Concurrent Presentation of Subacute Thyroiditis and Graves Disease
by An-Luo Phon1,2, Pi-Ling Hsiao2, Wen-Sen Lin2, Lee-Min Wang2*
1Department of Internal Medicine, Division of Endocrinology and Metabolism, Taiwan
2Ministry of Health and Welfare, Shuang-Ho Hospital, Taipei, Taiwan
*Corresponding Author: Lee-Min Wang, Ministry of Health and Welfare, Shuang-Ho Hospital, Taipei, Taiwan
Keywords: Subacute Thyroiditis; Graves Disease; Physical Examination; Outpatient Department; Hyperthyroidism.
Received Date: 02 July 2025
Accepted Date: 07 July 2025
Published Date: 10 July 2025
Citation: Phon AL, Hsiao PL, Lin WS, Wang LM (2025). Concurrent Presentation of Subacute Thyroiditis and Graves Disease. Ann Case Report. 10: 2336. https://doi.org/10.29011/2574-7754.102336
Abstract
Subacute thyroiditis is the leading cause of painful thyroid, primarily affecting middle-aged women. Initial hyperthyroidism results from the release of preformed thyroid hormone, followed by a subsequent hypothyroid phase and eventual restoration of normal thyroid function. Graves' disease, an autoimmune condition, predominantly targets the thyroid gland but can also impact other organs, such as the eyes and skin. It is the most prevalent cause of hyperthyroidism. In this report, we present the case of a 51-year-old woman exhibiting symptoms of hyperthyroidism and a tender thyroid gland. Subacute thyroiditis was tentatively diagnosed based on clinical presentation, with laboratory tests and a Tc-99m thyroid study supporting the impression of Graves' disease. Symptoms improved with treatment, and the patient was discharged with arrangements for outpatient department follow-up. Conclusion: Graves Disease is the most common cause of hyperthyroidism and is an autoimmune-related disease. Subacute thyroiditis, typically provoked by a viral infection, is the most common cause of painful thyroiditis. Initial management of both conditions is similar.
Introduction
Thyroiditis referred to inflammation of thyroid gland. It may be painful or painless. Subacute thyroiditis (SAT) is the most common cause of thyroid/neck pain.[1] SAT is also called subacute granulation or undervalue thyroiditis, a self-limited disease with subsequent full thyroid function restoration, is characterized by three phases: (1)Active thyrotoxic phase; (2) reactive hypothyroidism;(3) return to normal thyroid Function.[2] SAT affects women three to five times more often than men. The overall incidence is reported at 12 people out of every 100,000. It is most common in middle age, followed by young adulthood, and decreases in frequency with increasing age. There is a seasonal incidence, with most cases occurring in the summer.[3] Viral infections are presumed as a cause of SAT.Many patients may have a history of viral upper respiratory infection 2 to 8 weeks before developing thyroiditis.[1] Some studies have suggested that the seasonal distribution of thyroiditis coincides with the peak incidences of coxsackievirus (groups A & B) and echovirus infections. It is also associated with mumps, measles, influenza, SARS-CoV-2, and other viruses.[4,5] Grave’s disease is the most common cause of hyperthyroidism accounting for 60% to 80% of hyperthyroid cases. Signs of extrathyroidal manifestations of Graves’ disease include ophthalmopathy like eyelid retraction, proptosis, periorbital edema, chemosis, scleral injection, exposure keratitis. Thyroid dermopathy and osteopathy or thyroid acropachy were also observed in some individuals Onycholysis (Plummer nails) and clubbing are very rare.[6] Simultaneous occurrence of subacute thyroiditis and Graves’ disease was noted in rare circumstance.[7]
Case Presentation
A 51-year-old indigenous Taiwanese woman, previously healthy, began experiencing palpitations, malaise, fatigue, and tremors 10-14 days ago. These symptoms were accompanied by an anxious mood, difficulty swallowing progressing to shortness of breath, heat intolerance, profuse sweating, gastric discomfort, urinary frequency, and difficulty standing up from a sitting position. She also noticed a weight loss of approximately 7kg within two weeks. She denied experiencing nausea, vomiting, abdominal pain, or diarrhea but endorsed recent respiratory symptoms including a runny/stuffy nose and productive cough at night. She is a non-smoker but drinks
occasionally. She disclosed a habit of consuming iodine-rich foods such as seaweed and kelp but denied taking any medications, including traditional Chinese medicine, except for a once-daily vitamin tablet. Her younger sister was diagnosed with hyperthyroidism at age 49 (Figure 1). She initially visited a cardiologist at another center and was then referred to our endocrinologist's outpatient department. On physical examination, tachycardia without murmurs, tremors, and moist Palmerston surfaces were noted. No sympathetically, abnormal respiratory sounds, tenderness or guarding of the abdomen, leg swelling, or focal neurological signs were observed. Vital signs showed a heart rate (HR) of 108 beats per minute. There was no obvious enlargement of the thyroid gland, no audible bruit, the gland moved with agglutination, mild tenderness on palpation, and no skin attachment. (Figure 2) Laboratory findings revealed white blood cells (ABCs) 4800 cells/µL, hemoglobin 13.5g/AL, and platelets 191000 cells/µL. Biochemical profiles showed sodium (Na) 142 me/L,potassium (K) 4.0 me/L, blood urea nitrogen (BUN) 16.1 mg/AL, Creator 0.36 mg/AL,Kasparov transfer (AST) 35 U/L, tronning T 9.8 Eng/L, NT-pro BNP 488 pg/L, erythrocyte sedimentation rate (ESR) 33 mm/hr, and C-reactive protein (CRP) 5.84 mg/L. Thymine (T4) was > 24µg/AL, thyroid stimulating hormone (TSH) was < 0.005 µIU/L, and anti-ASH receptor antibodies (Antichrist) were > 40.0 IU/L. (Table 1)
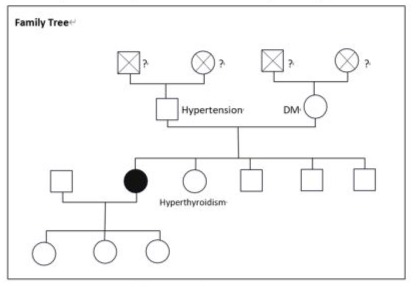
Figure 1: Family Tree
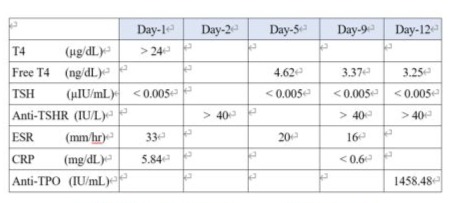
Table 1: Thyroid hormones and ESR level
A supine chest X-ray showed mild Keri-bronchial infiltration over both lung fields, and a 12-lead ECG revealed sinus tachycardia at a rate of 108/min. Thyroid ultrasound demonstrated a diffused multinodular goiter with cystic changes. Right lobe measured 2.14cm x 5.89cm x 2.43cm, and (1). 0.81cm x 0.43cm nodule with ill-defined margin, partial cystic solid part 50%, (2). 0.24cm nodule with ill-defined margin, partial cystic solid part 70%, and (3). 0.31cm nodule with ill-defined margin, partial cystic solid part 50%. Left lobe measured 2.13cm x 5.90cm x 2.08cm; and 0.32cm x 0.2cm nodule with ill-defined margin, partial cystic solid part 60%. Isthmus measured 0.71cm with hypervascularity. All the cysts are wider than taller in shape. (Figure 2)
Figure 2: No obvious enlargement of the thyroid gland
Treatment was initiated with good hydration, oral propranolol 30mg/day in three divided doses, and oral methimazole 5mg thrice daily. Symptomatic treatment for runny nose, neck pain, and productive cough were also prescribed. Free T4 was 4.62ng/dL, and TSH was < 0.005µIU/mL on the fifth day of admission, prompting the replacement of 2 methimazole with propylthiouracil 100mg thrice daily. A Tc-99m thyroid study after intravenous injection of 6mCi revealed diffusely increased uptake of the agent in the gland with visualization of the pyramidal lobe. The impression was likely hyperthyroidism due to Graves' disease. (Figure 3)
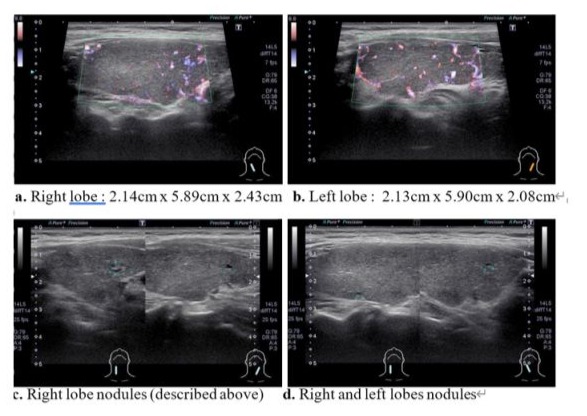
Figure 3: Thyroid Ultrasound
Treatment was initiated with good hydration, oral propranolol 30mg/day in three divided doses, and oral methimazole 5mg thrice daily. Symptomatic treatment for runny nose, neck pain, and productive cough were also prescribed. Free T4 was 4.62ng/dL, and TSH was < 0.005µIU/mL on the fifth day of admission, prompting the replacement of methimazole with propylthiouracil 100mg thrice daily. A Tc-99m thyroid study after intravenous injection of 6mCi revealed diffusely increased uptake of the agent in the gland with visualization of the pyramidal lobe. The impression was likely hyperthyroidism due to Graves' disease. (Figure 4)
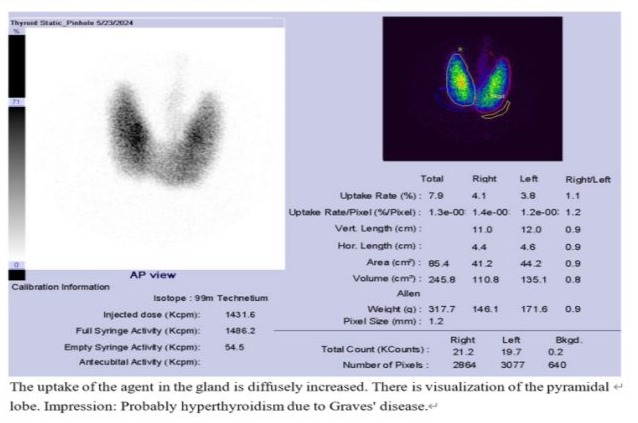
Figure 4: Tc-99m Thyroid Scan
The patient exhibits typical symptoms of hyperthyroidism, including heat intolerance, palpitations, anxiety, fatigue, weight loss, muscle weakness, tremors, tachycardia, warmmoist skin, urinary frequency, and unintentional weight loss. However, there are no signs of lid lag or axial proptosis, nor increased bowel movements. Upper gastrointestinal pan-endoscopy was performed due to epigastric discomfort lasting for months, revealing gastroesophageal reflux disease (LA grade-A) and a duodenal ulcer. Endoscopic biopsy was conducted for suspected esophageal metaplasia and gastric erosion. A 24-hour Holter’s ECG showed sinus rhythm as the basic rhythm, with occasional arterial premature complexes (183) including one repetitive APC and one ventricular premature complex without ventricular tachycardia. An ophthalmologist consultation found no evidence of thyroid eye disease except for xerophthalmia.
Discussion
Hyperthyroidism is a pathological syndrome in which tissue is exposed to excessiveamounts of circulating thyroid hormone. The most common cause of this syndrome is Graves' disease, followed by toxic multinodular goiter, and solitary hyperfunctioning nodules. Autoimmune postpartum and subacute thyroiditis, tumors that secrete thyrotropin, and drug-induced thyroid dysfunction, are also important causes.[8] The patient's hyperthyroidism is confirmed by elevated levels of T4 and free T4 (above upper limits) and a suppressed TSH level (< 0.005 IU/mL). Further investigation aims to determine the underlying cause. Graves' disease, being the most common cause, is supported by persistently elevated levels of anti-TSHR antibodies, antithyroid peroxidase (anti-TPO), and the findings from the Tc-99m thyroid scan.
The diagnosis of subacute thyroiditis is established based on the symptoms of hyperthyroidism, along with a history of preceding respiratory symptoms (such as a runny/stuffy nose and productive cough), seasonal coincidence, and physical examination findings of a tender thyroid on palpation. Laboratory testing typically reveals abnormalities in the thyroid panel (elevated T4/Free T4, low TSH) as well as elevated inflammatory markers such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). Thyroid sonographic findings may also support the diagnosis. Other potential causes of painful thyroiditis, such as suppurative thyroiditis and thyroiditis resulting from radiation or trauma, were excluded based on the patient's history and clinical presentation. Additionally, other causes of thyrotoxicosis, including overdosing or inadvertent ingestion of oral levothyroxine, the Jod-Basedow phenomenon resulting from exogenous iodine exposure (e.g., amiodarone or IV contrast media), and post-partum thyroiditis were also ruled out. Subacute thyroiditis is a clinical diagnosis. Neck pain with tender thyroid gland on the exam is sufficient enough to establish the diagnosis.[1] The thyroid gland might be slightly enlarged or normal with a diffuse or focal hypoechogenic appearance on ultrasonography.The presence of ill-defined hypoechoic thyroid lesions without a round or oval shape is diagnostic for subacute thyroiditis in the proper clinical setting.[9] Color Doppler sonographyshows decreased flow during the hyperthyroid phase in subacute thyroiditis, compared with Graves' disease's increased flow.[10] Tc-99m pertechnetate scintigraphy in the thyroid was markedly reduced during the acute stage of SAT. [11] The TSH receptor antibody titer is a biomarker that is more specific for Graves' disease, although it may also be elevated to some extent in subacute thyroiditis. Subacute thyroiditis may trigger autoreactive B cells to produce TSH receptor antibodies, resulting in TSH receptor antibody-associated thyroid dysfunction in some patients.[12] Almost all patients with Hashimoto's thyroiditis and nearly 75% of individuals with diagnosed Graves' disease has detectable TPO antibodies (TPOAbs). [13] These autoantibodiesare also frequently present in euthyroid subjects, particularly women, even within the normal range for thyrotropin (TSH). TPOAbs levels are one of the predictive factors for conversion from euthyroidism to thyroid dysfunction.[14] The primary objectives of subacute thyroiditis treatment are pain relief and symptom management. Subacute thyroiditis usually resolves, and patients return to a normal euthyroid state in 3 or 4 months. Rarely do patients have hypothyroidism which can be transient or permanent.1 Recurrence is uncommon but can occur in up to 2% of patients.
Hypothyroidism may also become permanent in 5% of cases.[15] Treatment for Graves' disease depends on its presentation. It consists of rapid controlling of symptoms and reduction of thyroid hormone secretion. There are three options to reduce thyroid hormone synthesis. These options are: (1). Antithyroid drugs which block thyroid
hormone synthesis and release, (2). Radioactive iodine (RAI) treatment of the thyroid gland, (3). Total or subtotal thyroidectomy [16]. The longer that antithyroid drug (ATD) therapy is used, the lower the relapse rate is in patients with Graves' disease. Long-term ATD treatment may be considered in Graves' patients who do not show complications or an economic burden from hyperthyroidism. [16] All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Conclusion
Graves' disease is the most common cause of hyperthyroidism and is an autoimmune-related disease. Subacute thyroiditis, typically provoked by a viral infection, is the most common cause of painful thyroiditis. Initial management of both conditions is similar. Subacute thyroiditis is a self-limiting disease, which may leave some patients hypothyroid. The management of Graves' disease aims to reduce the hyperactivity of the thyroid gland, either medically or surgically. Healthcare providers should keep in mind the possibility of the simultaneous occurrence of subacute thyroiditis and Graves' disease in rare circumstances.
References
- Tabassom A, Chippa V, Edens MA. (2023) De Quervain Thyroiditis. StatPearls.
- Zornitzki T, Mildiner S, Schiller T, Kirzhner A, Ostrovsky V, Knobler H. (2022) Subacute Thyroiditis – Still a Diagnostic Challenge: Data from an Observational Study. Int J Environ Res Public Health. 19: 9388.
- Moini J, Pereira K, Samsam M. (2020) Epidemiology of Thyroid Disorders.
- Michas G, Alevetsovitis G, Andrikou I, Tsimiklis S, Vryonis E. (2014) De Quervain thyroiditis in the course of H1N1 influenza infection. Hippokratia. 18: 86-87.
- Martino E, Buratti L, Bartalena L, Mariotti S, Cupini C, Aghini-Lombardi F, Pinchera A. (1987) High prevalence of subacute thyroiditis during summer season in Italy. J Endocrinol Invest. 10: 321-323.
- Pokhrel B, Bhusal K. (2023) Graves Disease. StatPearls.
- Kageyama N, Kinoshita M, Daimon M. (2018) A Case of Thyrotoxicosis due to Simultaneous Occurrence of Subacute Thyroiditis and Graves’ Disease. Case Reports in Endocrinology.
- Cooper DS. (2003) Hyperthyroidism. Lancet. 362: 459-468.
- Park SY, Kim EK, Kim MJ, Kim BM, Oh KK, Hong SW, Park CS. (2006) Ultrasonographic characteristics of subacute granulomatous thyroiditis. Korean J Radiol. 7: 229-234.
- Hiromatsu Y, Ishibashi M, Miyake I, Soyejima E, Yamashita K, et al. (1999) Color Doppler ultrasonography in patients with subacute thyroiditis. Thyroid. 9: 1189-1193.
- Hiromatsu Y, Ishibashi M, Nishida H, Kawamura S, Kaku H, et al. (2003) Technetium-99 m sestamibi imaging in patients with subacute thyroiditis. Endocr J. 50: 239-244.
- Iwaku K, Noh J, Watanabe Y, Sugino K, Ito K. (2023) Painless thyroiditis as a precursor to the relapse of Graves’ disease during remission. Thyroid Science. 10: 100003.
- Czarnocka B, Eschler DC, Godlewska M, Shoenfeld Y, Meroni PL, Gershwin ME. (2014) Thyroid autoantibodies: Thyroid peroxidase and thyroglobulin antibodies. Auto-antibodies. 3: 365-373.
- Amouzegar A, Ghaemmaghami Z, Beigy M, Gharibzadeh, Mehran L, et al. (2017) Natural course of euthyroidism and for early diagnosis of thyroid dysfunction: Tehran thyroid study. Thyroid. 27: 616-625.
- Slatosky J, Shipton B, Wahba H. (2000) Thyroiditis: differential diagnosis and management. Am Fam Physician. 61: 1047-1054.
- Park SY, Kim BH, Kim M, Hong AR, Park J, Park H, et al. (2021) The longer the antithyroid drug is used, the lower the relapse rate in Graves' disease: a retrospective multicenter cohort study in Korea. Endocrine. 74: 120-127.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.