Comparative Analysis of the Influence of Titanium Surface Treatment with Different Acid Treatment Protocols on Bacterial Adhesion: an in Vitro Study
by Flavia Tatiane Barbosa Lima1,Vilton Zimmermann de Souza1*, Rafael Manfro1, Carlos Nelson Elias3, Elizabeth Ferreira Martinez2
1Division of Oral Implantology, Faculdade São Leopoldo Mandic, Campinas, SP, Brazil
2Division of Cell Biology, Faculdade São Leopoldo Mandic, Campinas, SP, Brazil
3Departament of Materials Science, Instituto Militar de Engenharia, Rio de Janeiro, RJ, Brazil
*Corresponding author: Vilton Zimmermann de Souza, Faculdade São Leopoldo Mandic, Campinas, SP, Brazil Rua José Rocha Junqueira, 13, CEP 13045-610, Campinas, SP, Brazil
Received Date: 01 July 2025
Accepted Date: 08 July 2025
Published Date: 10 July 2025
Citation: Lima FTB, Souza VZD , Manfro R, Elias CN, Martinez EF (2025) Comparative Analysis of the Influence of Titanium Surface Treatment with Different Acid Treatment Protocols on Bacterial Adhesion: an in Vitro Study. J Surg 10: 11375 https://doi.org/10.29011/2575-9760.011375
Abstract
Background: Modifications in the surface of dental implants have demonstrated an important role in the optimization of osseointegration.
Objectives: The objective of this research was to evaluate in vitro if different titanium surfaces treatments promoted by acid solution at different times influence on the adhesion and viability of two bacterial species (S. aureus and S. mutans).
Materials and Methods: Commercially pure titanium discs of Grade 4 (6mm X 2mm) were treated with acid solution (hydrochloric, nitric and sulfuric) at 20 (P20) and 60 (P60) minutes, obtaining, respectively, mean roughness of 0.610 (± 0.037) μm and 0.773 (± 0.033) μm. As control, a machined surface not subjected to any treatment was used. In order to determine bacterial adhesion at different surfaces, the bacterial strains were cultivated in each sample at the density of 1X108 CFU/ml and incubated for 4 h at 37 ºC, in microaerophilic conditions. For quantification of live and dead adherent bacteria, the fluorescence technique with Live/
Dead Baclight viability kit was used. The total area, as well as the area of live and dead bacteria were quantified by means of the Image program J. The statistical analysis was performed by means of Analysis of Variance followed by Tukey test, conducted at the significance level of 5%.
Results: For S. aureus, the Tukey test identified that the smallest counts and the smallest percentage of area were observed on the machined surface, while the highest values were found on the P20 surface. When considering the culture of S. mutans, the count and percentage of area were also lower on the machined surface, with no statistically significant difference between the values obtained P20 and P60 surfaces.
Conclusion: According to the results of the research, it was concluded that the microbial characteristics impacted the viability values. Despite the highest surface roughness of P60 surface, colonization of S.aureus was lower when compared to P20.
Keywords: Bacterial Adhesion; Dental Implant; Surface Roughness, Mucointegration; Fibrointegration
Introduction
Surface treatments of dental implants have demonstrated an important role in optimizing osseointegration, significantly changing concepts. Based on technological developments and studies based on immunohistochemistry and electron microscopy, it was established that these characterizations positively influence the behavior of osteoblastos [1-5]. However, the long-term prognosis of dental implants depends not only on osseointegration, but also on the quality of the seal between the mucosa and the implant abutment [6-8]. Studies have shown that final roughness, regardless of surface treatment, between 0.7um and 2.0um allowed direct adhesion of the osteoblast to the surface of the implants, while roughness lower than these values f avored the adhesion of fibroblasts [9-12]. One of the critical points of implant rehabilitations is the gingival sealing around the prosthetic components. Unlike natural teeth, which have perpendicular fibers adhered to the dental tissue, implants only have circumferential fibers, as the vast majority of prosthetic components are machined, smooth, and do not allow fibers to adhere. For this reason, the maintenance of cervical margins is mainly due to the volume of gingival tissue, often requiring grafting procedures with connective tissue to prevent aesthetic or peri-implant health changes. Therefore, after the installation of dental implants, it is possible that pathogenic microorganisms from the oral cavity, in the sealing region between the gingival tissue and the implant platform, initiate gingival inflammation called mucositis, which can progress to cup-shaped bone resorption, characterizing the peri- implantitis, especially in individuals with poor oral hygiene [13-22]. In order to change the direction of the cervical gingival fibers, the treatment of prosthetic components has been considered and much discussed in the literature. With the consolidation of surface treatments for dental implants, literature has turned its attention to the sealing between the prosthetic component and gingival tissue. Published works have demonstrated that small roughness positively alters the behavior of collagen fibers. As most of these studies are still in vitro, bacterial behavior was not observed in relation to these treated prosthetic abutments and whether bacterial adhesion could somehow compromise the behavior of fibroblastos [23-28]. The objective of this work was to evaluate in vitro bacterial adhesion on titanium surface discs with roughness less than 0.7 μm, simulating the surface treatment on prosthetic abutments.
Materials and Methods
This work has received exemption from the Research Ethics Committee for Human Beings of the São Leopoldo Mandic Institute and Research Center, Campinas/SP as it is exclusively laboratory research, without the involvement of human beings or materials (Protocol 2016/06460, annex 1 ).
Samples: For this work, were used commercially pure grade 4 titanium discs (n = 18), 6 mm in diameter and 2 mm thick, supplied by the company Conexão Sistemas de Próteses (Arujá, São Paulo). For the treatment of the titanium discs, it was used a solution of sulfuric, nitric and hydrochloric acids, with times of 20 minutes (n = 6) and 60 minutes (n = 6). Discs without surface treatment called machined were used as a control (n = 6). Acid concentrations are not described as this is confidential company information. Roughness was measured using a contact profilometer (Mitutoyo, model Surftest SJ200, Brazil, Suzano). Four linear measurements were made on each sample in accordance with the DIN ISO 1302 standard, and the arithmetic average of the absolute values of each disc (Ra) was calculated. The average roughness (Ra) of the machined surface was 0.278 (± 0.035) μm, and after acid treatment,
0.610 (± 0.037) μm was obtained for a time of 20 minutes and 0.773 (± 0.033) μm for a time of 60 minutes. Figure 1 illustrates the morphological characteristics of the obtained surfaces. The discs were sterilized in ethylene oxide (Acecil, Campinas, São Paulo) and used in the following experiments. Figure 1 - Scanning Electronic Microscopy and Interferometry. A: machined surface, B: surface with 20 minutes of acid treatment, C: surface with 60 minutes of acid treatment. Original magnification: 15,000X. Detail insertion magnification of 70,000X.
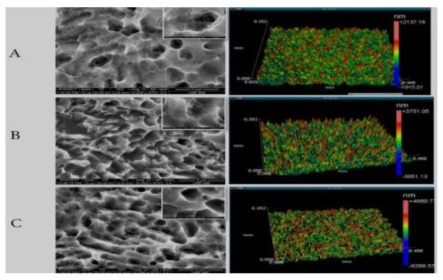
Figure 1: Scanning Electron Microscopy and Interferometry.
A: Machined Surface, B: Surface With 20 Minutes Of Acid Treatment, C: Surface With 60 Minutes of Acid Treatment. Original Magnification: 15000X. Detail Inset Magnification 70000X.
Bacterial Adhesion Test
For the present study, standard ATCC (American Type Culture Collection, USA) strains of Streptococcus mutans (ATCC 25175) and Staphylococcus aureus (ATCC 25923) were used, isolated and stored in the Microbiology Laboratory of the São Leopoldo Mandic Dental Research Center (Campinas) , SP. These strains are kept frozen for subsequent activation in BHI broth culture medium (Brain Heart Infusion, Himedia, India), where they remained for 24 hours in a bacteriological oven at 37 oC, under microaerophilic conditions. After this period, with the aid of a platinum loop, a portion of the culture medium was collected and cultivated in a Petri dish containing BHI agar, so that the strains to be studied could grow, under the conditions described above, in the bacteriological greenhouse. From colonies grown overnight, broths were obtained containing a final density of 15x108 cells/ml, corresponding to factor n. 5 on the McFarland scale (Nefelobac, McFarland Nephelometric Scale, Brazil). To determine bacterial adhesion to different surfaces, bacterial strains were grown on each sample and incubated for 4 hours at 37o C under microaerophilic conditions, as previously described. Samples were gently washed with sterile saline (0.9%) and it was used a BacLight LIVE/DEAD viability kit (Molecular Probe, OR, USA). The kit includes two fluorescent nucleic acids, SYTO9 and propidium iodide. SYTO9 (green fluorescence) identifies viable bacteria, while propidium iodide (Red Fluorescence) identifies non-viable bacteria. To assess viability, 1μL of solution from each area was added to 3mL of sterile saline (0.90%) and then mixed. 70 μL of the solution was dispensed onto each surface and incubated for 15 minutes in the dark at room temperature. Bacterial colonies were examined under a fluorescence microscope (Zeiss, Germany) using a lens (40X). The excitation and emission wavelengths of SYTO9 and propidium iodide were 488 nm and 525 nm, respectively. For each sample, 6 images were taken at standardized positions at each point of the calibration curve. To determine the viability of bacterial species adhered to each type of surface, the area (Arbitrary Units, AU) was measured green zones (viable cells) and red zones (non-viable cells) and the total area of t he image (merged), in each surface analyzed for each calibration point of the fluorescence curve, using the ImageJ program (National Institute Of Health, NIH, 167 USA).
The experiments were carried out in triplicate, for each experiment and bacterial species.
Statistical Analysis
To assess viability, 1μL of solution from each area was added to 3mL of sterile saline (0.90%) and then mixed. 70 μL of the solution was dispensed onto each surface and incubated for 15 minutes in the dark at room temperature. Bacterial colonies were examined under a fluorescence microscope (Zeiss, Germany) using a lens (40X). The excitation and emission wavelength of SYTO9 and propidium iodide was 488 nm and 525 nm, respectively. For each sample, 6 images were taken at standardized positions at each point of the calibration curve. To determine the viability of bacterial species attached to each type of surface, the area (Arbitrary Units, AU) of the green zones (viable cells) and red zones (non-viable cells) and the total image area (merged) were measured on each surface analyzed for each calibration point of the fluorescence curve, using the ImageJ program (National Institute of Health, NIH, USA). The experiments were carried out in triplicate, for each experiment and bacterial species. After the data had been assessed for normality and homoscedasticity, comparisons between the three different titanium surfaces in terms of cell count and percentage of area occupied by viable and non-viable S. aureus and S. mutans were performed by average analysis variance for a criterion, followed by Tukey tests. Statistical calculations were performed using a significance level of 5%, using SPSS 23 software (SPSS Inc., Chicago, IL, USA).
Results
Through one-way analysis of variance, it was found that the count and percentage of area occupied by viable S. aureus (p<0.001) and S. mutans (p=0.042) were significantly affected by the type of surface. When considering the S. mutans culture, the count and percentage of area were also lower on the machined surface, with no statistically significant difference between the values obtained for the P20 and P60 surfaces (Table 1 and graphs 1 and 3). For S. aureus, the Tukey test identified that the lowest counts and the lowest percentages of area were observed on the machined surface, while the highest values were found on the P20 surface (Table 1 and graphs 2 and 4). The count and area percentage values were intermediate for the P60 surface.
Table 1: Mean values and standard deviations of counts (AU) and percentage of area showing viable S. aureus and S. mutans on different surfaces.
Caption: ANOVA: Analysis of Variance. Averages followed by different letters within the same column differ significantly from each other.
|
Surface |
Viable S. aureus |
Viable S. mutans |
||
|
Count (AU) |
Area (%) |
Count (AU) |
Area (%) |
|
|
Machined |
6.148 (1.387) a |
9.58 (2.16) a |
11.291 (2.478) a |
17.60 (3.86) a |
|
P20 |
20.702 (5.078) c |
32.27 (7.91) c |
17.216 (3.554) b |
26.83 (5.54) b |
|
P60 |
13.871 (1.571) b |
21.62 (2.45) b |
16.698 (3.555) b |
26.03 (5.54) b |
|
ANOVA |
p < 0.001 |
p < 0.001 |
p = 0.042 |
p = 0.042 |
Source: Own authorship.
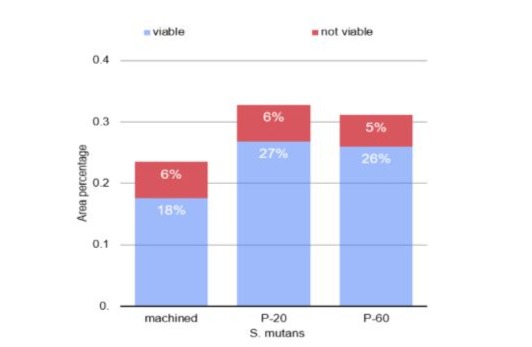
Graph 1: Column diagram of the percentage of area in which viable and non-viable S. mutans were attached to different surfaces.
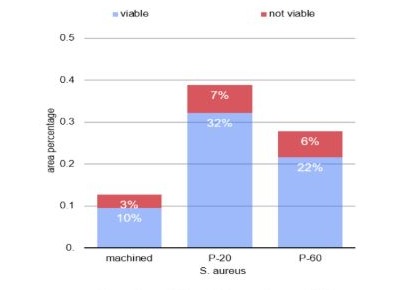
Graph 2: Column diagram of the percentage of area in which viable and non-viable S. aureus were attached to different surfaces.
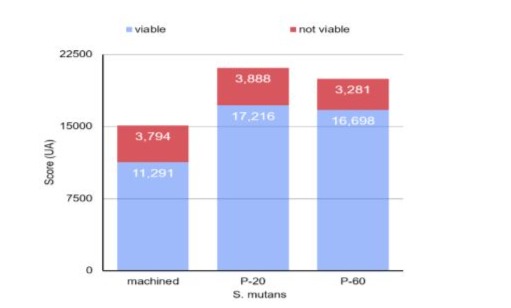
Graph 3: Column diagram of the count of viable and non-viable S. mutans on different surfaces.
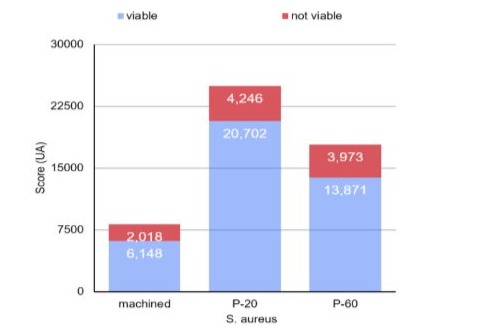
Graph 4: Column diagram of viable and non-viable S. aureus counts on different surfaces.
Regarding the count and percentage of area data presenting S. aureus (p = 0.573) and S. mutans (p = 0.872), the unfeasible analysis of variance in one criterion demonstrated that there was no statistically significant difference between the surfaces (table 2 and graphs 2 and 4).
|
Superfície |
Non-viable S. aureus |
Non-viable S. mutans |
||
|
Count (AU) |
Area (%) |
Count (AU) |
Area (%) |
|
|
Machined |
2.018 (998) a |
3.15 (1.56) a |
3.794 (1.568) a |
5.91 (2.45) a |
|
P20 |
4.246 (3.996) a |
6.62 (6.23) a |
3.888 (1.916) a |
6.06 (2.99) a |
|
P60 |
3.973 (2.657) a |
6.19 (4.14) a |
3.281 (2.202) a |
5.11 (3.43) a |
|
ANOVA |
p = 0.573 |
p = 0.573 |
p = 0,872 |
p = 0.872 |
Table 2: Mean values and standard deviations of counts (AU) and percentage of area showing non-viable S. aureus and S. mutans on different surfaces.
Surface
Caption: ANOVA: Analysis of Variance. Averages followed by the same letters within the same column do not differ significantly from each other.
Discussion
Changes in the relationships between the diameters of the implant platforms and the prosthetic components known as the swift platform allowed the maintenance of peri-implant hard tissues at the level of the implant platforms and even above them. This effect caused the industry to change the structure of the cervical region of implants, both in macrogeometric with specific thread designs and in microgeometric with treatment up to the implant platform. The presence of roughness in this region, contrary to what was expected, did not demonstrate a greater presence of bacteria and an increase in peri-implant diseases. The literature shows a lack of conclusive studies on this subject, and more work is needed to achieve an ideal surface for prosthetic components on implants, promoting the health of the peri-implant connective tissue based on tissue adhesion and, consequently, a better seal between the tissue soft and the prosthetic component [6]. The influence of microroughness on cellular behavior around implants is well defined in the literature. Some of these studies have demonstrated that lower roughnesses of less than 0.7um allow fibroblasts to adhere, suggesting that they would alter the behavior in the region of the prosthetic components, and could improve sealing in the peri-implant groove through the formation of collagen fibers perpendicular to the components. Controlled changes in the roughness of the components allow a change in the orientation of the gingival fibers and adhesion to the component. Although these changes will influence the healing of the periimplant gum, many doubts are still present, whether regarding the type of treatment, the ideal roughness and the bacterial behavior and induction of peri-implant disease [29,30]. In vitro studies have demonstrated that this fact may be possible and that roughnesses close to 0.2 um would have a satisfactory performance [6,30,28]. On the other hand, moderate surface roughness, while favoring the osseointegration of implants, can also provide greater bacterial colonization and, consequently, peri-implantitis [31]. In fact, studies demonstrate that machined surfaces promote less bacterial colonization [32,24,33]. In this study, for S. aureus there was a difference between the groups, while for S. mutans there was no difference between the surfaces when analyzed as viable cells. However, in the evaluation of non-viable cells, there was no difference between the surfaces, including in relation to the machined surface, for both bacteria. When comparing the P-20 and P-60 surfaces, the surface with the lowest roughness showed greater bacterial colonization in the analysis of viable cells for both S. mutans and S. aureus, similar to the results obtained [19]. This result also follows the behavior of fibroblast cells that demonstrate greater adhesion in this type of roughness. In a human study, it was concluded that the laser-treated surface is promising in positively influencing peri-implant connective tissue wound healing. The results demonstrated that the topographic nature of healing pillars can positively influence mucosal healing and molecular expression [25,27,34,22,44]. Rough surfaces show greater adhesion of certain bacterial species only in the initial moments. At more advanced stages, smooth and rough surfaces behave similarly in terms of the number of colonies present in the biofilm [35-38,17]. Individual and systemic characteristics inherent to each individual also seem to have a decisive effect on bacterial colonization, even having a greater impact than the material of the prosthetic abutments itself [39,29]. A study in dogs demonstrated that implants maintained suprabony with rough cervical treatment and without surface treatment demonstrated better quality of peri-implant tissues, including vertical bone increase, without showing the presence of disease in any of the cases [40,41]. In another study in dogs, the results suggest that the healing of hard and soft tissues around implants and abutments is similar when comparing sandblasted surfaces with surfaces machined and turned with nanotubes. Both resulted in similar soft tissue contact values as well as connective tissue fiber orientation [26]. As there is no direct competition between bacteria and fibroblast cells, despite the study showing greater adhesion of bacteria in the samples, it cannot be concluded that the roughness of the prosthetic components would actually increase the risk of infectious inflammatory peri-implant disease [42-44].
Conclusion
Despite the limitations of the study, it is possible to conclude that increasing the roughness of titanium discs treated with acid etching at different times increases bacterial adhesion of S aureus and S mutans in vitro.
Acknowledgements
The authors wish to thank Mrs. Pollyanna Tombini Montaldi for her excellent technical expertise and assistance.
Authors’ contributions
FTB and EFM interpreted and analyzed the data collected, contributed to the drafting of the paper and revised it critically, and were major contributors in writing the manuscript. RM, VZS, and CNE contributed to the concept/design of the study and the final manuscript. VZS and RM critically revised and contributed to the final manuscript. All authors read and approved the final version to be published.
Funding
The funding received for this study was from the researcher himself.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
This work has received exemption from the Research Ethics Committee for Human Beings of the São Leopoldo Mandic Institute and Research Center, Campinas/SP as it is exclusively laboratory research, without the involvement of human beings or materials (Protocol 2016/06460, annex 1 ).
Consent for publication Not applicable.
Competing interests
Flavia Tatiane Barbosa Limaa;Vilton Zimmermann de Souzaa, Rafael Manfroa, Carlos Nelson Eliasc, Elizabeth Ferreira Martinezb* state that they have no conflicts of interest.
References
- Al-Radha AS, Dymock D, Younes C, O’Sullivan D (2012) Surface properties of titanium and zirconia dental implant materials and their effect on bacterial adhesion. J Dent 40: 146-153.
- Albrektsson T, Chrcanovic B, Jacobsson M, Wennerberg A (2017) Osseointegration of implants a biological and clinical overview. JSM Dent Surg 2: 1-6.
- Albrektsson T, Wennerberg A (2019) On osseointegration in relation to implant surfaces. Clin Implant Dent Relat Res 1: 4-7.
- Alfarsi MA, Hamlet SM, Ivanovski S (2014) Titanium surface hydrophilicity enhances platelet activation. Dent Mater J 33: 749-756.
- Quirynen M, Bollen CM, Papaioannou W, Van Eldere J, van Steenberghe D (1996) The influence of titanium abutment surface roughness on plaque accumulation and gingivitis: short-term observations. Int J Oral Maxillofac Implants 11: 169-178.
- de Avila ED, Avila-Campos MJ, Vergani CE, Spolidório DM, Mollo Fde A Jr (2016) Structural and quantitative analysis of a mature anaerobic biofilm on different implant abutment surfaces. J Prosthet Dent 428436.
- Valderrama P, Jones AA, Wilson TG Jr, Higginbottom F, Schoolfield JD, et al. (2010) Bone changes around early loaded chemically modified sandblasted and acid-etched surfaced implants with and without a machined collar: a radiographic and resonance frequency analysis in the canine mandible. Int J Oral Maxillofac Implants 25: 548-557.
- Valderrama P, Bornstein MM, Jones AA, Wilson TG, Higginbottom FL, et al. (2010) Effects of implant design on marginal bone changes around early loaded, chemically modified, sandblasted Acid-etchedsurfaced implants: a histologic analysis in dogs. J Periodontol 82: 1025-1034.
- Tomas A, Ann W ( 2004) Oral implant surfaces: Part 1 - review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont 17: 536-543.
- Tomas A, Ann W (2004) Oral implant surfaces: Part 2 - review focusing on clinical knowledge of different surfaces. Int J Prothodont 17: 544564.
- Ferreira Ribeiro C, Cogo-Müller K, Franco GC, Silva-Concílio LR, Sampaio Campos M, et al. ( 2016) Initial oral biofilm formation on titanium implants with different surface treatments: An in vivo study. Arch Oral Biol 69: 33-39.
- Velasco-Ortega E, Ortiz-García I, Jiménez-Guerra A, Monsalve-Guil L, Muñoz-Guzón F, et al. (2019) Comparison between Sandblasted Acid-Etched and Oxidized Titanium Dental Implants: In Vivo Study. Int J Mol Sci 3; 20.
- Alves DC, Carvalho PS, Martinez EF (2014) In vitro microbiological analysis of bacterial seal at the implant-abutment interface using two morse taper implant models. Braz Dent J 25: 48-53.
- Dorkhan M, Yücel-Lindberg T, Hall J (2014) Adherence of human oral keratinocytes and gingival fibroblasts to nano-structured titanium surfaces. BMC Oral Health 14: 75.
- Faveri M, Mayer MP, Feres M, de Figueiredo LC, Dewhirst FE, et al. (2008) Microbiological diversity of generalized aggressive periodontitis by 16S rRNA clonal analysis. Oral Microbiol Immunol 23: 112-118.
- Ghinassi B, D’Addazio G, Di Baldassarre A, Femminella B, Di Vincenzo G, et al. (2020) Immunohistochemical Results of Soft tissues Around a New Implant Healing-Abutment Surface: A Human Study. J Clin Med 9: 1009.
- Han A, Tsoi KH, Rodrigues FP, Leprince JG, Palin WM (2016) Bacterial adhesion mechanisms on dental implant surfaces and the influencing factors. Int J Adhes Adhes 69: 58-71.
- Marsich E, Travan A, Donati I, Turco G, Kulkova J, et al. (2013) Biological responses of silver-coated thermosets: an in vitro and in vivo study. Acta Biomater 9: 5088-5099.
- Ramaglia L, Di Spigna G, Capece G, Sbordone C, Salzano S, et al. (2015) Differentiation, apoptosis, and GM-CSF receptor expression of human gingival fibroblasts on a titanium surface treated by a dual acidetched procedure. Clin Oral Investig 19: 2245-2253.
- Teughels W, Van Assche N, Sliepen I, Quirynen M (2006) Effect of material characteristics and/or surface topography on biofilm development. Clin Oral Implants Res 17: 68-81.
- Wang Y, Zhang Y, Jing D, Shuang Y, Miron RJ (2016) Enamel matrix derivative improves gingival fibroblast cell behavior cultured on titanium surfaces. Clin Oral Investig 20: 685-695.
- Wassmann T, Kreis S, Behr M, Buergers R (2017) The influence of surface texture and wettability on initial bacterial adhesion on titanium and zirconium oxide dental implants. Int J Implant Dent 3: 32.
- Baltriukienė D, Sabaliauskas V, Balčiūnas E, Melninkaitis A, Liutkevičius E, et al. (2014) The effect of laser-treated titanium surface on human gingival fibroblast behavior. J Biomed Mater Res A 102: 713-720.
- Chrcanovic BR, Albrektsson T, Wennerberg A (2016) Turned versus anodised dental implants: a meta-analysis. J Oral Rehabil 43: 716728.
- de Souza VZ, Manfro R, Joly JC, Elias CN, Peruzzo DC, et al. (2019) Viability and collagen secretion by fibroblasts on titanium surfaces with different acid-etching protocols. Int J Implant Dent 5: 41.
- Nicu EA, Van Assche N, Coucke W, Teughels W, Quirynen M (2012) RCT comparing implants with turned and anodically oxidized surfaces: a pilot study, a 3-year follow-up. J Clin Periodontol 39: 1183-1190.
- Blázquez-Hinarejos M, Ayuso-Montero R, Jané-Salas E, López-López J (2017) Influence of surface modified dental implant abutments on connective tissue attachment: a systematic review. Arch Oral Biol 80: 185-192.
- Velasco-Ortega E, Alfonso-Rodríguez CA, Monsalve-Guil L, EspañaLópez A, Jiménez-Guerra A, et al. (2016) Relevant aspects in the surface properties in titanium dental implants for the cellular viability. Mater Sci Eng C Mater Biol Appl 64: 1-10.
- Alam F, Balani K (2017) Adhesion force of staphylococcus aureus on various biomaterial surfaces. J Mech Behav Biomed Mater 65: 872880.
- Rodriguez y Baena R, Arciola CR, Selan L, Battaglia R, Imbriani M, et al. (2012) Evaluation of bacterial adhesion on machined titanium, Osseotite® and Nanotite® discs. Int J Artif Organs 35: 754-761.
- Mombelli A, Müller N, Cionca N (2012) The epidemiology of periimplantitis. Clin Oral Implants Res 6: 67-76.
- Badihi Hauslich L, Sela MN, Steinberg D, Rosen G, Kohavi D (2013) The adhesion of oral bacteria to modified titanium surfaces: role of plasma proteins and electrostatic forces. Clin Oral Implants Res 24: 49-56.
- Renvert S, Lindahl C, Roos Jansåker AM, Persson GR (2011) Treatment of peri-implantitis using an Er:YAG laser or an air-abrasive device: a randomized clinical trial. J Clin Periodontol 38: 65-73.
- Garrett PW, Johnston GW, Bosshardt DD, Jones AA, Sasada Y, et al. (2020) Hard and soft tissue evaluation of titanium dental implants and abutments with nanotubes in canines. J Periodontol 91: 516-523.
- Bürgers R, Gerlach T, Hahnel S, Schwarz F, Handel G, et al. (2010) In vivo and in vitro biofilm formation on two different titanium implant surfaces. Clin Oral Implants Res 21: 156-164.
- Busscher HJ, Weerkamp AH, van der Mei HC, van Pelt AW, de Jong HP, et al. (1984) Measurement of the surface free energy of bacterial cell surfaces and its relevance for adhesion. Appl Environ Microbiol 48: 980-983.
- Caous JS, Lövenklev M, Fäldt J, Langton M (2013) Adhesion of Streptococcus mitis and Actinomyces oris in co-culture to machined and anodized titanium surfaces as affected by atmosphere and pH. BMC Oral Health 8; 13-14.
- Chen CJ, Ding SJ, Chen CC (2016) Effects of Surface Conditions of Titanium Dental Implants on Bacterial Adhesion. Photomed Laser Surg 34: 379-388.
- Absolom DR, Lamberti FV, Policova Z, Zingg W, van Oss CJ, et al. (1983) Surface thermodynamics of bacterial adhesion. Appl Environ Microbiol 46: 90-97.
- Roffel S, Wu G, Nedeljkovic I, Meyer M, Razafiarison T,et al, (2019) Evaluation of a novel oral mucosa in vitro implantation model for analysis of molecular interactions with dental abutment surfaces. Clin Implant Dent Relat Res 1: 25-33.
- Teughels, Wim (2006) “Effect of material characteristics and/or surface topography on biofilm development.” Clinical oral implants research 17: 68-81.
- Wennerberg A, Albrektsson T (2009) Effects of titanium surface topography on bone integration: a systematic review. Clin Oral Implants Res 4:172-84.
- Yamane K, Ayukawa Y, Takeshita T, Furuhashi A, Yamashita Y, et al. (2013) Bacterial adhesion affinities of various implant abutment materials. Clin Oral Implants Res 24: 1310-1315.
- de Souza VZ, Manfro R, Teixeira LN, Elias CN, Joly JC, et al. (2025) Histological and Immunohistochemical Analysis of Peri-implant Tissue Regeneration at the Interface of Prosthetic Abutments Treated with Anodizing: A Prospective, Randomized Controlled Clinical Trial on the Early Postoperative Period. The International journal of oral & maxillofacial implants 2025: 1-27
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.