Concurrent Atrial Flutter and Complete Heart Block: Diagnostic Challenges and Management Considerations
by by Lubna Alruwaili1*, Hadeel Ghazal1, Mohmmad Alotaibi1, Ahmed Aljizeeri1-3, Abdulmohsen Almusaad1,3
1Cardiac Science, King Abdulaziz Cardiac Centre, Ministry of National Guard Health Affairs, Riyadh, Saudi Arabia
2College of Medicine, King Saud University for Health Science, Riyadh, Saudi Arabia.
3King Abdullah International Medical Research, Riyadh, Saudi Arabia
*Corresponding author: Lubna Alruwaili, King Abdulaziz Cardiac Center, Ministry of National Guard Health Affairs Riyadh, Saudi Arabia
Received Date: 24 March 2025
Accepted Date: 29 March 2025
Published Date: 31 March 2025
Citation: Alruwaili L, Ghazal H, Alotaibi M, Aljizeeri A, Almusaad A (2025) Concurrent Atrial Flutter and Complete Heart Block: Diagnostic Challenges and Management Considerations. Ann Case Report. 10: 2238. https://doi.org/10.29011/2574-7754.102238
Abstract
Atrial flutter coexisting with complete heart block is a rare combination of arrhythmias. It requires a challenging approach to underlying predisposing conditions and an ambiguous optimum management strategy. Few cases of such have been reported so far, and there is debate on the appropriate therapy approach. We are reporting a case of new-onset atrial flutter with complete heart block in a patient under 60 years of age. We aimed to explore a differential diagnosis of this unusual combination. Our management approach for cardiac resynchronization therapy with defibrillator (CRT-D) emphasizes critical therapeutic aspects in such challenging scenarios.
Keywords: Complete Heart Block; Atrial Flutter; Sarcoidosis; Arrythmia; Conduction Disease.
Introduction
Atrial flutter, a supraventricular arrhythmia, is one of the most frequent heart rhythm disorders. It is a macro re-entrant circuit traversing the Cavo tricuspid isthmus of the right atrium, manifesting in a high atrial rate and a stable or fluctuating ventricular rate. A complete heart block is defined as an irregular heart rhythm caused by a malfunction in the cardiac conduction system, where there is no conduction through the atrioventricular node (AVN), leading to full dissociation of the atria and ventricles. Typically, it presents with severe bradycardia as a result of coronary ischemia or progressive degeneration in the myocardial conduction system [1]. The coexistence of these contradictory electrical phenomena may suggest significant underlying pathology affecting multiple cardiac conduction structures. Several potential causes have been prescribed so far, some of which are inflammatory conditions such as myocarditis, digoxin toxicity, degenerative conduction system disease such as Lev’s disease, and infiltrative cardiomyopathy diseases like sarcoidosis and amyloidosis. We aimed to address the possible etiologies and management strategies of such cases.
Case Presentation
A 56-year-old male with pre-existing diabetes, dyslipidemia, hypertension, and heart failure with mildly reduced ejection fraction was diagnosed in 2022 when the patient presented with symptoms of new-onset heart failure, including shortness of breath upon exertion, orthopnoea, and generalized body swelling. Admitted and worked up at that time for new-onset heart failure. The workup included echocardiography, which was reported with regional wall motion abnormalities in the septal, anterior, and apical akinesis, with a moderately reduced Ejection fraction of 40-45 %. The patient’s ECG at that time showed normal sinus rhythm with a PR interval of 300 (Figure 1). Cardiac catheterization showed mild, nonobstructive coronaries; therefore, the patient was labelled as having non-ischemic cardiomyopathy. Advanced cardiac imaging showed a mildly dilated left ventricle with moderate systolic dysfunction, LVEF = 41%. Delayed patchy myocardial enhancement imaging showed the presence of 25-50% sub-endomyocardial enhancement in the anterior, anteroseptal, and apex, strongly suggesting cardiac sarcoidosis (Figure 2). The patient was discharged on anti-failure medication, including loop diuretics and a maintenance dose of 2.5 bisoprolol. However, in June 2024, the patient was presented to our emergency department with the main complaint of lower limb swelling. The patient denied any symptoms of shortness of breath, syncope, dizziness, or fatigue. Upon an emergency workup, the patient’s ECG showed a new-onset 3rd-degree heart block with atrial flutter (Figure 3). The patient’s vitals were remarkable, with a heart rate of 38 and blood pressure of 70/50. He was started on dopamine infusion and bedside transcutaneous pacing. His bisoprolol was held, and the initial laboratory investigation was sent with no significant abnormalities. He was admitted for further workup and management. The patient was urgently pushed to the Cath lab, where a temporary pacemaker was inserted. The patient’s ECG strip showed continuous pacing, proving the patient was pace-dependent. A Repeated Echocardiograph done in July 2024 was significant for a further drop in EF, 35-40%, and mild to moderate valvular aortic stenosis. The patient was shifted to the electrophysiology lab afterward, where he underwent successful insertion of a CRT-D to the mid-posterior coronary sinus, extending to the lateral wall, programmed to DDD-adaptive at 60 bpm. The patient was shifted to the floor in stable condition with a biventricular-paced rhythm. Given his CHAD Vasc score of 2, he was started on appropriate anticoagulation. An extensive workup to investigate the underlying cause of the coexistence of these contradictory electrical phenomena included an amyloidosis workup, which came back negative for urine light chains and immunofixation with unremarkable serum protein electrophoresis. Infectious causes, including COVID-19, were negative. The patient’s chest CT showed bilateral hilar lymphadenopathy. In the light of excluding other medical causes, the patient was presumed to have sarcoidosis, given his clinical scenario and sarcoidosis diagnosis score of 5. Thus, the pulmonology team was involved, and a bronchial biopsy was done, which came back negative for non-caseating granuloma. A whole-body PET scan showed no evidence of active inflammation. One week following device implantation, the patient underwent synchronized cardioversion with successful restoration of sinus rhythm.
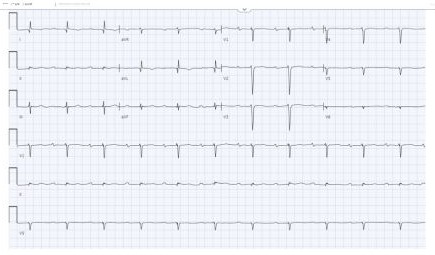
Figure 1: Normal sinus rhythm with 1st-degree heart block.
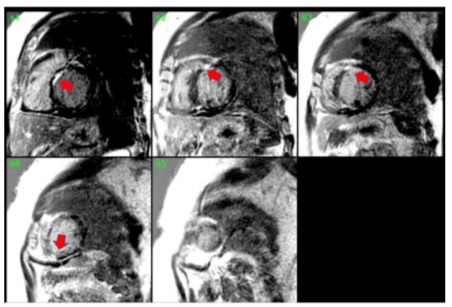
Figure 2: Delayed myocardial enhancement imaging showed the presence of 25-50% sub-endomyocardial patchy enhancement in the anterior, anteroseptal, and apex sarcoidosis.
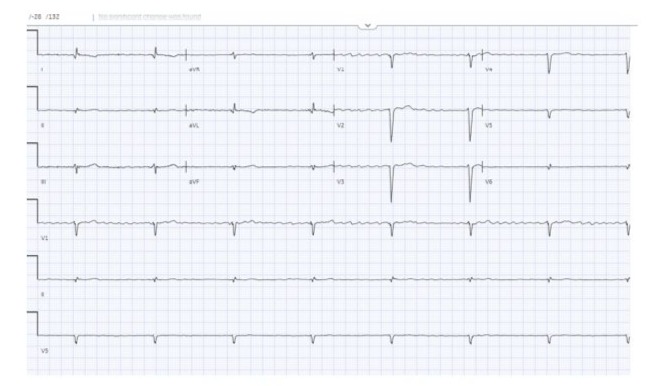
Figure 3: 3rd-degree heart block with atrial flutter.
|
Test |
Result |
Reference Range |
|
TSH |
1.35 mIU/L |
0.35–4.49 mIU/L |
|
AST |
21 U/L |
5–35 U/L |
|
ALT |
24 U/L |
5–55 U/L |
|
PTH |
21.732 pmol/L |
2.65–12.73 pmol/L |
|
Free Kappa |
28.41 mg/L |
3.30–19.40 mg/L |
|
Free Lambda |
24.14 mg/L |
5.71–19.30 mg/L |
|
Free Kappa / Free Lambda Ratio |
1.18 |
0.26–1.65 |
|
Phosphorus |
1.11 mmol/L |
0.66–1.07 mmol/L |
|
Adjusted Calcium |
2.28 mmol/L |
2.1–2.55 mmol/L |
|
Potassium |
5.0 mmol/L |
3.5–5.1 mmol/L |
|
Sodium |
135 mmol/L |
135–145 mmol/L |
|
Albumin |
28 g/L |
35–50 g/L |
|
BNP |
244.1 pmol/L |
<28.9 pmol/L |
|
Magnesium |
0.88 mmol/L |
0.66–1.07 mmol/L |
|
1,25-Dihydroxy Vitamin D |
232.1 pmol/L |
62.6–228 pmol/L |
|
IgG |
8.46 g/L |
7.51–15.60 g/L |
|
IgA |
1.678 g/L |
0.845–4.990 g/L |
|
U24 Calcium |
5.60 mmol/day |
2.5–7.5 mmol/day |
|
Urine Microalbumin |
54.50 mg/L |
<30 mg/L |
|
Urinary Calcium |
3.73 mmol/L |
|
|
Alpha 1 |
2.6 g/L |
3.5–8.5 g/L |
|
Alpha 2 |
7.0 g/L |
5.1–8.5 g/L |
|
Beta 1 |
3.6 g/L |
3.4–5.2 g/L |
|
Beta 2 |
2.3 g/L |
2.3–4.7 g/L |
|
Gamma |
7.1 g/L |
8–13.5 g/L |
|
IFE |
Absent |
|
|
AC Ratio |
5.9 mg/mmol |
<2.4 mg/mmol |
|
Abbreviations: TSH: Thyroid stimulating hormone; AST: Aspartate transaminase; PTH: Parathyroid hormone; BNP: Brain natriuretic peptide; IFE: Immunofixation; AC: Albumin/Creatinine. |
||
Table 1: The possibility of biochemistry as a cause of the patient’s presentation.
Discussion
Cardiac arrhythmia is not an uncommon manifestation of underlying cardiomyopathy. However, simultaneous presentation of new-onset atrial flutter and complete heart block in nonischemic cardiomyopathy is quite rare. Currently, few cases have been reported. Given the diagnostic uncertainty, we pursued an extensive evaluation for possible causes: Our patient had an ECG with no evidence of previous infarction or pathological Q waves and a coronary angiogram-proven non-obstructive coronary artery disease, excluding ischemia as a possible etiology. While it is possible that Betablocker could progress 1st-degree AV block to complete heart block or could cause 3rd-degree heart block [2], it is still rare; therefore, our patient, who was on a maintenance dose of bisoprolol, was presumed to have had the conduction disease unmasked rather than caused by the bisoprolol. It is rather more common for patients with beta-blockers to present with slow atrial flutter/fibrillation instead of a complete heart block [3]. Moreover, half of the patients receiving beta-blocker-related AV conduction disorder are thought to restore normal conduction after drug cessation. Thus, Bisoprolol was discontinued, but complete heart block persisted after one week, making this an unlikely sole etiology [4]. Nonetheless, aside from mild proteinuria, all of our patient’s blood work was within normal range, including chemistry, electrolytes, and TSH levels (Table 1), eliminating the possibility of biochemistry as a cause of the patient’s presentation. Whilst it’s possible for cardiac amyloidosis to present with restrictive cardiomyopathy and conduction abnormalities, our patient’s protein electrophoresis and urine immunofixation were negative, therefore effectively excluding amyloidosis as a working diagnosis in the patient. Inexplicable high-grade atrioventricular block in a person under 60 should raise the alert of the possibility of underlying cardiac sarcoidosis. Cardiac sarcoidosis represents a prime consideration in this case. CMR, a high-spatial-resolution method, provides a valuable tool for the non-invasive diagnosis of cardiac sarcoidosis. A late gadolinium enhancement (LGE) suggests sarcoidosis-related cardiac involvement, with a high sensitivity (95%) and specificity (85%) for the diagnosis of cardiac sarcoidosis [5,6]. Similarly, our patient’s CMR showed patchy myocardial involvement and delayed enhancement in the absence of obstructive coronary lesion, which was highly suggestive of cardiac sarcoidosis. It might be necessary to do an endomyocardial biopsy to establish a definite diagnosis of cardiac sarcoidosis, as stated by the Heart Rhythm Society expert consensus in diagnosing arrhythmia associated with cardiac sarcoidosis [7]. However, because the disease is focal and patchy in nature, EMB has low sensitivity and only reveals non-caseating granulomas in less than 25% of CS patients [8,9]. The constellation of unexplained high-grade AV block in a middle-aged patient with reduced ejection fraction and hilar lymphadenopathy is highly suggestive of sarcoidosis despite negative biopsy and PET findings. One last possibility is progressive idiopathic fibrosis of the conduction system (Lev’s or Lenègre’s disease), which might explain the evolution from first-degree to complete heart block. However, these conditions rarely affect atrial tissue sufficiently to cause flutter, making this diagnosis less likely in isolation. Other potential reversible causes, such as infectious causes, including COVID-19 and other viral myocarditis, were also sought and ruled out. Some histopathological studies have demonstrated that patients with complete AV block often have concurrent atrial pathology that can predispose to atrial arrhythmia. Ohkawa et al. and Yamashita et al. have shown correlations between atrial lesions and AV conduction disorders, suggesting that shared underlying pathology might affect both structures simultaneously. This concept of “atrialmalfunctioning a trio-Hisian block” may explain the rhythm combination in our patient [10,11], yet remains a less likely cause than sarcoidosis in light of our patient’s presentation.
Yet our patient was a candidate for conventional right ventricular pacing. We favoured choosing CRT-D due to the lower negative impact and side effects on the heart, such as heart failure and atrial fibrillation/flutter, which our patients had previously encountered before cardioversion to restore sinus rhythm [11-13]. Moreover, current data suggest that in patients with depressed left ventricular function, RV pacing negatively impacts the left ventricular function and structure, leading to further deterioration in LV function and possibly to atrial arrhythmias [14,15]. Though patients with normal left ventricular function can usually tolerate continuous RVA pacing [15], the possibility of underlying sarcoidosis placed our patient at high risk for malignant arrhythmias, justifying defibrillator capability. Consequently, we inserted CRT-D rather than CRT-P.
Conclusion
This case illustrates that concurrent atrial flutter and complete heart block should prompt consideration of specific underlying pathologies, particularly infiltrative cardiomyopathies. While a definitive diagnosis remains challenging in some cases, a systematic approach to the differential diagnosis can guide appropriate management. In patients with this rare arrhythmia combination and reduced ejection fraction, CRT-D represents a reasonable therapeutic strategy that addresses both the bradyarrhythmia and the potential risk of tachyarrhythmias. Long-term monitoring remains essential, as the underlying etiology may become more apparent over time with disease progression.
Acknowledgments: The authors have no acknowledgments to declare.
Ethical considerations: All identifying information has been anonymized to protect the patient’s privacy and confidentiality. Institutional approval was not required for this type of publication as per the policies of our institution
Conflict of interest: There’s no conflict of interest to declare.
References
- Knabben V, Chhabra L, Slane M. (2022) Third-degree atrioventricular block. StatPearls.
- Prystowsky EN. (1988) The effects of slow channel blockers and beta blockers on atrioventricular nodal conduction. J Clin Pharmacol. 28:6– 21.
- Marcu DTM, Adam CA, Dorobanțu DM, Șalaru DL, Sascău RA, et al. (2022) Beta-blocker-related atrioventricular conduction disorders: a single tertiary referral center experience. Medicina (Kaunas). 58:320.
- Zeltser D, Justo D, Halkin A, Rosso R, Ish-Shalom M, et al. (2004) Drug-induced atrioventricular block: prognosis after discontinuation of the culprit drug. J Am Coll Cardiol. 44:105–8.
- Aitken M, Chan MV, Urzua Fresno C, Farrell A, Islam N, et al. (2022) Diagnostic accuracy of cardiac MRI versus FDG PET for cardiac sarcoidosis: a systematic review and meta-analysis. Radiology. 304:566–79.
- Cheng RK, Kittleson MM, Beavers CJ, Birnie DH, Blankstein R, et al. (2024) Diagnosis and management of cardiac sarcoidosis: a scientific statement from the American Heart Association. Circulation. 149: e1197–216.
- Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, et al. (2014) HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 11:1305–23.
- Ardehali H, Howard DL, Hariri A, Qasim A, Hare JM, et al. (2005) A positive endomyocardial biopsy result for sarcoid is associated with poor prognosis in patients with initially unexplained cardiomyopathy. Am Heart J. 150:459–63.
- Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, et al. (2007) The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 116:2216–33.
- Ohkawa S, Sugiura M, Itoh Y, Kitano K, Hiraoka K, et al. (1981) Electrophysiologic and histologic correlations in chronic complete atrioventricular block. Circulation. 64:215–31.
- Yamashita T, Murakawa Y, Ajiki K, Omata M. (1997) Incidence of induced atrial fibrillation/flutter in complete atrioventricular block: a concept of ‘atrial-malfunctioning’ atrio-Hisian block. Circulation. 95:650–4.
- Zhang D, Huang X. (2020) Treatment of atrial fibrillation with thirddegree atrioventricular block by pacing His bundle and left bundle branch: case report. Medicine (Baltimore). 99: e21097.
- Pastore G, Zanon F, Baracca E, Aggio S, Corbucci G, et al. (2016) The risk of atrial fibrillation during right ventricular pacing. EP Europace. 18:353–8.
- Xie JM, Fang F, Zhang Q, Chan JY, Yip GW, et al. (2012) Left atrial remodeling and reduced atrial pump function after chronic right ventricular apical pacing in patients with preserved ejection fraction. Int J Cardiol. 157:364–9.
- Sweeney MO, Hellkamp AS. (2006) Heart failure during cardiac pacing. Circulation. 113:2082–8.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.