Comparative Outcomes Amniotic Tissue Membrane Versus Standard Care in Diabetic Foot Ulcer Management: A Real-World Retrospective Cohort Study
by Windy Cole1*, Marshall Medley2, Carlos Encinas3
1Research, Kent State University, College of Podiatric Medicine, USA
2Chief Medical Officer, Biolab Holdings, USA
3Chief Science Officer, Biolab Holdings, USA
*Corresponding author: Windy Cole, DPM, CWSP, Director of Wound Care Research, Kent State University, College of Podiatric Medicine, USA
Received Date: 30 October 2025
Accepted Date: 10 November 2025
Published Date: 15 November 2025
Citation: Cole W, Medley M, Encinas C (2025) Comparative Outcomes Amniotic Tissue Membrane Versus Standard Care in Diabetic Foot Ulcer Management: A Real-World Retrospective Cohort Study. J Diabetes Treat 10: 10149. https://doi.org/10.29011/2574-7568.010149
Introduction
Diabetes remains a major public health concern in the United States, affecting approximately 38.4 million people of all ages, or 11.6% of the population, as of the most recent CDC estimates [1]. Among adults aged 18 years and older, the prevalence rises to 14.7%, with nearly 8.7 million adults unaware they have the disease [1]. If current trends continue, 1 in 3 U.S. adults may be diagnosed with diabetes by 2050 [1].
One of the most serious complications of diabetes is the diabetic foot ulcer (DFU), which affects up to 34% of people with diabetes during their lifetime [2]. Approximately 50% of DFUs become infected, and each year, about 100,000 Americans with diabetes undergo lower-limb amputation due to non-healing or infected ulcers [3]. DFUs are the leading cause of non-traumatic lower extremity amputations in this population, accounting for 80% of such procedures [3].
The prognosis following major amputation is poor, with five-year mortality rates approaching 80% in some populations. Survivors often experience significant functional limitations, psychological distress, and a high risk of ulcer recurrence, particularly in the contralateral limb [2].
DFUs significantly impair patient quality of life and place a substantial burden on healthcare systems through prolonged and costly treatment regimens [4,5]. Despite the availability of standard and advanced therapeutic options including offloading, debridement and wound dressings DFUs often remain resistant to healing, underscoring the need for innovative treatment strategies[6]. Moreover, key risk factors such as ulcer duration, ulcer size, number of active ulcers, ambulatory status and number of comorbid conditions are strongly associated with progression to amputation [7].
Despite increasing interest in biologic therapeutics such as Amniotic Tissue Membranes (ATMs) for the treatment of chronic wounds, substantial Real-World Evidence (RWE) to support their use in complex patients with DFUs is lacking. In an effort to demonstrate the value and utility of real-world evidence in the wound care space, the authors analyzed the efficacy of a human ATM product (Membrane Wrap™, BioLab Holdings, Mesa,
Arizona) in the treatment of complex, hard to heal DFUs compared to Standard of Care (SOC) treatments alone.
Methods
This multicenter, retrospective cohort study evaluated the effectiveness of a human amniotic tissue membrane versus standard of care in treating chronic diabetic foot ulcers. This study was conducted under a waiver of informed consent and HIPAA authorization granted by Sterling Institutional Review Board #13631 on April 23, 2025. The study inclusion and exclusion criteria are listed in Table 1.
|
Category |
Criteria |
|
Inclusion |
Adults ≥18 years with Type 1 or Type 2 diabetes |
|
Inclusion |
Treated with Membrane Wrap™ plus SOC or SOC alone |
|
Inclusion |
Treatment between January 1, 2022 and June 30, 2025 |
|
Inclusion |
Diabetic foot ulcers (DFUs) > 2 cm² in area |
|
Exclusion |
No follow-up wound assessment |
|
Exclusion |
Use of other wound care agents |
De-identified Electronic Health Record (EHR) data were extracted from the Intellicure database (Intellicure, LLC, The Woodlands TX), encompassing the period from February 15, 2017, to June 30, 2025. Clinical data were sourced from the U.S. Wound Registry (USWR), a national repository of research-grade wound care data. Patients received either ATM plus standard of care or SOC alone.
Table 1: Study Population.
Data quality was ensured through manual validation procedures. Outliers and missing values were retained to preserve the robustness of the dataset. Patients were excluded if outcome data were unavailable due to loss to follow-up, death, or transfer of care.
Subjects were categorized into two groups: those treated with ATM and those receiving SOC without cellular or tissue-based products. Diabetic patients with foot pressure ulcers were reclassified as having diabetic foot ulcers. Wound location was determined using descriptive clinical entries and ICD-10 codes, primarily L89.6. The most severe tissue exposure per wound was identified across all visits. Small wounds were retained due to their negligible influence on outcome classification.
Wound outcomes were categorized as “Healed,” “Healing,” or “Worsening” based on the standardized wound size change rate (r). Wounds with a final area ≤ 0.5 cm² and r ≤ −0.0001 were classified as “Healed.” Wounds with r ≥ 0.0001 were labeled “Worsening,” while all others were considered “Healing.”
Matching was performed at the wound level using 16 covariates, including demographic characteristics, clinic attributes, comorbidities, and wound-specific features. A 1:1 nearest neighbor matching algorithm based on Mahalanobis distance was implemented using R version 4.5.1 and the MatchIt package (v4.7.2). Propensity score methods were also applied to match SOC patients to ATM-treated counterparts based on wound type, wound size, and comorbidities. The study period for matched comparisons extended from January 2022 through October 2025.
Descriptive statistics were used to summarize matched cohorts. Outcome differences between groups were assessed using signed rank tests. A two-sided Type I error rate of 0.05 was maintained for all statistical analyses.
All data were de-identified and handled in accordance with applicable ethical standards and data protection regulations.
Results
Patient and Wound Demographics
A total of 85 patients with 92 wounds were included in the matched cohort analysis. Patient demographics can be found in (Table 2). The mean age was slightly higher in the ATM group (73.83 ± 9.85 years) compared to the SOC group (71.93 ± 9.52 years), though not statistically significant (p = 0.0622). The majority of patients in both groups were over 65 years of age (84.8% vs. 80.4%, p = 0.7237), and predominantly male (78.3% vs. 80.4%, p = 1.0000). Full baseline patient and wound characteristics can be found in (Image 1).
Race data were incomplete, with substantial missingness (41 in ATM, 12 in SOC). Among available data, most patients identified as White (80.0% in ATM, 76.5% in SOC). BMI was similar between groups (29.07 ± 4.05 ATM vs. 31.19 ± 8.14 SOC, p = 1.0000), though BMI data were missing for nearly all ATM patients.
Arrival methods were comparable, with ambulatory status being the most common (50.0% vs. 47.8%). Wound age at first treatment was significantly longer in the ATM group (181.59 ± 132.99 days) than in the SOC group (130.59 ± 142.30 days, p = 0.0040).
All patients had diabetes. Comorbidities such as peripheral artery disease, chronic kidney disease, congestive heart failure, dementia, autoimmune disease, paralysis, and malnourishment were similarly distributed across groups, with no statistically significant differences.
The number of concomitant wounds varied, with most patients having one or two wounds. A higher proportion of ATM patients had four wounds (13.0% vs. 4.3%), while more SOC patients had three wounds (19.6% vs. 6.5%)
|
Characteristic |
Membrane Wrap (N=39) |
Standard Care (N=46) |
p-value |
|
Age (years) |
73.83 ± 9.85 |
71.93 ± 9.52 |
0.0622 |
|
Age > 65 years |
84.8% |
80.4% |
0.7237 |
|
Sex: Female |
21.7% |
19.6% |
1.0000 |
|
Sex: Male |
78.3% |
80.4% |
|
|
Race: White |
80.0% |
76.5% |
|
|
Race: Black or African American |
0.0% |
17.6% |
|
|
Race: Other / Declined / Missing |
High missingness |
High missingness |
|
|
BMI (kg/m²) |
29.07 ± 4.05 |
31.19 ± 8.14 |
1.0000 |
|
Arrival: Ambulatory |
50.0% |
47.8% |
|
|
Arrival: Walker |
21.7% |
21.7% |
|
|
Arrival: Wheelchair |
19.6% |
21.7% |
|
|
Arrival: Stretcher/Bedridden |
8.7% |
8.7% |
|
|
Wound Age at First Treatment (days) |
181.59 ± 132.99 |
130.59 ± 142.30 |
0.0040 |
|
Diabetes |
100.0% |
100.0% |
|
|
Peripheral Artery Disease |
26.1% |
21.7% |
0.4795 |
|
Chronic Kidney Disease |
30.4% |
30.4% |
|
|
Congestive Heart Failure |
32.6% |
32.6% |
|
|
Dementia |
13.0% |
13.0% |
|
|
Autoimmune Disease |
4.3% |
2.2% |
1.0000 |
|
Paralysis |
2.2% |
2.2% |
|
|
Malnourished |
30.4% |
26.1% |
0.4795 |
|
Concomitant Wounds: 1 |
45.7% |
43.5% |
|
|
Concomitant Wounds: 2 |
32.6% |
30.4% |
|
|
Concomitant Wounds: 3 |
6.5% |
19.6% |
|
|
Concomitant Wounds: 4 |
13.0% |
4.3% |
|
|
Concomitant Wounds: 5 |
2.2% |
2.2% |
Table 2: Patient Demographics.
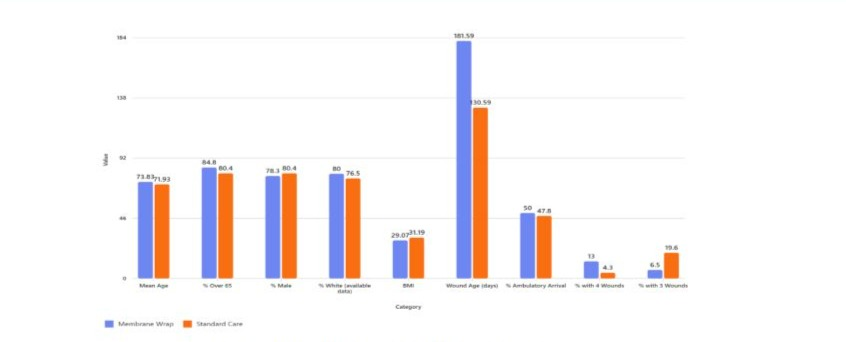
Image 1: Baseline Wound and Patient Characteristics.
Wound Healing
A total of 92 DFUs were analyzed, with 46 wounds in each of the ATM and SOC groups. Wound progress comparisons can be found in (Image 2).Wound location was predominantly heel and midfoot in both groups (78.3% ATM vs. 84.8% SOC), with minor representation from other foot regions. Tissue exposure was similar across groups, with subcutaneous tissue being the most common (73.9%).
Wounds in the ATM group had significantly longer wound duration (181.6 ± 133.0 days ATM vs. 130.6 ± 142.3 days SOC, p = 0.0040) compared to the SOC group. The ATM treated wounds were larger in wound size compared to the SOC group (13.5 ± 20.0 cm² vs. 11.3 ± 20.6 cm², p = 0.0224).
Granulation tissue coverage was more favorable in the ATM group, with 56.5% of wounds showing >50% granulation compared to 30.6% in the SOC group. Wound size reduction was comparable (−0.42 ± 0.53 ATM vs. −0.31 ± 0.89 SOC, p = 0.9952).
Clinical outcomes indicated a higher proportion of healing or healed wounds in the ATM group (84.8% ATM vs. 76.1% SOC), though not statistically significant (p = 0.2497).
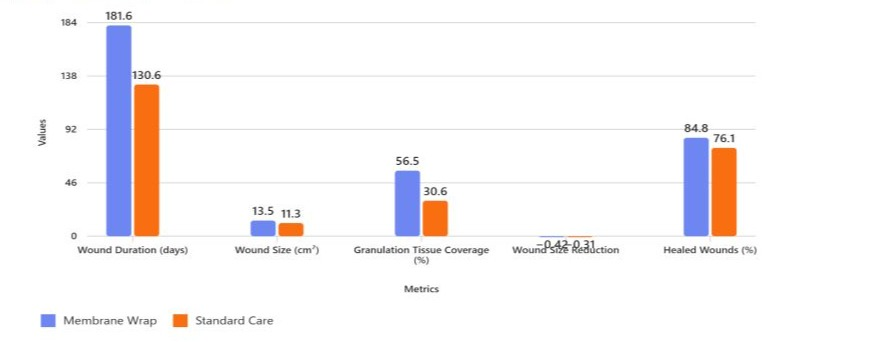
Image 2: Wound Progression Comparison
Discussion
The PICO framework-Patient, Intervention, Comparison, Outcome—is a cornerstone of evidence-based wound care, guiding clinical inquiry and research. Medicare supports its use by aligning coverage decisions with evidence generated through PICOstructured studies, particularly via Local Coverage Determinations (LCDs) and CPT coding for advanced wound therapies to ensure treatments are both medically necessary and cost-effective [8].
This study followed the PICO structured, iterative approach: beginning with an identified patient population (patients with complex, hard to heal DFUs) and a clearly defined clinical question (does the use of the ATM improve outcomes). The two participant cohorts, ATM and SOC, were assessed, and systematic evidence was gathered from electronic health records and USWR. The data was then critically assessed for quality and outcomes were reported.
Although statistical significance was not achieved across woundlevel comparisons, the findings suggest clinically meaningful trends favoring ATM treatment. Healing or healed outcomes were more frequent in the ATM group (84.8%) compared to standard care (76.1%), and a greater proportion of wounds exhibited >50% granulation tissue (56.5% vs. 30.6%), indicating enhanced wound bed preparation.
Importantly, ATM was applied to wounds with longer durations and larger sizes, yet outcomes remained comparable or better, suggesting potential benefit in more complex cases. These consistent trends across multiple wound metrics, despite variability and small sample sizes, reinforce the clinical utility of ATM.
The absence of statistical significance may be attributed to limited sample size, high variability in wound characteristics, balanced outcome distributions, missing granulation data in the control group, and the influence of multiple comparisons. Nonetheless, in wound care, even modest improvements in healing trajectories can substantially impact patient quality of life, reduce infection risk, and lower healthcare costs.
RWE plays a pivotal role in advancing wound care by capturing the complexity and variability inherent in clinical practice-elements often underrepresented in randomized controlled trials. While RCTs remain the gold standard for establishing efficacy, their controlled environments and narrowly defined patient populations can limit generalizability [9,10]. In contrast, RWE reflects diverse treatment settings and patient profiles, including individuals with comorbidities and varied therapeutic histories, thereby enhancing external validity and practical relevance [11]. Within the wound care domain—where personalized treatment plans and multimodal therapies are common—RWE supports evidence-based decisionmaking by grounding clinical choices in data derived from everyday practice.
By bridging the gap between controlled research environments and real-world clinical scenarios, RWE not only validates emerging therapeutic approaches but will also inform the development of guidelines that are both scientifically sound and contextually appropriate, ultimately improving patient-centered wound care.
Limitations
Although this study offers worthwhile RWE into the use of ATM products to treat chronic wounds, there are several limitations that should be noted. As an observational study conducted retrospectively, it is inherently subject to methodological constraints, including potential biases and confounding factors. The relatively small sample size may have limited the statistical power to detect meaningful differences between treatment groups. Additionally, missing data for key demographic and clinical variables restricts the depth and robustness of the analysis. Despite the use of propensity score matching, baseline imbalances persisted between groups; notably, the ATM group exhibited significantly longer wound duration and larger baseline wound size, which may have influenced outcomes. Given these limitations, the findings should be considered hypothesis-generating. Further research is needed to definitively establish clinical efficacy.
Conclusion
The observed trends seen in this retrospective study suggest potential clinical benefits. The Membrane Wrap group demonstrated a higher proportion of healing or healed wounds, greater granulation coverage, and treatment of more chronic and larger wounds. These findings may support the use of Membrane Wrap in complex DFU cases where conventional therapies are less effective. Clinicians should consider these results as hypothesis-generating and potentially indicative of benefit in real-world settings, warranting further investigation through larger, prospective studies.
Acknowledgements
The authors would like to express their sincere gratitude to the US Wound Registry team who contributed to the data collection process, particularly Dr. Caroline Fife, whose diligence and attention to detail were instrumental in ensuring the integrity of the dataset. We also extend our appreciation to Dr. Hongyu Miao, whose expertise in statistical analysis greatly enhanced the rigor and clarity of our findings. Their thoughtful guidance and analytical support were invaluable throughout the research process.
Author Disclosures: Marshal Medley, DO, FACOS, CWSP serves as Chief Medical Officer of Biolab Holdings. Carlos Encinas, PhD serves as Chief Science Officer of Biolab Holdings. Both authors have direct financial interests in the commercial success of Membrane Wrap™. Windy Cole, DPM, CWSP has received consulting fees from Biolab Holdings.
Funding and Sponsorship: This retrospective analysis was funded solely by Biolab Holdings.
References
- National Diabetes Statistics Report.
- McDermott K, Fang M, Boulton AJM, Selvin E, Hicks CW (2026) Etiology, Epidemiology, and Disparities in the Burden of Diabetic Foot Ulcers. Diabetes Care 46: 209-221.
- Reducing Disparities in Diabetic Amputations.
- Nussbaum SR, Carter MJ, Fife CE, DaVanzo J, Haughtet R, al. (2018) An economic evaluation of the impact, cost, and Medicare policy implications of chronic nonhealing wounds. Value Health 21: 27-32.
- Driver VR, Fabbi M, Lavery LA, Gibbons G (2010) The costs of diabetic foot: the economic case for the limb salvage team. J Am Podiatr Med Assoc 100: 335-341.
- Margolis DJ, Kantor J, Berlin JA (1992) Healing of diabetic neuropathic foot ulcers receiving standard treatment. A meta-analysis Diabetes Care 22: 692-654.
- Lu Q, Wang J, Wei X, Wang G, Xu Y (2021) Risk Factors for Major Amputation in Diabetic Foot Ulcer Patients. Diabetes Metab Syndr Obes 14: 2019-2027.
- Wound Care.
- Andrade C (2024) Poorly Recognized and Uncommonly Acknowledged Limitations of Randomized Controlled Trials. Indian Journal of Psychological Medicine 47: 83-85.
- Roger M, Ajeet SB, Hamilton A, Pritha D, Outhred T, et al. (2017) The limitations of using randomised controlled trials as a basis for developing treatment guidelines. Evidence Based Mental Health. 21: 4-6.
- Costa V, Custodio MG, Gefen E, Fregni F (2025) The relevance of the real-world evidence in research, clinical, and regulatory decision making. Front Public Health 13: 1512429.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.