, a 3-0 nylon monofilament was inserted through an arteriotomy and gently advanced into ICA. Monofilament was inserted into CCA but not MCA in the sham-operated group. After 60 minutes of occlusion, the monofilament was gently removed to allow reperfusion and the animals to recover.
Two different treatment protocols were followed to understand the role of Sirt1-induced autophagy during the delayed stroke. Curcumin alone and curcumin + EX-527 combination (Combination therapy was preferred to minimize the animal number and their suffering). Curcumin activates Sirt1-induced autophagy signaling, while EX-527, a Sirt1-specific inhibitor, inhibits Sir1. We performed all the drug administrations in the early day7.
Animals were sacrificed at the end of the day7. Curcumin (300 mg/kg in 5N NaOH + saline; pH-7.4) was administered via intraperitoneal (IP) route [20], and EX-527 (30 mg/kg in 1% DMSO in PBS) was administered via intracerebroventricular (ICV) route [12]. 5N NaOH in PBS and 1% DMSO in PBS was used as a vehicle for respective groups.
Neurological Deficit Evaluation
We evaluated the neurological deficit for infarct intensity using the 4-point scale described previously [21]. Rats having no abnormality were scored as grade 0, rats who failed to extend forelimb opposite to infarct were scored as grade 1, animals with decreased resistance to lateral push were scored as grade 2, rats which show hemiparesis were scored as grade 3, and the rats who died because of the severe lesion were scored as grade 4. Rats with neurological scores below grade 3 were excluded from the experimental groups.
Immunoblotting
Control and experimental rats (day1, day7, day7+curcumin, and day7curcumin+EX) s were euthanized with an overdose of carbon dioxide (CO2), and ipsilateral brains were excised (n=3). Brains were homogenized in RIPA buffer (150 mM NaCl, 2 mM EDTA, 10% glycerol, 1% NP40, 50 mM Tris-HCl (pH-7.4), and 0.5% Sodium deoxycholate). Tissue homogenates were centrifuged at 10,000 rpm for 20 min at 4°C to separate the supernatant. Protein concentration in the supernatant was determined by Bradford protein assay [22]. Total protein extract was resolved on 10-12% SDS-PAGE gels and transferred overnight onto a nitrocellulose membrane at 4° C using transfer buffer (25 mM Tris, 192 mM glycine, 20% methanol (v/v), adjust pH 8.3). The membranes were blocked for 90 minutes at room temperature with 5% nonfat milk in tris-buffered saline containing Tween-20 (TBS-T) at room temperature (RT) followed by overnight incubation at 4° C with respective primary antibody at 1:1000 dilution. Membranes were then washed and probed with respective horseradish peroxidase (HRP) labelled secondary antibody at 1:3000 dilutions for 90 min at RT. Chemiluminescence was captured using an enhanced chemiluminescent (ECL) detection system (Bio-Rad, USA).
Infarct Volume Measurement
Control and experimental rat brains (day1, day7, day7+curcumin, and day7curcumin+EX) were measured for infarction with standard tetrazolium staining (n=3). Brain tissues were frozen immediately at -20 for 30 min after decapitation. Frozen brain tissues were made into six coronal slices with 2 mm thickness and stained with 1% 2,3,5- triphenyl tetrazolium chloride (TTC) at 37° C for 30 min, followed by 4% paraformaldehyde (PFA) fixation. The infarct area in each slice was evaluated by scanned digital images with ImageJ software (NIH, US). The infarcted areas from each segment were added to derive the total infarct, multiplied by the thickness of the brain section to determine total infarct volume. Correction for edema of infarct was done as described elsewhere [23].
Histology and Immunohistochemistry
Control and experimental rat brains (day7, day7+curcumin, and day7curcumin+EX) (n=3) were fixed by trans cardiac perfusion with phosphate-buffered saline (PBS) and 4% PFA. Paraffin-embedded brain tissues were sectioned into 5-10 mm thick slices using the microtome (Leica, Germany). Brain sections were stained with hematoxylin and eosin (H&E) and then immunostained against cleaved caspase-3 using an IHC kit. Briefly, sections were deparaffinized and hydrated in a decreasing gradient of alcohol followed by endogenous peroxidase blocking with 3% hydrogen peroxide in methanol. After three washes in PBS, antigen retrieval was carried out with Tris-EDTA buffer (pH-9.0). The nonspecific binding was minimized by blocking sections with 0.25% BSA for 30 at RT. The sections were then incubated with primary antibody (1:100 dilution) for 2 h 30 min at RT. Further, brain sections were washed in PBS and incubated in polydetector HRP-labelled secondary antibody for 45 min at RT. Next, staining was visualized by covering the sections in 3’-3’ diaminobenzidine (DAB) buffer for 5 min, followed by counterstaining by hematoxylin. After subsequent dehydration in graded alcohol and xylene, sections were mounted, and images were captured under the light microscope (Olympus, Japan) with 400 X magnification.
Caspase Activity Assay
The caspase activity assay was performed to monitor key effector caspases (caspase-3,6 & 7) involved in apoptosis. Total 100 mg of protein lysates from control and experimental rat brains (day7, day7+curcumin, and day7curcumin+EX) (n=3) were dissolved in 100 ml of analysis buffer (100 mM NaCl, 10 mM DTT, 20 mM HEPES pH 7.4, 10%sucrose, 0.1% CHAPS, 1 mM EDTA) and incubated at 37 C for 6 h. After incubation 5 ml of Caspase Substrate (Ac-DEVD-AFC) was added to the lysates and made up to 1 ml of final concentration with analysis buffer, followed by incubation for 1 h at 37° C. The cleavage of AFC substrate was measured by fluorescence emission using fluorescence spectrophotometer (FluoroMax) at excitation 400 nm and emission 450–505 nm. The intensity of fluorescence signal represents the cumulative activity of effector caspases 3, 6 and 7.
Immunofluorescence
Brain sections from both control and experimental rat brains (day7, day7+curcumin, and day7curcumin+EX) (n=3) were dewaxed and rehydrated through 100-70% graded ethanol. Antigen retrieval was carried out in a microwave with 10 mM citrate buffer with 0.05% tween-20 (pH 6.0). After three washes in PBS, brain sections were blocked by 5% goat serum for 60 min at 37° C followed by incubation in primary antibody (1:100 dilution) for glial fibrillary acidic protein for 18 h at 4°C. After being washed with PBS, sections were covered with Alexa Fluor-485 conjugated goat anti-rabbit IgG (1:3000 dilutions) for 60 min at RT. Sections were mounted with antifade DAPI and Images were captured under the laser scanning confocal microscope (Carl-Zeiss, Germany).
Statistical Analysis
All the experimental data expressed as the mean ± SD and analysed for statistical significance using one-way ANOVA with Tukey’s multiple comparisons test. The experimental data shown are the representative of three independent experiments. Bars represent variation within the experimental samples. Graphs were plotted with GraphPad Prism 7.0 software (GraphPad Inc. US). Significance was shown as asterisks: * indicates p<0.05.
Results
Decrease in Expression of Sirt1/Autophagy Signaling on Day7 Post-Stroke
The sirt1-induced autophagy mechanism confers functional protection against CI. We sought to determine the role of Sirt1induced autophagy signaling on neuronal survival following day seven post-stroke. To explore the changes in Sirt1-induced autophagy signaling in the pathogenesis of ischemia, Sirt1 and autophagy mediators’ expression was assessed using immunoblot analysis. Immunoblotting results showed a significant decrease in the expressions of Sirt1 and most of the autophagy mediators (Beclin-1, Atg-3, Atg-5, and Atg-7) on day1 after MCAo. On the contrary, the ratio of Lc3-II/I was augmented on day1 compared to the sham control (Figure 1A-F, Sirt1<0.02; Beclin-1<0.001; Atg3<0.001; Atg-5<0.003; Atg-7<0.01; Lc3-II/I<0.001).
Further, the expressions of Sirt1 and autophagy mediators was decreased on day7 compared with the sham control (Figure 1A-F, Sirt1<0.001; Beclin-1<0.001; Atg-3<0.001; Atg-5<0.001; Atg-7<0.003). Notably, p-mTOR expression was inversely related to the autophagy mechanism. The expression of p-mTOR was upregulated on day 1 and day 7 when compared to control brains. (Figure 1G, p-mTOR<0.001).
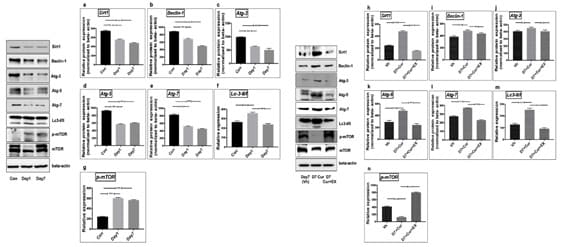
Figure 1: Curcumin treatment on day7 post-stroke up-regulates Sirt1 induced autophagy signaling. An equal amount of total protein sample (75ug) was used for immunoblotting. Respective total proteins and beta-actin were served as a loading control (Lc3-II/I, p-mTOR), while Sirt1, Beclin-1, Atg-3, Atg-5, and Atg-7 were normalized to beta-actin. (A-G) Representative graphs show changes in the expression of Sirt1/autophagy/mTOR signaling on day1 and day7 post-stroke. (H-N) The graphs from the brains of the vehicle (day7), curcumin, and curcumin + EX-527 treatment on day7 shows that curcumin treatment augmented Sirt1 induced autophagy on day7 and curcumin + EX-527 treatment show a substantial decrease in Sirt1 induced autophagy. The densitometry values are represented as mean ± SD (n=3). *p<0.05 versus control brain
Decrease in Infarct in Day7 Post-Stroke Rat Brains
Changes in neuronal morphology were confirmed by routine H&E staining (Figure 2A). Typical TTC staining was performed to measure the lesion volume changes, and the results indicate a significant amount of infarction in ipsilateral brains on day1 compared to day7 following MCAo (Figure 2B&C).
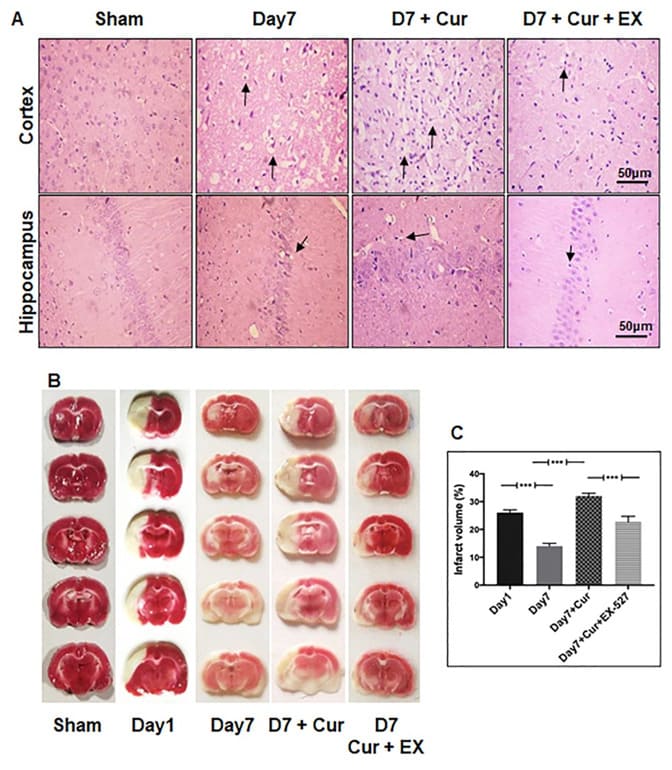
Figure 2: Curcumin treatment on day7 post-stroke promoted neuronal loss following CI. Rat brains were paraffin-embedded and sectioned into 5-10 mm thick slices (A) H&E staining shows the changes in neuronal morphology of cortex and hippocampus neurons (black arrow) in the brains of sham control, day7 post-stroke, curcumin, and curcumin + EX-527 treatment on day7 (magnification 400X; Scale Bar - 50mm). (B) Representative image of TTC staining from rat brains following MCAo at different time points. (C) Representative images from the brains of control, day1 post-stroke, day7 post-stroke, curcumin, and curcumin + EX-527 treatment on day7 after TTC staining. Curcumin + EX-527 treatment on day7 rescued infarct volume compared to curcumin treatment on day7. The data represented as a percentage of tissue loss (n=3). *p<0.05 versus day1post-stroke brain.
Increase in Caspase-3 Immunoreactivity and Effector Caspase Activity on Day7 Post-Stroke
Apoptotic cell death is the major contributing factor to neuronal cell loss following ischemia. The caspase cascade activation, including caspase-3, mainly mediates apoptotic neuronal cell loss. To understand the crosstalk between sirt1/autophagy signaling and apoptotic cell death, we immunostained paraffin-embedded brain sections of sham controls and day7 post-stroke rats against cleaved caspase-3. A Very few caspase-3 positive cells were observed from control cortex and hippocampus, while on day7, caspase-3 immunoreactivity was increased (Figure 3A). Caspase-3 immunoreactivity results were further validated by caspase activity assay (Figure 3B), which measures the cumulative activity of effector caspases-3,6, & 7. We observed a similar trend in effector caspase activity on day7 compared with control brains.
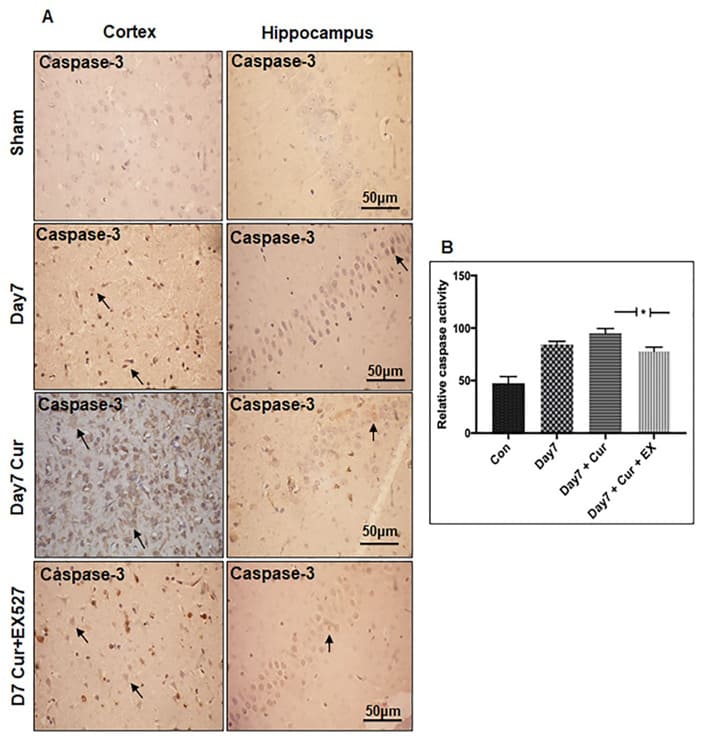
Figure 3(A): Subcellular localization of cleaved caspase-3 in the brains of the experimental rat following CI. Brain tissues from sham control and experimental rats were paraffin-embedded and sectioned into 5-10 mm thick slices. Immunohistochemistry staining for cleaved caspase-3 in the brains of rat cortex and hippocampus neurons from sham control and day7 post-stroke, curcumin, and curcumin + EX-527 treatment on day7. The black arrow represents changes in cleaved caspase-3 immunoreactivity for the respective group. Immunoreactivity for cleaved caspase-3 was depicted in brown (DAB). The neuronal nucleus is counterstained with hematoxylin (magnification 400X; Scale Bar - 50mm). (B) Graph shows changes in effector caspases activation from sham control and day7 poststroke, curcumin, and curcumin + EX-527 treatment on day7 (n=3).
Increase in Reactive Astrogliosis on Day7 Post-Stroke
The role of astrocyte reactivity/astrogliosis in response to delayed stroke was assessed using GFAP. Double immunofluorescence labeling was performed against GFAP and DAPI on paraffin-embedded brain sections from control and day7 rats. We observed an increase in astrocyte ramification and GFAP positive astrocytes number on day7 compared with the day1 following MCAo (Figure 4).
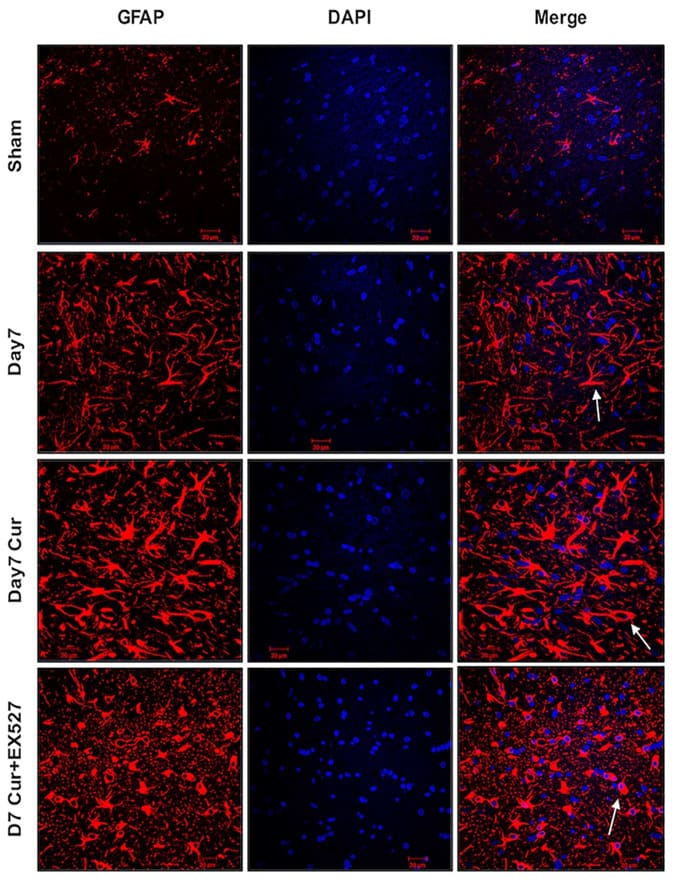
Figure 4: Immunofluorescence labelling of GFAP in the brains of the experimental rat following CI. Double immunofluorescence labeling for GFAP (red), and DAPI (blue), to assess astrogliosis in cortex neurons from sham control as well as brains from day7 poststroke, curcumin, and curcumin + EX-527 treatment on day7. Curcumin treatment on day7 promoted reactive astrogliosis, and combined use of curcumin + EX-527 reduced reactive astrogliosis on day7. The white arrow represents morphological changes in astrocytes from the respective group (n=3; Scale Bar - 20mm).
Curcumin Treatment Upregulate Sirt1-Induced Autophagy Signaling on Day7 Post-Stroke
We sought to determine whether the activation of Sirt1 induced autophagy could regulate neuronal survival on day7 following MCAo. Curcumin treatment was given for Sirt1 induced autophagy activation. Immunoblotting results showed the upregulation of Sirt1-induced autophagy and a decrease in phosphorylation of mTOR on day7 after curcumin treatment compared with the vehicle (day7 post-stroke) (Figure 1H-N, Sirt1<0.001; Beclin-1<0.001; Atg-3<0.03; Atg-5<0.001; Atg-7<0.001; Lc3-II/I<0.001, p-mTOR<0.001).
Curcumin Treatment Promote Infarct, Caspase-3 Immunoreactivity, and Reactive Astrogliosis, on Day7 PostStroke
Routine TTC staining results showed that infarct formation increased after curcumin treatment on day7 compared with the vehicle (Figure 2B&C). Interestingly, curcumin treatment increased cleaved caspase-3 and effector caspase activity. (Figure 3A&B). In addition, curcumin treatment increased reactive gliosis (Figure 4) compared with the vehicle. These effects were partly mediated by Sirt1-induced autophagy activation.
Suppression of Sirt1 Expression Down-Regulate Autophagy Signaling on Day7 Post-Stroke
To understand the interaction of SIRT1/autophagy pathway in delayed phase of CIR, rats were treated with the combination of curcumin + EX-527 on day7. This confirmed that Sirt1 mediated autophagy network was down-regulated and phosphorylation of mTOR was up-regulated compared with the curcumin-treated group (Figure 1H-N, Sirt1<0.001; Beclin-1<0.02; Atg-3<0.03; Atg-5<0.001; Atg-7<0.001; Lc3-II/I<0.001; p-mTOR<0.001).
Suppression of Sirt1 Expression Reduce Infarct, Caspase-3 Immunoreactivity, and Effector Caspase Activity on Day7 Post-Stroke
Changes in neuronal morphology was evaluated with H&E showed that curcumin + EX-527 treatment reduced neuronal loss on day7 compared with the curcumin-treated group. The characteristic TTC staining confirmed that combined use of curcumin + EX-527 rescued infarct volume compared with the curcumin group. (Figure 2B&C). The immunostaining of cleaved caspase-3 illustrated a few immunoreactive cells in the curcumin + EX-527 group compared with the curcumin-treated group (Figure 3A). The immunostaining results were further supported by caspase activity assay (Figure 3B). Our results showed that suppression of Sirt1/autophagy signaling reduced infarct and cleaved caspase-3, effector caspase activity on day7 following MCAo.
Suppression of Sirt1 Expression Reduced Reactive Astrogliosis on Day7 Post-Stroke
The GFAP immunostaining showed decreased astrocyte ramification on curcumin + EX-527 treatment compared with the curcumin-treated group. We did not observe any decrease in GFAP-positive astrocyte number after curcumin + EX-527 treatment compared with the curcumin-treated group (Figure 4). Our results showed that suppression of Sirt1/autophagy signaling reduced reactive astrogliosis on Day7 following MCAo.
Discussion
The mammalian Sirt1 is highly expressed in neurons and the adult brain [24]. Sirt1 plays a vital role in neuronal development, plasticity, and metabolism [24-27]. Genetic and pharmacological manipulation of Sirt1 activity is shown to have a protective effect in neurodegenerative disease models. For example, brain-specific knockdown of SIRT1 exacerbated Huntingtin disease pathology via BDNF/CREB signaling in the experimental mouse model. Moreover, Sirt1 activation has been proven to aid in neuronal survival [28-30], and inhibition of Sirt1 with EX-527 significantly increased neuronal apoptosis against cerebral ischemia in experimental animal models [31], indicating that SIRT1 plays a crucial role in mediating neuroprotection. Immunoblot results demonstrated decreased Sirt1 expression on day1 and day7 poststroke in rat MCAO model.
The functional role of autophagy in cerebral ischemia is controversial. Some findings demonstrated that autophagy promotes ischemic pathogenesis, and other results supported its neuroprotective role against ischemia [32-36]. As part of the early protective response, a basal autophagy mechanism may promote cell survival. However, defective/excessive autophagy may trigger neuronal cell death following ischemia [37,38]. In recent years, the correlation between the effect of Sirt1 activation in the induction of autophagy has been investigated in cardiomyocytes and renal ischemia [38,39]. Further, Sirt1 can induce autophagy mechanism by direct deacetylation of various autophagy mediators, including Atg5, Atg7, and Lc-3 [40,41]. Nicotinamide phosphoribosyltransferase, the rate-limiting enzyme in NAD biosynthesis, protects against ischemia via autophagy induction in Sirt1 dependent manner and also shown that autophagy induction seems to be neuroprotective during the early phase of reperfusion but might be harmful if it is persistently activated by ischemia [42]. Our study observed an increase in the Lc3-II/I ratio was observed on day1 post-stroke, which may have attributed to the impaired autophagosome transport mechanism leading to the accumulation of autophagosomes [43-45]. Additionally, a decrease in Lc3-II/I ratio and reduced infarct was observed on day7 post-stroke. Our findings on infarct volume are in line with previous observations, where infarct was shown to be reduced on day7 post-stroke [46].
Both autophagy and apoptosis can act cooperatively to induce neuronal cell death. Autophagy can act as an antagonist or promoter of apoptosis after ischemia-reperfusion [46,47]. Beclin-1/ Bcl-2 interaction serves as a crossroads to lead neuronal cells to autophagy or apoptosis [48,49]. Further, rapamycin’s protective effect following cerebral ischemia is by activation of the autophagy mechanism and inhibition of mTOR-mediated apoptotic signaling pathway [49,50]. Caspase-3 is one of the principal effector caspases that can promote or inhibit autophagy mechanism by directly interacting with various autophagy mediators, including Beclin-1, Atg-4d, and Atg-5 [51,52]. Our present observations might be consistent with this broader observation. We observed an increase in caspase-3 immunoreactivity and effector caspases activity (effector caspases 3,6 & 7) on day7 post-stroke.
Astrocytes become reactive in response to central nervous system injury. This process is known as reactive astrogliosis. An increase in GFAP expression is a crucial marker for astrogliosis in central nervous system injuries [53]. The glial scar/astrogliosis can prevent axonal sprouting, thereby preventing recovery. Recent observations have reported that astrogliosis starts about 7-10 days after ischemia and persists for a lifetime in stroke patients, reducing synaptic plasticity and recovery [54]. Interestingly, our study observed increased reactive astrogliosis on day7, which might indicate reduced cognition and synaptic plasticity.
Protective effects of curcumin have been proven against various neurodegenerative disorders [54,55]. For example, administration of curcumin after MCAO in rats resulted in the reduced cerebral infarction and edema [56,57]. Curcumin treatment confers neuroprotection by increasing the expression of Sirt1, which attenuates caspase-3-mediated apoptotic neuronal cell death in experimental stroke models [28,4]. Further, curcumin exerts neuroprotective effects by modulating autophagic activities through PI3K/Akt/mTOR pathway following CIR [58]. The neuroprotective effects of curcumin in these preclinical models were mainly evaluated on day1 and day3 following MCAo. Interestingly, our study observed increased Sirt1-induced autophagy, astrogliosis, infarct upon curcumin treatment on day7 post-stroke, while combined use of curcumin + EX-527 abolished the effects of curcumin.
If curcumin-mediated activation of Sirt1/autophagy signaling plays a biphasic role after stroke, caution is needed for targeting the Sirt1-induced autophagy. However, the ischemic zone is a dynamic and flexible area during the chronic phase of ischemia [17]. The biphasic role of Sirt1-mediated autophagy may be due to the neurovascular unit’s involvement of multiple signaling pathways. For this, careful characterization of curcumin dosage and the molecular mechanisms associated with acute recovery transition to delayed injury. More importantly, astrogliosis is a typical protective/detrimental response initiated by reactive astrocytes during the chronic phase of ischemia to prevent damage/repair. Here, the activation of Sirt1/autophagy signaling after curcumin treatment might interfere with the endogenous repair mechanisms by speeding up reactive astrogliosis and promoting more damage. A significant limitation of this study is that curcumin on Sirt1/ autophagy signaling was assessed at a single time point with a single dose of curcumin on day7 since the neuroprotective role of curcumin is well established in different settings. Besides the dosage and the route of administration, the time of administration and the involvement of multiple signaling pathways activation by curcumin during delayed-phase of CIR could be the reason for the discrepancy in our results [58-60]. The neuroprotective nature of curcumin is dose-dependent and various studies have proven that 300 mg/kg could provide the best protective effects. Further, curcumin absorption was considerably higher after intraperitoneal injection than other routes of administration [58,60,20]. However, we need to evaluate the effect of different dosages and multiple time points to characterize the role of curcumin and astrogliosis during the chronic phase of ischemia.
Conclusion
The present study concludes that curcumin treatment on day7 post-stroke may promote infarction and astrogliosis via activating Sirt1-induced autophagy. Sirt1-mediated autophagy may interfere with post-stroke recovery by promoting scar formation on day7 in the brains of the experimental rat.
Conflict of Interests
The authors declare that they have no conflicts of interest.
Acknowledgements
The authors are thankful to the financial assistance from the Department of Science and Technology, Government of India, DST- SERB Core grant, file Nos. CRG/2020/005021, CRG/2019/002570, and financial support to the University of
Hyderabad-IoE by the Ministry of Education, Government of India (F11/9/2019-U3 (A). DST-FIST, and UGC-SAP to the
Department of Biotechnology and Bioinformatics, BUILDERDBT-BT/INF/22/SP41176/2020 to the School of Life Sciences is gratefully acknowledged. Authors are duly acknowledged DST FIST and UGC SAP. Authors are thankful to Naimisha Rapaka for experimental support.
Data Availability
The datasets used and/or analyzed during this study are included in this manuscript.
Author’s Contribution
P.P.B conceived and designed the experiments. S.K.T performed all the in-vivo experiments and analyzed the data. S.K.T, VM & P.P.B. wrote the manuscript. P.P.B approved the final manuscript.
Ethical Approval & Consent to Participate
All the animal experiments were conducted in accordance with the Institutional and National Animal Ethical Committee (IAEC) – UH/IAEC/PPB/2018-I/27.
References
- Murata Y, Fujiwara N, Seo JH, Yan F, Liu X, et al. (2012) Delayed inhibition of c-Jun N-terminal kinase worsens outcomes after focal cerebral ischemia. J Neurosci 32: pp. 8112–5.
- Surguchov A, Bernal L, Surguchev AA (2021) Phytochemicals as regulators of genes involved in synucleinopathies. Biomolecules 11:
- Huang L, Chen C, Zhang X, Li X, Chen Z, et al. (2018) Neuroprotective effect of curcumin against cerebral ischemia-reperfusion via mediating autophagy and inflammation. J Mol Neurosci 64: 29–139.
- Li W, Suwanwela NC, Patumraj S (2017) Curcumin prevents reperfusion injury following ischemic stroke in rats via inhibition of NF‑κB, ICAM‑1, MMP‑9 and caspase‑3 expression. Mol Med Rep 16: 4710–4720.
- Miao Y, Zhao S, Gao Y, Wang R, Wu Q, et al. Curcumin pretreatment attenuates inflammation and mitochondrial dysfunction in experimental stroke: The possible role of Sirt1 signaling. Brain Res Bull 121: 9–15.
- Han J, Pan XY, Xu Y, Xiao Y, An Y, et al. (2012) Curcumin induces autophagy to protect vascular endothelial cell survival from oxidative stress damage. Autophagy 8: 812–825.
- Imai S, Armstrong CM, Kaeberlein M, Guarente L (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone Nature 403: 795–800.
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, et al. (2004) Modulation of NF‑κB‑dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23: 2369–2380.
- Vaziri H, Dessain SK, Eaton EN, Imai SI, Frye RA, et al. (2001) hSIR2(SIRT1) functions as an NAD‑dependent p53 deacetylase. Cell 107: 149–59.
- Donmez G (2012) The neurobiology of sirtuins and their role in Trends Pharmacol Sci 33: 494–501.
- Hernandez‑Jimenez M, Hurtado O, Cuartero MI, Ballesteros I, Moraga A, et al. (2013) Silent information regulator 1 protects the brain against cerebral ischemic damage. Stroke 44: 2333–2337.
- Yan W, Fang Z, Yang Q, Dong H, Lu Y, et al. (2013) SirT1 mediates hyperbaric oxygen preconditioning‑induced ischemic tolerance in rat J Cereb Blood Flow Metab 33: 396–406.
- Della‑Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, et al. (2009) Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience 159: 993–1002.
- Teertam SK, Prakash Babu P (2021) Differential role of SIRT1/MAPK pathway during cerebral ischemia in rats and humans. Sci Rep 11:
- In HL, Cao L, Mostoslavsky R, Lombard DB, Liu J, et al. (2008) A role for the NAD-dependent deacetylase Sirt1 in the regulation of Proc Natl Acad Sci U S A 105: 3374–3379.
- Teertam SK Phanithi PB (2022) Up‑regulation of Sirtuin‑1/autophagy signaling in human cerebral ischemia: Possible role in caspase-3 mediated apoptosis. Heliyon 18: e12278.
- Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, et al. (2006) Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med 12: 441–445.
- Besancon S, Guo S, Lok J, Tymianski M, Lo EH (2008) Beyond NMDA and AMPA glutamate receptors: Emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol Sci 29: 268–75.
- Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20: 84–91.
- Wu J, Li Q, Wang X, Yu S, Li L, et al. (2013) Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. PLoS One 8: e59843.
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, et al. (1986) Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke 17:472–476.
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‑dye Anal Biochem 72: 248–254.
- Lin TN, He YY, Wu G, Khan M, Hsu CY (1993) Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke 24: 117–121.
- Ramadori G, Lee CE, Bookout AL, Lee S, Williams KW, et al. (2008) Brain SIRT1: Anatomical distribution and regulation by energy J Neurosci 28: 9989–9996.
- Li XH, Chen C, Tu Y, Sun HT, Zhao ML, et al. (2013) Sirt1 promotes axonogenesis by deacetylation of Akt and inactivation of GSK3. Mol Neurobiol 48: 490–499.
- Ramadori G, Lee CE, Bookout AL, Lee S, Williams KW, et al. (2008) Brain SIRT1: Anatomical distribution and regulation by energy J Neurosci 28: 9989–9996.
- Nemoto S, Fergusson MM, Finkel T (2005) SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha}. J Biol Chem 280: 16456–16460.
- Miao Y, Zhao S, Gao Y, Wang R, Wu Q, et al. (2016) Curcumin pretreatment attenuates inflammation and mitochondrial dysfunction in experimental stroke: The possible role of Sirt1 signaling. Brain Res Bull 121: 9–15.
- Teertam SK, Jha S, Prakash Babu P (2019) Up‑regulation of Sirt1/ miR‑149‑5p signaling may play a role in resveratrol induced protection against ischemia via p53 in rat brain. J Clin Neurosci 72: 402-411.
- Lv H, Wang L, Shen J, Hao S, Ming A, et al. (2015) Salvianolic acid B attenuates apoptosis and inflammation via SIRT1 activation in experimental stroke rats. Brain Res Bull 115: 30–36.
- Yan W, Fang Z, Yang Q, Dong H, Lu Y, et al. (2013) SirT1 mediates hyperbaric oxygen preconditioning‑induced ischemic tolerance in rat J Cereb Blood Flow Metab 33: 396–406.
- Liu X, Tian F, Wang S, Wang S, Xiong L (2018) Astrocyte autophagy flux protects neurons against oxygen‑glucose deprivation and ischemic/reperfusion injury. Rejuvenation Res 21: 405–415.
- Xin H, Katakowski M, Wang F, Qian JY, Liu XS, et al. (2017) MicroRNA cluster miR‑17‑92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke 48: 747–753.
- Wang P, Liang J, Li Y, Li J, Yang X, et al. (2014) Down-regulation of miRNA‑30a alleviates cerebral ischemic injury through enhancing Beclin 1‑mediated autophagy. Neurochem Res 39: 1279–1291.
- Feng D, Wang B, Wang L, Abraham N, Tao K, et al. (2017) Preischemia melatonin treatment alleviated acute neuronal injury after ischemic stroke by inhibiting endoplasmic reticulum stress-dependent autophagy via PERK and IRE1 signalings. J Pineal Res 62: e12395.
- Ni Y, Gu WW, Liu ZH, Zhu YM, Rong JG, et al. (2018) RIP1K contributes to neuronal and astrocytic cell death in ischemic stroke via activating autophagic-lysosomal pathway. Neuroscience 371: 60–74.
- Shi R, Weng J, Zhao L, Li XM, Gao TM, Kong J (2012) Excessive autophagy contributes to neuron death in cerebral ischemia. CNS Neurosci Ther 18: 250–260.
- Nadtochiy SM, Yao H, McBurney MW, Gu W, Guarente L, et al. (2011) SIRT1‑mediated acute cardioprotection,” Am J Physiol Heart Circ Physiol 301: H1506-1512.
- Kume S, Uzu T, Horiike K, Chin‑Kanasaki M, Isshiki K, et al. (2010) Calorie restriction enhances cell adaptation to hypoxia through Sirt1dependent mitochondrial autophagy in mouse aged kidney. J Clin Inves 120: 1043–1055.
- Ren Q, Hu Z, Jiang Y, Tan X, Botchway BOA, et al. (2019) SIRT1 protects against apoptosis by promoting autophagy in the oxygen glucose deprivation/reperfusion‑induced injury. Front Neurol 10: 1289.
- Wang P, Guan YF, Du H, Zhai QW, Su DF, et al. (2012) Induction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyltransferase in cerebral ischemia. Autophagy 8: 77–87.
- Boland B, Kumar A, Lee S, Platt FM, Wegiel J, et al. (2008). Neurobiology of disease autophagy induction and autophagosome clearance in neurons: Relationship to autophagic pathology in alzheimer’s disease. J Neurosci 28: 6926‑6937.
- Hou K, Xu D, Li F, Chen S, Li Y (2019) The progress of neuronal autophagy in cerebral ischemia stroke: Mechanisms, roles and research methods. J Neurol Sci 400: 72–82.
- Nixon RA, Yang DS (2011) Autophagy failure in alzheimer’s diseaselocating the primary defect. Neurobiol Dis 43: 38–45.
- Popp A, Jaenisch N, Witte OW, Frahm C (2009) Identification of ischemic regions in a rat model of stroke. PLoS One 4: e4764.
- Xing S, Zhang Y, Li J, Zhang J, Li Y, et al. (2012) Beclin 1 knockdown inhibits autophagic activation and prevents secondary neurodegenerative damage in the ipsilateral thalamus following focal cerebral infarction. Autophagy 8: 63–76
- Wang P, Xu TY, Wei K, Guan YF, Wang X, et al. (2014) ARRB1/βarrestin-1 mediates neuroprotection through coordination of BECN1dependent autophagy in cerebral ischemia. Autophagy 10: 1535–
- Qi Z, Dong W, Shi W, Wang R, Zhang C, et al. (2015) Bcl‑2 phosphorylation triggers autophagy switch and reduces mitochondrial damage in limb remote ischemic conditioned rats after ischemic Transl Stroke Res 6: 198–206.
- Carloni S, Girelli S, Scopa C, Buonocore G, Longini M, et al. (2010) Activation of autophagy and Akt/CREB signaling play an equivalent role in the neuroprotective effect of rapamycin in neonatal hypoxia Autophagy 6: 366–377.
- Nazarinia D, Aboutaleb N, Gholamzadeh R, Nasseri Maleki S, Mokhtari B, et al. (2019) Conditioned medium obtained from human amniotic mesenchymal stem cells attenuates focal cerebral ischemia/ reperfusion injury in rats by targeting mTOR pathway. J Chem Neuroanat 102: 101707.
- Tsapras P, Nezis IP (2017) Caspase involvement in autophagy. Cell Death Differ 24: 1369–1379.
- Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32: 638–647.
- Sofroniew MV, Vinters HV (2010) Astrocytes: Biology and pathology. Acta Neuropathol 119: 7–35.
- Tiwari SK, Agarwal S, Seth B, Yadav A, Nair S, et al. (2014) Curcuminloaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in alzheimer’s disease model via canonical Wnt/βcatenin pathway. ACS Nano 8: 76–103.
- Nguyen TT, Vuu MD, Huynh MA, Yamaguchi M, Tran LT, et al. (2018) Curcumin effectively rescued parkinson’s disease-like phenotypes in a novel drosophila melanogaster model with dUCH knockdown. Oxid Med Cell Longev 2038267.
- Thiyagarajan M, Sharma SS (2014) Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in Life Sci 74: 969–985.
- Huang L, Chen C, Zhang X, Li X, Chen Z, et al. (2018) Neuroprotective effect of curcumin against cerebral ischemia-reperfusion via mediating autophagy and inflammation. J Mol Neurosci 64: 129–139.
- Zhao J, Zhao Y, Zheng W, Lu Y, Feng G, et al. (2008) Neuroprotective effect of curcumin on transient focal cerebral ischemia in rats. Brain Res 1229: 224–232.
- Lo EH (2008) A new penumbra: Transitioning from injury into repair after stroke. Nat Med 14: 497–500.
- Dohare P, Garg P, Jain V, Nath C, Ray M (2008) Dose dependence and therapeutic window for the neuroprotective effects of curcumin in thromboembolic model of rat. Behav Brain Res 193: 289–297. s
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.