Critical Appraisal of Studies on Sore Throat Symptoms for A Resolution Strategy on Children’s Patients to Assure Clinical Quality and Safety as Outcomes
by Antonio Steardo*
RpharmS, Cchem Via Cupa Parisi 2 84133 Salerno, Italy.
*Corresponding author: Antonio Steardo, RpharmS, Cchem via Cupa Parisi 2 84133 Salerno, Italy
Received Date: 18 November, 2025
Accepted Date: 10 December, 2025
Published Date: 16 December, 2025
Citation: Steardo A (2025) Critical appraisal of studies on sore throat symptoms for a resolution strategy on children’s patients to assure clinical quality and safety as outcomes. Rep GlobHealth Res 8: 221. https://doi.org/10.29011/2690-9480.100221
Abstract
Problem description
Sore throat is a common ailment among children, often causing concern for parents and caregivers. The condition can result from viral infections, bacterial infections, or environmental irritants. Proper diagnosis and treatment are crucial to ensure the children well-being and prevent complications. Sore throat symptoms in children can be quite distressing, with complaints ranging from mild discomfort to severe pain. Parents frequently seek medical advice to alleviate their children suffering, making it essential for healthcare providers to stay updated on the best practices and Evidence-Based-Medicine approaches for managing sore throat.
Available knowledge
As previous consideration outlines, it is imperative to explore the most effective diagnostic tools and treatment options available for children presenting with sore throat symptoms. Healthcare professionals must weigh the benefits and limitations of various strategies to make informed decisions that will optimize patient care. Additionally, the NICE guideline recommends [1],
• Managing acute sore throat without needing antibiotics.
• Antibiotic selection for back-up or immediate prescription.
• Self-care.
Keywords: Sore throat; critical appraisal; quality and safety outcomes; paediatry.
Rationale
Clinical scenario
A child presents symptoms of a sore throat. The fist approach is an Evidence-Based-Medicine decision regarding the diagnosis and treatment of this patient. Furthermore, cultural diagnostic tests should be employed to ensure the precise identification of sore throat etiopathological features.
Clinical Question and its specific aims
If a child presents sore throat symptoms. What is the best way to treat him? What is the best diagnostic tool? What’s the best intervention? A literature critical appraisal is the best way to assure the appropriate cure this heath issue by medical quality and safety. 1) What is the result? 2) Is it valid? 3) Will it help me to cure patients?.
Specific aims Purpose of the project and of this report
The literature provided a critical assessment of three scientific studies on paediatric sore throat.
PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers.
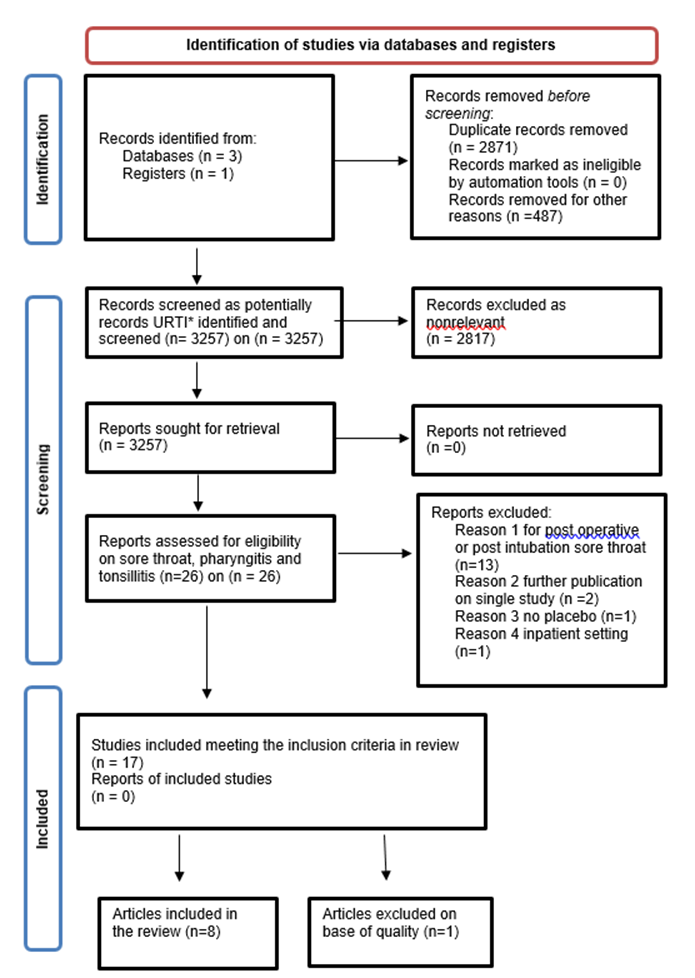
Figure 1: PRISMA flowchart set results evaluation in chosen papers [2]. *URTI means upper respiratory tract infection.
Context
Critical appraisal on the chosen three papers
Retrieving in scientific literature the following three papers leads to a critical appraisal.
1. Little P., Williamson I., Warner G., Gould C., Grantley M., Kinmonth AL. "Open randomised trial of prescribing strategies in managing sore throat." BMJ. 1997 Mar 8;314(7082):722-7. PMID: 9116551. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2126131/
2. Hayward G., Thompson M., Heneghan C., Perera R., Del Mar C., Glasziou P. "Corticosteroids for pain relief in sore throat: systematic review and meta-analysis." BMJ. 2009 Aug 6;339: b2976. doi: 10.1136/bmj. b2976. Review. Erratum in: BMJ. 2010;340. doi: 10.1136/bmj.c692. PMID: 19661138. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2722696/
3. Van Limbergen J., Kalima P., Taheri S., Beattie TF. "Streptococcus A in paediatric accident and emergency: are rapid streptococcal tests and clinical examination of any help?" Emerg Med J. 2006 Jan;23(1):32-4. PMID: 16373800. https://pmc.ncbi.nlm.nih.gov/articles/PMC2564123/.
Interventions10
Intervention study methods, rationale, and analysis on three chosen papers using PRISMA guideline [3,4,5].
Critical appraisal paper one
"Open randomized trial of prescribing strategies in managing sore throat." BMJ 1997; 314 doi: https://doi.org/10.1136/bmj.314.7082.722 (Published 08 March 1997) P Little.
Study Design: Open Randomised Trial.
Clinical question: Is any antibiotic therapy better than no treatment at all? Is the therapeutic use of antibiotic treatment necessary to obtain symptoms regression?
Patient: The trial participants were: 716 patients aged four years and over with sore throat and with throat abnormal medical sign. These symptoms characterised the sore throat. 1) inflamed tonsils or pharynx, 2) purulent exudate, 3) faucal or palatal inflammation and cervical adenopathy. Patients’ initial temperature was major than 37.5 C°, at beginning of the clinical trial. Throat abnormal signs were in the was important enough to admit children under 12 years old, who are less likely to moan. General Practitioners recruit participants on their own that could an observational bias source.
Intervention: 246 patients received Antibiotics in the intervention group (10-day prescription of penicillin V), or Erythromycin (if sensitive to penicillin), 250 mg four times daily (125 mg for 3¬5-year-old patients) [6].
Comparison: The control factors are no antibiotic treatment at all, in the first control group. The administration of the Antibiotic started late in the second control group for 230 patients. Patients, assigned to the second control group, received the same Antibiotic of group 1 after three days if symptoms did not regress.
Outcomes: 1) Kruskal-Wallis χ2 test on symptoms duration and 2) Pearson χ2 test on symptoms prevalence have no % composed the outcome assessments. They display that both symptoms duration and incidence have no meaningful difference between these three groups. Group 1 is the Intervention Group, and the other two Groups are the comparators Groups. The comparator also evaluates side effect prevalence due to Antibiotic therapy. Symptoms duration is between 4 and 5 days in every group. The studies conclude that Antibiotic therapy has a marginal effect on sore throat cure. Moreover, the outcomes documented, on 582 subjects (81% of participants), lead to symptoms satisfaction. Twenty-five patients received the Likert-Scale questions to assess psychosocial issues after two weeks. Patients had high agreement on the two occasions (> 14/20), with good correlations rank (> 0.64). This study is an analytic experimental randomised trial study. It aims to qualify the relations: 1) antibiotic therapy use, 2) no therapy use at all, 3) delayed therapy beginning in the groups of patients. These factors determine the validity of antibiotic use on sore throat cure. This study design has a level of evidence type 1. It is robust evidence for the Level of Clinical Evidence 3. The internal validity is based on patients' randomisation between three groups. The study has a baseline characteristics table, which reports the allocation sequence. This study assesses how variables could affect the outcomes. The two comparator groups give two
different methods to assess the cure for severe symptoms. Pharyngitis and tonsillitis affected 84% of participants. The other type of sore throats represents the variables in the study. The trial assesses selection bias on percentage difference by age, sex, the appearance of symptoms three days before the trial begins. Detection bias can occur, as patients collect the data on diaries and phone. Symptoms collection on diaries can affect accuracy due to observational bias. A paragraph, on data entry and analysis, does by type I error analysis to discuss the validity of this process. The article displays the following data: "Spearman r=0.94; P-value < 0.001, median difference 0 days, interquartile range for the difference 0 to 1 day, range to 2 days".6
Critical appraisal paper two
"Corticosteroids for pain relief in sore throat: systematic review and meta-analysis." BMJ 2017; 358 doi: https://doi.org/10.1136/bmj.j3887 (Published 20 September 2017) Cite this as: BMJ 2017;358: j3887 Behnam, Sadeghirad.
Study design: Systematic Review and Metanalysis.
Patients: In eight trials, the population affected by exudative or severe sore throat comprised 369 children, 347 adults, and 743 patients in total. Three hundred forty-eight patients (47% of the population) had an exudative sore throat. Three hundred thirty patients (44% of the population) were positive for group A β-Haemolytic Streptococcus Test.
Intervention: Patients therapy is Antibiotic, Analgesia and Corticosteroid, in all eight trials.
Comparison: Placebo in the control groups, it designs the likelihood of symptoms resolution at different time extension, additionally.
Outcome: The study outlines the outcome in the objective section saying that Corticosteroids, in addition to Antibiotic therapy, provides the likelihood of symptomatic relief and pain onset during sore throat therapy.
Is it unlikely that necessary, and relevant studies misses?
It is unlikely that the relevant study misses from this study. The study material derives from Medline (From 1966 to 2008); Embase (From 1983 to 2008); Cochrane Library, the Database of reviews of effectiveness (DARE) and National Health System National Economics Database. Wei, Kinderman, Marvez-Valls, O'Brien and Bulloch trial consider steroid against placebo. Research methods used are all valid to include relevant or essential studies.
Were the included studies sufficiently valid for the type of question asked?
The included study is a randomised controlled study only. The validity of this study is high in a hierarchy of evidence for Evidence-Based-Medicine [1]. The inclusion of patients affected by a different type of sore throat causes the heterogenicity of this systematic review. Overall, all the included studies were valid.
Were the criteria used to select articles for inclusion?
All patients, treated by corticosteroids resolved symptoms after 24-48 hours, definitively. Corticosteroids decreased symptoms even after 6 hours. The united analysis shows a significant heterogenicity between the data. The criteria to select articles were valid and essayed in PRISMA 1.
How does the review show results?
This study displays data. It explains that: 1) In more than four trails, by a high statistical value (9,5% confidence interval 2.0 hours to 5.1 at 24 hours), more than three times relative risk is 3.3 confirming the efficacy of Corticosteroids. Corticosteroids, in addition to analgesia therapy, gave a slow resolution in three trial (1.7, 1.3 to 2.1) at 48 hours. The use of Corticosteroids reduced the onset of the symptom relief in six trials. The validity of data displays: (95% confidence interval of 3.4 to 9.3, P<0.001). Although, the data was heterogeneous, the time resolution analysis and comprehensive analysis across all the studies assess all the other outcomes. Tables and graphics in the study accompany results assessment. The graphic visual analogues were pain score at 72 hours after the intervention.
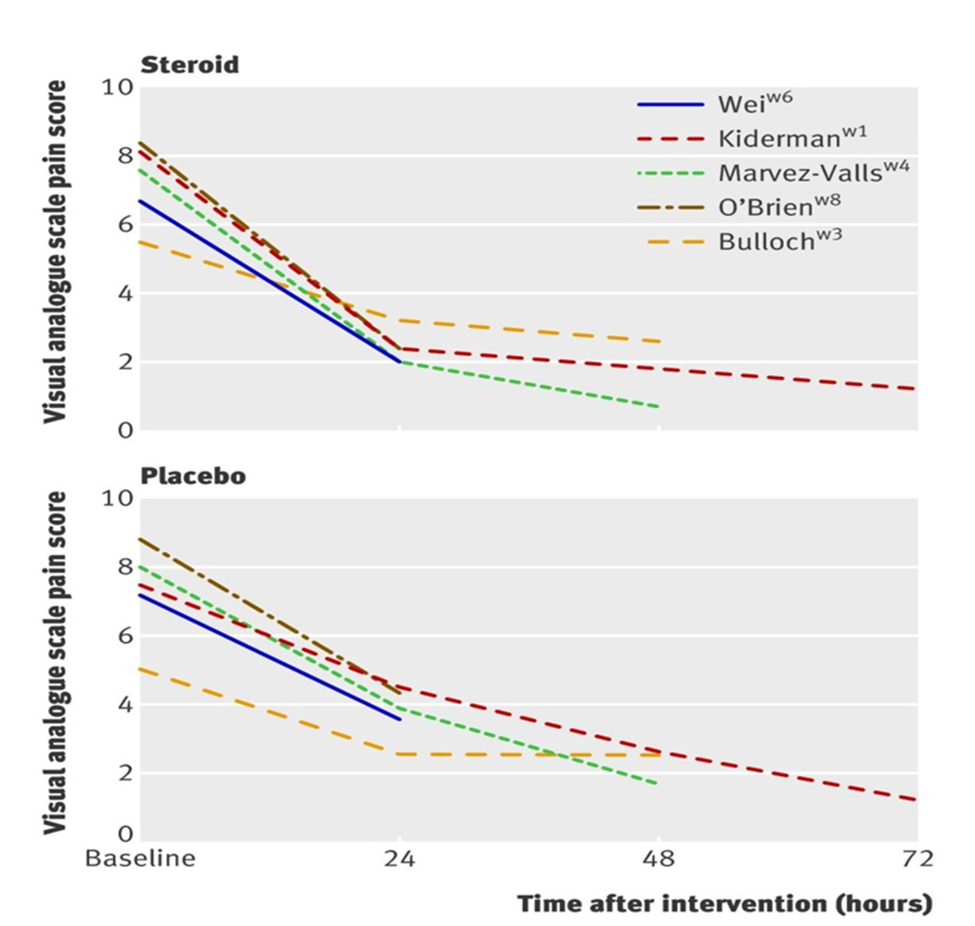
Figure 2 in paper two: The Corticosteroid use is like placebo as graphics displays by visual analogues pain score at 72 hours after the intervention.
From Hayward G., Thompson M., Heneghan C., Perera R., Del Mar C., Glasziou P. "Corticosteroids for pain relief in sore throat: systematic review and meta-analysis." BMJ. 2009 Aug 6;339: b2976. doi: 10.1136/bmj. b2976. Review. Erratum in: BMJ. 2010;340. doi: 10.1136/bmj.c692. PMID: 19661138. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2722696/
Table 1 includes the characteristics of trials included in the meta-analysis: participants' age, intervention (type and dose of Corticosteroids), control (Saline 1 ml or placebo), and Antibiotics used (Penicillin G or Erythromycin). Additional analgesia treatment: Paracetamol or unregulated, unrecorded Paracetamol or Ibuprofen. Symptoms relieving emerges in all the trials evaluation.
Table 2 shows the studies' methodological quality. The evaluation assesses the bias of the survey. Furtherly, the selection bias evaluation determined by the selection and the allocation. Moreover, the randomisation and the intervention on evaluation assess the discontinuation bias. The data collection assessment evaluates observational bias quality. Founts of bias can damage the scientific value of the study [7].
Forest plots are the most exact evaluation method included in this study.
In the second figure forest plots, at page four, displays Corticosteroids effect at 24 and 48 hours. Kiperman, Niland, Taser, Wei study at 24 hours (Heterogeneity: I2=44%) and Kiperman, Niland, Taser study at 48 hours (Heterogeneity: I2=0%), respectively. The intervention, by a wide confidence interval, does no worse than the control group. All the studies show a (fixed) relative risk value in favour of steroid. The diamond graphic in the plot shows that the metanalysis is statically significant for Corticosteroids against placebo [4,5].
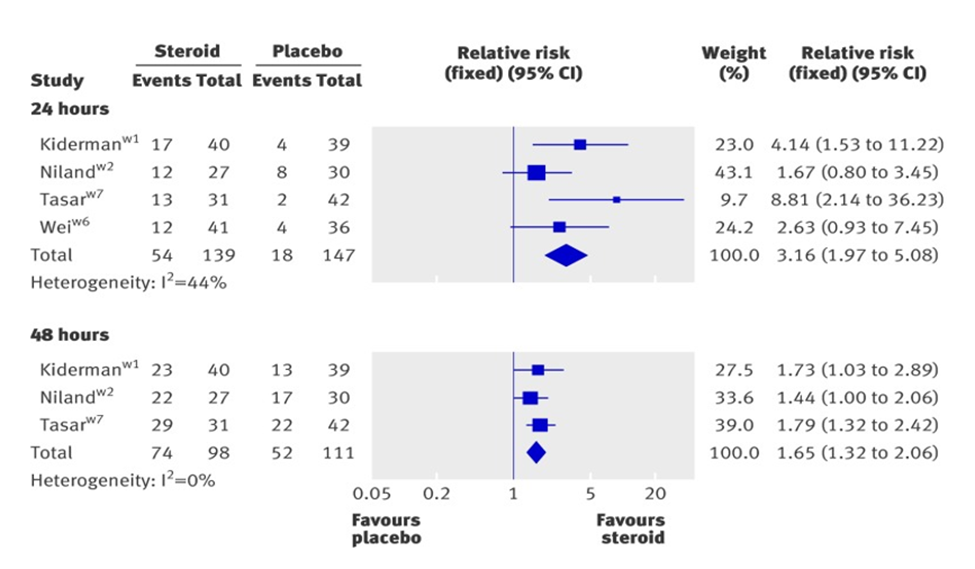
Figure 3: Complete resolution of pain at 24 or 48 hours.
From "Corticosteroids for pain relief in sore throat: systematic review and meta-analysis." BMJ 2017; 358 doi: https://doi.org/10.1136/bmj.j3887 (Published 20 September 2017) Cite this as: BMJ 2017;358: j3887 Behnam, Sadeghirad.
In the third figure, forest plots, at page five, displays that intervention works in Marvel-Valls, O'Brien, Olympia, Tasar, Wei studies. Their heterogeneity is I2=72% by a mean difference (random) and by 95% of the confidence interval. Bullock study is a small study. Its plot shows that the intervention works by a wide confidence interval. The diamond plot passes the of no unity or no effect line. The effect analysis is favourable to Corticosteroids against placebo. Their efficacy considers the hours necessary to start pain relief [4,5].
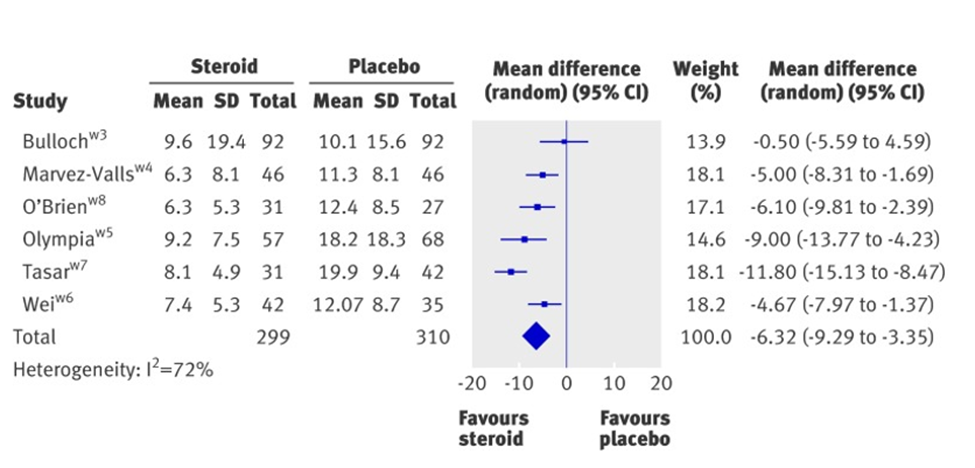
Figure 4: Effect of corticosteroids on mean time to onset of pain relief in hours.
From "Corticosteroids for pain relief in sore throat: systematic review and meta-analysis." BMJ 2017; 358 doi: https://doi.org/10.1136/bmj.j3887 (Published 20 September 2017) Cite this as: BMJ 2017;358: j3887 Behnam, Sadeghirad.
Critical appraisal paper three
"Streptococcus A in paediatric accident and emergency: are rapid streptococcal tests and clinical examination of any help?" Van Limbergen J., Kalima P., Taheri S., Beattie TF.
Study design: Diagnostic Study.
Clinical question: are Rapid Streptococcal Tests accurate diagnostic tools to confirm Streptococcus A infection? Was the Diagnostic Test evaluated in a representative group of patients like those seen in practice?
The representative spectrum of 213 patients considers, with clinical signs of pharyngitis to participate in the diagnostic test study. The diagnostic test aims to evaluate firstly: the Streptococcus test like Diagnostic Test with or without throat culture; secondly: the clinical diagnosis and its contribution to clinical improvement. Patients were all children. A strep-wise logistic analysis analysed the regression of thirteen clinical features. Quikvue+ Step A test process patients sampling. Its manufacturer Quidel Inc., San Diego, CA assigns to the test for a sensitivity level and specificity level of 95% and 98%, respectively.
Was the reference standard ascertained regardless of the index test result?
What is test adherence to the clinical case? The tests were positive on thirty-three patients for Rapid Streptococcal Test or throat cultural medium. Rapid Streptococcal Test individuated twenty-one patients only. Between these last ones, eleven samples were a false positive. The study assigned a low sensitivity for clinical diagnosis 57% (34% to 78%) and a low specificity 71% (61% to 80%) for the same pattern. Among the patients affected by sore throat with a prevalence of 15.9%, rapid Streptococcal test sensitivity was 65.6% (95% CI 46.8% to 81.4%) and its specificity 99.4% (96.7% to 99.9%). The post-test likelihood ratio was 19.6 and negative likelihood 0.60. Clinical diagnosis, used as a predictive value, was 30% valid [9].
Was there an independent, blind comparison between the index test and an appropriate reference standard of diagnosis?
The Rapid Streptococcal Test, validated by a follow-up culture test, is an optimal reference standard for confirming results. The outcome of negative Rapid Streptococcal Tests and pyrexia gave the diagnosis of sore throat by a (P<0.05). Results of clinical diagnosis of Group A β-Haemolytic Streptococcus Pharyngitis has not any limited value in the diagnosis of sore throat if they are cultural medium based. The manufacturer gave to the product and high sensitivity. While this Rapid Streptococcal Test shows low sensitivity in this study, overall, they are not an appropriate, predictive, for diagnostic tool by themselves without any further validation.
Are test characteristics presented?
The characteristics addresses in the sample paragraph, and the sensitivity and specificity shown, along with their predictive value. The tests are replicable, and the interpretation of the results is accurate, on thirteen clinical features related to sore throat symptoms even though the manufacturer company declares a different sensitivity and specificity from reality, as the study shows.
Were the methods for performing the test described in enough detail to permit replication?
The study uses the sensitivity and specificity examination as also post-test probability assesses the test appropriately. It audits rapid tests performed to evaluate their efficacy to validate the diagnosis, giving them low diagnostic evidence [6,7]. Even if they are replicable. On the other hand, in-depth laboratory analyses ensure a replicable and accurate diagnostic tool.
Context [10]
Contextual elements set the assessment of the interventions considering Evidence-Based-Medicine like primary outset. The appropriateness of the outcomes derives from an accurate statistical analysis. It carefully ensures quality and safety on therapeutic approach to sore throat assessing diagnostic tools and pharmacological treatment.
Study of the Intervention(s) [10]
The study found a significant correlation between positive screening results and sore throat prevalence. It also emphasized the need for more comparative analyses to address differences on intervention quality by its efficacy. As the critical appraisal assess.
Measures [10]
Measures to study the intervention processes and its outcomes, include the rationale for their selection, operational definitions, validity and reliability. This defines the validity and accuracy of the methods in this study. The method for ongoing evaluation covers contextual elements that influence the outcomes and the efficiency. It systematically assures chosen appraised papers for completeness and accuracy by data assessment. Furtherly, the accurate statistic approaches testimonies difference. They’re among the therapeutic outcomes.
Analysis
Data interpretation methods include qualitative and quantitative approaches. They examine therapeutic results and quantitative and quantitative data by comparing three selected papers. These methods analyse data variation, efficacy, and safety through critical appraisal analysis, highlighting clinical differences. It evidences differences between two different pharmacological approaches as also between two different diagnostic tools, by their efficacy and accuracy to evaluate sore throat most specific approach by its assessment.
Specific aims
The aim of this research project project is to critical appraisal on three sore throat chosen papers to assess the most appropriate therapeutics strategy in paediatric patients. It considers the assumption, the intervention on this heath problem, and its contextual elements. It aims to give the appropriate therapeutic approach. (As it has been considered at page 2 on point 3.2)
Ethical considerations
The study uses sensitivity and specificity examination, as well as post-test probability, to assess the difference between rapid diagnostic test and exudative laboratory test appropriately. It reviews rapid tests performed to evaluate their efficacy in validating the diagnosis, indicating their low diagnostic evidence on rapid tests. Awhile paper approaches symptoms diagnosis measurement by comparing them through robust statistic assessment. Therapeutics treatment evaluation, in paper one, respect ethics principles as it compares pharmacological treatment with symptoms resolution by themselves. Non-maleficence ethic principle appears in this item. Moreover, even if study consider minors it respect the beneficence ethics principle. Ethical aspects aim to implement and study the appropriate interventions addressed to patients. They include ethics aspects properly to review the chosen studies. Therefore, it has no potential ethical conflicts and no conflict of interest.
Results
Are the results similar from study to study?
• Kiderman, Niland, Tasar, Wei trail pain relief at 24 hours by relative risk assessment (relative risk 3.2, 95% confidence interval 2.0 to 5.1, P<0.001). The number needed to treat is 3.7 (2.8 to 5.9). The heterogeneity is: I2=44.
• Hayward G., Thompson M., Heneghan C., Perera R., Del Mar C., Glasziou P. trial pain relief at 48 hours relative risk assessment (relative risk 1.7, 9.5% confidence interval 1.3 to 2.1 P<0.001). The number needed to be treated: 3.3 (2.4 to 5.6). The heterogeneity is: I2=0%.
• Marvez-Valls, O'Brien, Olympia, Tasar, Wei, Bulloch and Tarsar trials show high the mean time difference on the pain onset relief by more than 6 hours between Corticosteroids against no treatment. (Ninety-five percent confidence interval 9.3 to 3.4, to P<0.001). The heterogeneity is: I2=73%.
The results are heterogeneous, but it is comprehensive, accordingly to what it has been examined yet. Moreover, further recommendations, comments and questions complete this critical appraisal in its final lay summary and in two bullet points essays.4,5 (As it reports at page 17-18 on points 14.1 and 14.2).
Does consideration fit with pre-existing guidelines that you may want to refer?
- NICE guidance [NG84] "Sore throat (acute): antimicrobial prescribing" expresses a favourable opinion on my point of view about the antibiotic prescription. It classifies pain fever by Centor Score Criteria and fever pain score to identify the likelihood of a bacterial infection in patients affected by sore throat. (It contains the table for paediatric Antibiotic choice and dosage. It reports as first choice Phenoxymethylpenicillin, and the alternative antibiotics for penicillin intolerance or allergy, Clarithromycin and Erythromycin). It confirms what the bullet-point summary outlines [6].
- The BMJ Rapid Recommendations article: "Corticosteroids for sore throat: a clinical practice guideline” uses GRADE (Grading of Recommendations, Assessment, Development and Evaluations framework) to assess by Evidence-Based-Medicine criteria the use of Corticosteroids [7,8] in sore throat therapy. It does not endorse its use, confirming what it has been in the bullet point summary on children. Material supports this evidence derives from a systematic review (elevated level of clinical evidence) of a large, randomised trial. The study population comprises children aged five years and older, as well as adults. It is applicable on 1) emergency and primary care patients, 2) a viral or bacterial sore throat, 3) severe and not severe sore throat with antibiotics treatment. It does not apply to infectious causes or diseases such as 1) Mononucleosis, 2) Immunocompromised patients, 3) sore throat following surgery or intubation and 4) children under five years old. As noted in the report. This guideline suggests moderating the use of Corticosteroids by a benefit harm assessment. Although, Corticosteroids resolved pain due to sore throat symptoms at 24 and 48 hours (GRADE high to moderate-quality evidence) 8 in an increased proportion of patients. Most guidelines recommend Paracetamol or Ibuprofen instead of Corticosteroids as first approach. Even if Corticosteroid is unlikely to resolve symptoms and so days out of school. (GRADE moderate-quality evidence) 8. Corticosteroids combined with antibiotics to treat bacterial can sore throat. A brief steroid treatment or a single dose assists in resolving painful throat symptoms. This therapeutic strategy reduces the chances of adverse effects. There is no difference in corticosteroid use between primary care and emergency care. It reduces antibiotic co-administration (GRADE low-quality evidence) [6]. Corticosteroids relieve pain and speed up symptom resolution. (GRADE low-quality evidence) [8].
- "Point-of-care diagnostic testing in primary care for strep A infection in the sore throat". MedTech innovation briefing [MIB145]. This briefing describes eleven technologies. It assesses diagnostic confidence on strap A infection. Key uncertainties improve lack of confidence to use this type of diagnostic tool. It confirms the suggestions. Consequently, diagnostic cultural medium tests used with along clinical evaluation and laboratory analysis confirm the type of pathogen. It would implement pharmacy, primary and emergency medicine quality, and safety on prescribing [9].
Patient and Public Involvement
Children and their family members can be involved in the creation and dissemination of information materials as the communication of adverse events and the promotion of a culture of transparency. Furthermore, young patients and their families can receive psychological, health and organizational support on the difficulties related to manage this common health problem. In conclusion Patient and Public Involvement approach patients and public health facilities in a central position, promoting a culture of collaboration, transparency and participation. It gives benefits for all participants involved in this quality and safety improvement for the best therapeutics approach.
The work is useful to assess the proper diagnostic and treatment of this common health issue using scientific critical appraisal. Even if further particular cases could rise interest on new research. Its sustainability ascertains therapeutics solution in a constant and definite way. The study can open the interest on further research on this topic like next steps. Medical practice gets an important lead from this critical appraisal. Further studies, on this field, could start to solve sore throat complication symptoms for children’s care assuring by medical quality and safety by a proper scientific evaluation.
Summary
Results can be summarised in the following bullet points summaries.
Final recommendations on therapeutic approaches, a bullet-point summary.
1. Clinical evaluation is necessary to start assessing sore throats. A laboratory culture, not a rapid test, needs to identify the causes of illness.
2. Plan the appropriate therapeutic strategy to cure children on evidence-based assessment.
3. Antibiotics prescription effects in a marginal way the resolution of sore throat.
4. Corticosteroids and Antibiotic provide a resolution of symptoms in severe and exudative sore throat.
5. Do not exceed in administering medicines to satisfy different patients' expectations.
Bullet-point summary recommendations regarding rapid testing.
1. The laboratory analysis should confirm quick test results.
2. Clinical evaluation carries to the appropriate assessment of symptoms.
3. Clinical evaluation assigns the proper therapeutic strategy assessing the severity of symptoms.
4. Do not over-administer Antibiotic or Corticosteroid if it is not necessary to satisfy children expectation without appropriate clinical evaluation. The cultural laboratory analysis, as well as the clinical assessment, could confirm any valid approach.
Interpretations
The comparison of these three chosen scientific studies gives the interpretation by the critical appraisal. Awhile further assessment is in bullet point section conclude with a lay summary section. Certainly, cost-effectiveness outlines strategic trade-offs, including a costs reduction by assuring secure therapy. (As it displays at page 20 on point 15).
Limitation
This study is an accurate evaluation as it considers internal and external validity. Its results are replicable. Bias measurement and statistical analysis outline robust results. Critical appaired studies have some limitation on internal validity such as confounding, bias, and heterogeneous measurements.
An accurate and detailed data assessment minimizes and adjust general limitations. As it is reported during the whole critical appraisal sections (As it displays on the whole point 6 from page 4).
Conclusion
Quality and Safety Recommendations for Evidence-Based Medicine Case Resolution, a lay summary
Furtherly, this study contains three articles that give the appropriate method to assess the cure of sore throat. In this way, it is possible to decide how to cure any children presents symptoms of a sore throat accurately. It assures an Evidence-Based-Medical cure by quality and safety assessment.
Antibiotic use is not necessary whether the symptomatology is not advanced and infectious, indeed. Use corticosteroid moderately only for severe sore throat. The rapid test used in primary and pharmacy care appears to lack sufficient medical evidence to support its effectiveness. It is not an accurate diagnostic tool. Clinical evaluation, along with laboratory cultural analysis, suggests the efficient therapeutic approach to evaluate the severity of symptoms. Antibiotics, Corticosteroids, and Analgesics administration based on etiopathological assessment is important to approach this health issue properly. On balance, do not exceed in drug administration, on paediatric patients. The unappropriated treatment can result in ineffective and harmful due to adverse effects. National Health Service should improve the updates of data on this topic. An information sheet could promote an improvement on quality and safety on paediatric assistance. This article focuses on Public and Patient Interests (PPI) by outlining efficacy and safety of sore throat treatments. It considers public health, social, and economic factors, as well as the concerns of patients and families regarding this common disease. It also outlines steps to treat sore throats in pediatric patients, aiming to minimize care errors and reduce costs. Dissemination can go along with PPI to spread results. Other contests can also use this simple method to improve quality. Further study in the field can start to assess medical complications in this assessed case.
Funding and Conflict of Interest Statement
No sources of funding that supported this work. The author discloses from any conflict of interest, that he solely contributed to outlining the study. Furthermore, no funding has been used to realize this work.
References
- https://www.nice.org.uk/guidance/ng84.
- https://www.prisma-statement.org/prisma-2020-flow-diagram.
- Oxford (UK) CEBM Levels of Evidence 2011.
- Narinder, Kaur, Gosall, Gurpal, Singh (2015) The Doctor's Guide to Critical Appraisal.
- The Practice of Evidence-Based Health Care Module Workbook" Paul P. Glasziou, Chris Del Mar, Janet Salisbury 2018
- Sore throat (acute): antimicrobial prescribing (2018), NICE guideline: NG84.
- Aertgeerts B, Agoritsas T, Siemieniuk RAC, Burgers J, Bekkering GE (2017) Corticosteroids for sore throat: a clinical practice guideline. BMJ 358:j4090.
- Alonso-Coello P, Schunemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, et al. (2016) GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. BMJ 353:i2016.
- Point-of-care diagnostic testing in primary care for strep A infection in sore throat” Nice Guideline 2018.
- Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F (2015) SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf 25:986–992.
Critical Apparised Papers
1. Little P., Williamson I., Warner G., Gould C., Grantley M., Kinmonth AL. "Open randomised trial of prescribing strategies in managing sore throat." BMJ. 1997 Mar 8;314(7082):722-7. PMID: 9116551. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2126131/
2. Hayward G., Thompson M., Heneghan C., Perera R., Del Mar C., Glasziou P. "Corticosteroids for pain relief in sore throat: systematic review and meta-analysis." BMJ. 2009 Aug 6;339: b2976. doi: 10.1136/bmj. b2976. Review. Erratum in: BMJ. 2010;340. doi: 10.1136/bmj.c692. PMID: 19661138. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2722696/
3. Van Limbergen J., Kalima P., Taheri S., Beattie TF. "Streptococcus A in paediatric accident and emergency: are rapid streptococcal tests and clinical examination of any help?"
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.