Clonapure® vs Creatine Monohydrate on ATP Synthesis in a Human Neuron Cell line
by Alejandra Hernández-Bueno1, Tamara Lapeña-Luzón2, Paula MoraMorón1, Lidia Tomás-Cobos2, Patricia Moreno-Puente1, Sandra García- Benlloch2, Laura Soriano-Romaní2, José Angel Marañón1*
1Tradichem Group, Spain
2AINIA Technological Centre, Spain
*Corresponding author: José Angel Marañón, Tradichem Group, Conde de Lemos 8, 28013, Madrid, Spain
Received Date: 22 July 2025
Accepted Date: 06 August 2025
Published Date: 08 August, 2025
Citation: Hernández-Bueno A, Lapeña-Luzón T, Mora-Morón P, Tomás-Cobos L, Moreno-Puente P, et al. (2025) Clonapure® vs Creatine Monohydrate on ATP Synthesis in a Human Neuron Cell line. J Neurol Exp Neural Sci 7: 158. https://doi.org/10.29011/2577-1442.100058
Abstract
The brain is a highly energetic complex organ, consuming 20% of total resting energy despite representing only about 2% of total body mass. Beyond sports endurance and muscle, existing evidence suggests that creatine supplementation may also improve brain function, particularly under conditions where cellular bioenergetics are compromised, such as metabolic stress, sleep deprivation, or certain diseases. We conducted a study using human dopaminergic neuronal cells to evaluate the effect of Clonapure®, versus standard creatine monohydrate supplementation. Cells were cultured in a medium containing D-galactose instead of glucose to force neurons to rely on mitochondrial ATP synthesis via oxidative phosphorylation, thereby inducing metabolic stress and decreasing energy production.
Clonapure®, even at its lowest doses, significantly enhanced neuronal ATP levels, with results notably higher than creatine monohydrate. Creatine monohydrate was not able to stimulate ATP synthesis at the same levels, even at higher concentrations. We hypothesize that Clonapure® increases energy production more efficiently due to its phosphocreatine content, which donates phosphagen immediately to support ATP synthesis, and to a possible synergistic effect between creatine monohydrate, phosphate salts and phosphocreatine. These results in compromised cells may be extrapolated to conditions demanding higher ATP levels, including altered brain energetics and mitochondrial dysfunction.
Keywords: Creatine, Clonapure®, Phosphocreatine, ATP, brain bioenergetics, SLC6A8, central nervous system, neurons, cognition.
Introduction
Creatine supplementation has been widely used to improve sports endurance and performance for many years with robust evidence behind. Its main mechanism of action is to immediately provide adenosine triphosphate (ATP) in muscle cells through phosphocreatine (PCr) in energy demands. Muscle consumes a big amount of energy via ATP.
The brain is a highly energetic complex organ, consuming 20% of total resting energy despite accounting for only about 2% of total body mass [1]. Neurons demand a constant supply of ATP for several cellular processes related to normal brain function and cognition, such as synaptic functioning, maintenance of ion gradients and neurotransmitter exocytosis [2].
The creatine transporter CrT1 (creatine transporter type 1 or SLC6A8) is a specific, sodium-dependent and active transporter [3] expressed in diverse cells that facilitates the uptake of creatine from the circulation into tissues [4,5]. Studies in rats have shown that SLC6A8 is expressed, among others, in neurons, oligodendrocytes and microcapillary endothelial cells of the blood-brain barrier (BBB), enabling creatine entry into the central nervous system (CNS) [6]. Apart from acquiring peripheral creatine from transporters, the brain can endogenously synthesize creatine in a small percentage, such as in the liver, pancreas and kidney [7,8]. The mechanism of synthesis includes the enzyme L-arginine: glycine amidino transferase (AGAT), which uses arginine and glycine as substrates to produce guanidinoacetate. Subsequently, guanidinoacetate methyltransferase (GAMT) converts guanidinoacetate into creatine [3,6].
Because of the partial ability to cross the BBB via microcapillary endothelial cells expressing the creatine transporter SLC6A8, creatine supplementation appears to have limited effects on brain creatine levels in healthy young subjects. Some interventions in human healthy volunteers reported minimal improvements on brain creatine (5-10%) from supplementation with different dosages, such as 20 g/day for 1-4 weeks or 2-5 g/day for 8 weeks [9-11]. A review by Dolan et al. [12] analyzing the effects of supplementation with various dosages reported an increase of 3-10% in brain stores. These findings suggest that under physiological conditions, creatine uptake into the brain may be limited. However, local cellular mechanisms and differential transporter expression, such as the presence of SLC6A8 in neurons but not in astrocytes, may influence distribution and responsiveness to supplementation at the cellular level.
Nevertheless, increasing evidence suggests that creatine intake can improve brain function, primarily under certain conditions or challenges -such as sleep deprivation, stress, depression, aging, or brain injuries-, which could lead to a reduction in brain creatine stores [13-16]. Long-term and high-dose creatine supplementation has been shown to augment brain creatine levels [8] higher than threshold [17].
Moreover, creatine supplementation has demonstrated to enhance cognitive and memory performance, especially in older adults and during periods of metabolic stress, and to decrease symptoms of poor sleep in both human and animal studies [18] Increasing brain creatine content has been associated with improvements in the recovery from traumatic brain injury in both children and adults [8,18]. Additionally, creatine supplementation increased neuronal energy supply in healthy subjects [19,20].
A systematic review by Avgerinos et al. [5] provides full evidence that creatine supplementation improves reasoning, intelligence and short-term memory in older individuals. Recent studies suggest that creatine may act as a neurotransmitter (NT) in the CNS [7,21,22] as it has been detected in synaptic vesicles from the mouse brain, along with classical NT. Creatine concentrations were found to be lower than NT such as GABA and glutamate but higher than acetylcholine and serotonin (5-hydroxytryptamine) [23].
Additionally, creatine may function as a central neuromodulator, due to its interaction with various molecules, including N-methyl-D-aspartate receptor (NMDAr), Na+/K+ ATPase, serotonin receptors and postsynaptic GABA receptors, and to its participation in critical roles of central neurotransmission [24,25]. Creatine acts as an NMDAr antagonist, attenuating glutamate excitotoxicity without side effects [26,27]. Creatine as well appears to reduce the loss of GABAergic interneurons, which may contribute to neuroprotection [28]. Moreover, creatine decreases extracellular glutamate accumulation and excitotoxicity mediated by glutamate through the stimulation of synaptic uptake [29,30].
Creatine has been identified as a neuroprotective factor for dopaminergic neurons against neurotoxicity [31]. Creatine supplementation prevents defects of hippocampal neurogenesis induced by chronic stress, through modulation of the Wnt/ GSK3β/β-catenin pathway [32]. Due to the requirements of ATP in cognition, we conducted a comparative study to evaluate the effect of Clonapure® (CLP) and creatine monohydrate (CRM) alone on ATP synthesis in a human neuronal cell model.
CLP is an ingredient composed of a combination of CRM, PCr (creatine-phosphate) and phosphate salt. PCr is the active form of creatine that provides ATP. CLP is believed to efficiently enhance ATP synthesis [33].
Materials and Methods
Samples
- Clonapure® (CLP) Micronized, kindly provided by Florida Human Nutrition, a Tradichem group Company
- Creatine Monohydrate (CRM) is synthetically derived, with 99.8% purity.
- Prayphos™ DCPD 308 SP FG; Dicalcium Phosphate Dihydrate (Phosphate)
Stock solutions were prepared by dissolving the samples in culture medium and filtering them through a 0,2 µm filter prior to the use. The pH was adjusted to 7.0. The assay dilutions (5 mM, 1 mM, 500 µM and 100 µM) were prepared from stock solutions.
Cell line
SH-SY5Y (ECACC 94030304), a human neuroblastoma cell line, was cultured in a 1:1 mixture of Ham’s F12 (Gibco™ 11765054) and EMEM (ATCC 30-2003), supplemented with 2 mM L-glutamine, 1% non-essential amino acids (NEAA), 15% fetal bovine serum (FBS) (Biowest), and 1% penicillin/streptomycin. Cultures were maintained at 37ºC in a humidified atmosphere containing 5% CO2 under standard conditions.
For neuronal differentiation toward a cholinergic phenotype, SHSY5Y cells were treated with 10 µM retinoic acid (RA) in medium containing 2% FBS for 6 days, with medium renewed every 2-3 days. To further induce dopaminergic-like differentiation, RA was withdrawn, and cells were subsequently exposed to 100 nM phorbol 12 myristate 13-acetate (PMA) in medium containing 0,5% FBS for an additional 2 days.
Biocompatibility studies
Viability of samples was evaluated in SY-SY5Y cell line. Cells were seeded in 96-well plates and incubated with 6 different concentrations of the samples at 37ºC and 5% CO2. DMSO was included as a positive control of death. Cell viability was evaluated after 24 hours of incubation using a fluorometric assay with alamarBlue™ Cell Viability Reagent (Life Technologies). Fluorescence was measured at λ excitation = 540 nm and λ emission = 590 nm using a Fluoroskan FL plate spectrofluorometer. Viability was calculated with the following formula:
% Viability = (Fluorescence units’ sample / Fluorescence units’ control) x 100
Intracellular ATP levels
Intracellular ATP levels were measured using the CellTiter-Glo Luminescent Cell Viability Assay (Promega), according to the manufacturer’s instructions. SH-SY5Y cells were seeded in 96well plates and subjected to neuronal differentiation. Cells were first cultivated in a glucose medium; subsequently, cells were incubated for 24 hours in a glucose-deprived medium (DMEM without glucose, supplemented with 10 mM D-galactose, 0,5% FBS, and 1% penicillin/streptomycin) to force cells to rely on mitochondrial oxidative phosphorylation for ATP production [35– 38].
Following the deprivation period, cells were exposed to different concentrations of the test compounds (5 mM, 1 mM, 500 µM and 100 µM) for 2 hours. After treatment, the plate was equilibrated to room temperature for 30 minutes. An equal volume of CellTiterGlo Reagent was then added to each well, and the content was mixed for 2 minutes on an orbital shaker to ensure cell lysis. Luminescence was subsequently measured using a luminometer, and the values were normalized to the untreated control, which was set as 1.
Statistical Analysis
Results were expressed as the mean and standard error of the mean (SEM) from two independent experiments; each performed in at least duplicate. Statistical analysis was carried out using Student’s t-test to compare each treatment condition against the untreated control. A p-value < 0.05 was considered statistically significant.
Results
Biocompatibility studies on SH-SY5Y cells
In order to evaluate the toxicity of samples in SH-SY5Y cells for the next ATP studies, a biocompatibility assay was carried out. 80% of viability was established as the minimum necessary for considering the sample non-toxic. Figure 1 shows the results of the biocompatibility assay at 24h of incubation.
The results indicate that all three APIs (CLP, CRM and Phosphate) maintained cell viability above the 80% threshold across all tested concentrations, confirming biocompatibility under the conditions used.
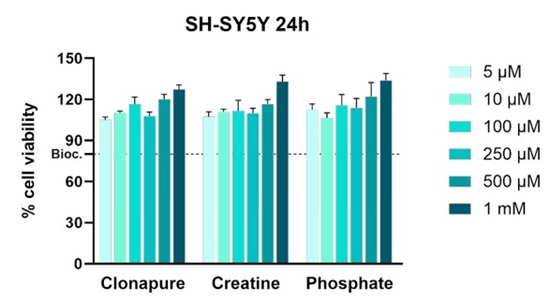
Figure 1: Biocompatibility assay results at 24h. Untreated cells were set as 100% viability. The dashed line indicates the 80% viability threshold, commonly used to define acceptable biocompatibility.
Intracellular ATP levels
Figure 2 shows the relative ATP levels in dopaminergic-like SHSY5Y cells treated with the APIs at three different concentrations, compared to untreated cells (vehicle).
In the glucose medium, there were no changes in ATP levels. A glucose-free medium was evaluated to observe possible improvements in cell response. Gohil et al. have shown that cells grown in a galactose-containing medium maximize mitochondrial ATP production via mitochondrial respiration in a larger fraction for ATP synthesis [34]. Slow oxidation of galactose to pyruvate does not produce sufficient ATP, thereby promoting cells to rely on oxidative phosphorylation (OXPHOS) to generate ATP to maintain cellular homeostasis.
In this model, CLP significantly increased intracellular ATP synthesis at 100 µM and 500 µM. At the highest tested concentration, slight ATP synthesis was reported, possibly due to a saturation phenomenon or feedback regulatory mechanisms. Notably, CLP elicited a more consistent ATP-boosting effect than CRM alone, which only enhanced ATP synthesis levels significantly at 500 µM.
The ATP synthesis induced by CLP at 100 µM was 11% (p < 0.05) higher than basal levels, significantly greater compared to CRM, which increased ATP synthesis by 2.7% relative to basal (Figure 3). In contrast, phosphate did not produce any measurable increase in ATP levels at any of the concentrations tested, confirming that phosphate salts alone do not enhance ATP synthesis.
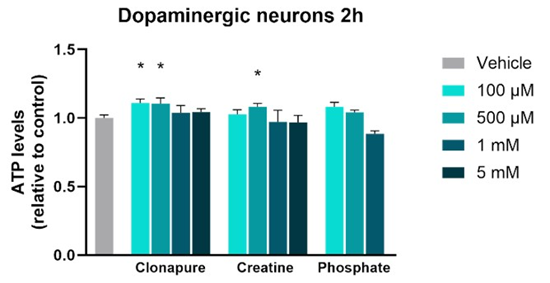
Figure 2: Relative ATP levels in dopaminergic-like SH-SY5Y cells after treatment with Clonapure®, creatine and phosphate at four concentrations (100 μM, 500 μM, 1 mM and 5 mM). Data are expressed as mean ± SEM. *p < 0.05.
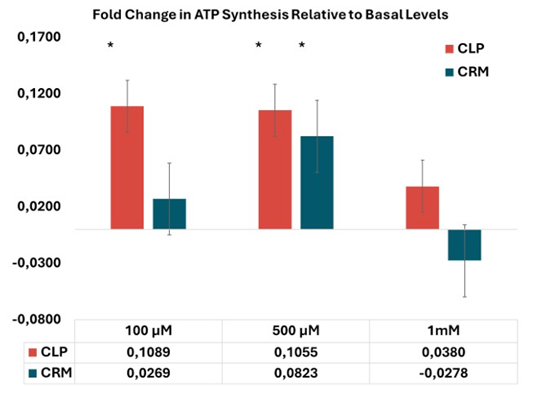
Figure 3: Percentage of ATP synthesis in dopaminergic-like SHSY5Y cells treated with the APIs at three different concentrations, relative to untreated cells (considered as basal levels).
Discussion
There is enough evidence that creatine supplementation has promising effects on muscle cells. Beyond muscle, creatine has also been shown to support brain processes, with proven neuroprotective effects both In Vitro and In Vivo [18].
Dietary PCr supplementation may influence brain health by supporting energy homeostasis; however, its direct effects on the BBB remain complex and not yet fully understood. While creatine, a component of PCr, is known to cross the BBB with difficulty, its accumulation in the brain after systemic administration is still being researched. Although creatine can be synthesized within the brain, supplementation may be particularly beneficial in conditions involving impaired synthesis or transport function.
Moreover, creatine supplementation is believed to be potentially beneficial for diverse clinical conditions, including neurodegenerative diseases. This is due to the effect that creatine may have in oxidative stress and mitochondrial damage [8] Existing and increasing evidence shows that creatine ameliorates the mitochondrial dysfunction commonly observed in neurodegenerative diseases [5].
Mitochondrial dysfunction can refer to any alteration of normal mitochondrial activity, particularly in ATP production, which is reduced [39]. Among the causes of mitochondrial dysfunction are overexpression of reactive oxygen species (ROS), hypoxia, or alteration of calcium homeostasis. As creatine supplementation increases PCr and subsequently provides ATP quickly, it might mitigate energy deficits associated with mitochondrial dysfunction.
Alterations in mitochondrial dynamics, including defects in mitochondrial trafficking and fusion/fission [40] are implicated in the pathogenesis of neurodegenerative diseases, such as Alzheimer’s disease (AD), Huntington’s disease (HD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS) [41]. Additionally, mitochondrial DNA mutations, respiratory chaindeficient cells and other mitochondrial abnormalities also exist in age-related neurodegenerative diseases, such as AD [15,42,43].
Creatine participates in the stabilization of energy metabolism and has demonstrated neuroprotective effects in diseases such as PD, HD and ALS, due to its ability to improve mitochondrial deficits associated with each condition [44]. Furthermore, a study showed that creatine is neuroprotective against toxin-induced lesions, that it might stabilize mitochondrial creatine kinase, and that creatine supplementation may reduce susceptibility to mitochondrialmediated apoptosis [44]. Creatine kinase activity participates in regulating mitochondrial respiration [45]. It is well known that factors such as stress, hypoxia, mental fatigue or sleep deprivation alter brain energetics, leading to a decrease in ATP. These conditions can influence the efficacy of creatine supplementation in increasing brain creatine content [18].
Additionally, several mental health disorders have been characterized to have abnormalities in brain bioenergetics, associated with low creatine levels in specific regions of the brain [46]. There is existing evidence supporting the use of creatine in the treatment of depression, as research have demonstrated improvements in related symptoms [47-49]. Limited research also suggests that creatine levels may be decreased in various regions of the brain in individuals with generalized anxiety disorder and post-traumatic stress disorder. Nevertheless, further investigation is needed to evaluate the use of creatine supplementation in these patients [50,51]. Creatine supplementation has also been shown to play a protective role and ameliorate symptoms of concussion and traumatic brain injury [52,53], evidence indicating that these benefits may be mediated via mitochondrial processes, including the sustainment of membrane potential, reduction of ROS and calcium and maintenance of ATP concentrations [8].
As mentioned before, sleep deprivation is well established to alter brain bioenergetics and affect cognitive function. In this context, creatine supplementation appears to enhance cognitive function compared to placebo, although efficacy is still limited [8]. Turner et al. examined the effects of oral CRM supplementation on neurophysiological function during acute oxygen deprivation in healthy young adults, demonstrating its effectiveness in the restoration of cognitive decline associated with hypoxia. In this study, CRM is believed to provide an abundant pool of PCr as a source of energy, balancing the ATP-generation/ATP-consumption ratio within the cell. Creatine may enhance anaerobic energy processes by prolonging the transfer of high-energy phosphates to neurons when glycolysis is compromised by hypoxia. This mechanism may help maintain ATP levels, preventing the typical decline observed during oxygen deprivation [16].
Overall, growing evidence shows that creatine supplementation has a positive effect on cognition, particularly when brain bioenergetics are compromised, such as in situations of stress or sleep deprivation. In the presence of glucose, many cancerous cells inhibit OXPHOS and promote glycolysis, even when oxygen is available [54]. In our study, glucose was replaced with D-galactose, forcing dopaminergic neurons to rely exclusively on mitochondrial ATP production via OXPHOS, a slower and less efficient pathway. Under these conditions, cells become more sensitive to molecules that affect mitochondria, and they experience metabolic stress. Dopaminergic neurons are particularly vulnerable to oxidative stress and mitochondrial dysfunction, both affecting ATP production. Ultimately, it leads to cell failure.
Under these conditions, CLP showed greater improvements in dopaminergic neuron cells treated with D-galactose instead of glucose, compensating for the loss of energy in a stress situation. We hypothesize that, in situations in which ATP production is depleted or modified, CLP enhances mitochondrial energy production due to its PCr content, which donates a phosphate group immediately to boost ATP synthesis.
Additionally, we found that the lowest dose of CLP tested (100µM) led to a significant increase in ATP levels in neuronal cells, approximately 11% above baseline (p < 0.05). In contrast, CRM alone, even at its most effective concentration (500µM), did not stimulate ATP synthesis as effectively as CLP. This corresponds to an estimated bioavailable effective dose of 13 mg for CLP versus 65.57mg/L for CRM in neurons. Low doses are especially relevant for brain-targeted creatine supplementation, given the limited expression of CT1 at the BBB, and the downregulation of both CT1 and the endogenous creatine synthesis. These factors may reduce the efficacy or contribute to resistance over time, as well as the need to minimize systemic side effects.
Long-term supplementation may suppress endogenous creatine synthesis via end-product inhibition, as the expression of L-arginine: glycine amidino transferase (AGAT), the rate-limiting enzyme for creatine biosynthesis in the brain, is downregulated in the presence of high intracellular creatine levels. This feedback mechanism helps maintain cellular energy homeostasis and prevents overaccumulation [18,55]. Limited CT1 expression at the blood–brain barrier often requires high creatine doses to achieve detectable increases in brain creatine levels. Current clinical studies show wide variability in dosing regimens, ranging from 5 g/day to 20 g/day [3,8], highlighting the need for greater consistency and deeper understanding of the optimal dosing strategy for brain-targeted effects. This is critical for the effective design of neuroprotective or cognitive-enhancement interventions using creatine.
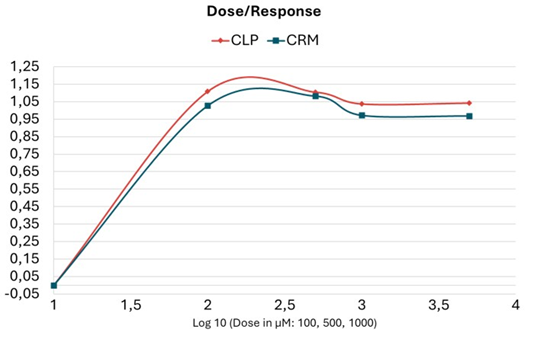
Figure 4: Dose-response plot.
The dose–response analysis (Figure 4) reveals that CLP exhibits a more efficient activation, at all and any tested dosage of the phosphagen system in dopaminergic neuron cells, enabling faster ATP replenishment through the use of PCr. CRM administration reached a higher saturation threshold at 500 µM, compared to CLP. From this concentration, ATP levels started to decline. Notably, stimulation of ATP synthesis remained more stable among the increasing concentrations of CLP, suggesting an immediate and sustained energy-supplying effect. This enhanced stability may be attributed to the synergistic combination of CRM, creatine phosphate, and phosphate salt within the CLP formulation.
PCr resynthesise is tightly linked to the efficiency of aerobic metabolism. In the neuronal system, enhanced mitochondrial function and PCr availability could contribute to greater cognitive resilience and attentional capacity under stress. Additionally, reduced intracellular acidification may create a neuroprotective environment, as excessive acidosis disrupts synaptic transmission and promotes excitotoxicity. Extracellular acidification has been shown to impair neurotransmission and induce neuronal dysfunction and toxicity through H⁺ and lactate accumulation [56,57] Moreover, enhanced aerobic metabolism may facilitate greater neuronal plasticity and synaptic adaptation. These effects could underlie some of the cognitive benefits associated with sustained PCr availability and efficient ATP buffering in the brain.
In this context, our findings highlight the potential of CLP to support brain bioenergetics under conditions favoring oxidative phosphorylation. These results obtained in compromised and metabolic stressed cells may be extrapolated to conditions with altered brain energetics and mitochondrial dysfunction, such as stress situations, anxiety, sleep deprivation, hypoxia or certain diseases mentioned above. Our data suggest that CLP supports the maintenance of brain creatine levels more efficiently than CRM alone, by preserving PCr levels and enhancing ATP buffering capacity. CLP may contribute to improved neuronal plasticity and synaptic adaptation, key processes underlying cognitive performance.
Further In Vivo studies are required to confirm the physiological relevance of these effects and to determine the therapeutic potential of CLP in brain health and cognitive performance.
References
- Andreone BJ, Lacoste B, Gu C (2015) Neuronal and vascular interactions. Annu Rev Neurosci 38: 25-46.
- Chen YH, Lin S, Jin SY, Gao TM (2025) Extracellular ATP is a Homeostatic Messenger That Mediates Cell-Cell Communication in Physiological Processes and Psychiatric Diseases. Biol Psychiatry 97(1): 41-53.
- Rae CD, Bröer S (2015) Creatine as a booster for human brain function. How might it work? Neurochem Int 89: 249-259.
- Wyss M, Kaddurah-Daouk R (2000) Creatine and creatinine metabolism. Physiol Rev 80(3):1107-1213.
- Avgerinos KI, Spyrou N, Bougioukas KI, Kapogiannis D (2018) Effects of creatine supplementation on cognitive function of healthy individuals: A systematic review of randomized controlled trials. Exp Gerontol 108: 166-173.
- Braissant O, Henry H (2008) AGAT, GAMT and SLC6A8 distribution in the central nervous system, in relation to creatine deficiency syndromes: a review. J Inherit Metab Dis 31(2): 230-9.
- Braissant O (2012) Creatine and guanidinoacetate transport at bloodbrain and blood-cerebrospinal fluid barriers. J Inherit Metab Dis 35(4): 655-664.
- Forbes SC, Cordingley DM, Cornish SM, Gualano B, Roschel H, et al. (2022) Effects of Creatine Supplementation on Brain Function and Health. Nutrients 14(5): 921.
- Wilkinson ID, Mitchel N, Breivik S, Greenwood P, Griffiths PD, et al. (2006) Effects of creatine supplementation on cerebral white matter in competitive sportsmen. Clin J Sport Med 16(1): 63-67.
- Merege-Filho CAA, Otaduy MCG, de Sá-Pinto AL, de Oliveira MO, de Souza Gonçalves L, et al. (2017) Does brain creatine content rely on exogenous creatine in healthy youth? A proof-of-principle study. Appl Physiol Nutr Metab Physiol Appl Nutr Metab 42(2): 128-134.
- Rawson ES, Lieberman HR, Walsh TM, Zuber SM, Harhart JM, et al. (2008) Creatine supplementation does not improve cognitive function in young adults. Physiol Behav 95(1–2): 130-134.
- Dolan E, Gualano B, Rawson ES (2019) Beyond muscle: the effects of creatine supplementation on brain creatine, cognitive processing, and traumatic brain injury. Eur J Sport Sci 19(1): 1-14.
- McMorris T, Harris RC, Howard AN, Langridge G, Hall B, et al. (2004) Creatine supplementation, sleep deprivation, cortisol, melatonin and behavior. Physiol Behav 90(1): 21-8.
- McMorris T, Mielcarz G, Harris RC, Swain JP, Howard A, et al. (2007) Creatine supplementation and cognitive performance in elderly individuals. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 14(5): 517-528.
- Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, et al. (2006) High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet 38(5): 515-517.
- Turner CE, Byblow WD, Gant N (2015) Creatine supplementation enhances corticomotor excitability and cognitive performance during oxygen deprivation. J Neurosci Off J Soc Neurosci. 35(4): 1773-1780.
- Joncquel-Chevalier Curt M, Voicu PM, Fontaine M, Dessein AF, Porchet N, et al. (2015) Creatine biosynthesis and transport in health and disease. Biochimie119: 146-165.
- Candow DG, Forbes SC, Ostojic SM, Prokopidis K, Stock MS, et al. (2023) “Heads Up” for Creatine Supplementation and its Potential Applications for Brain Health and Function. Sports Med 53(1): 49-65.
- Persky AM, Brazeau GA (2001) Clinical pharmacology of the dietary supplement creatine monohydrate. Pharmacol Rev 53(2): 161-176.
- Klivenyi P, Ferrante RJ, Matthews RT, Bogdanov MB, Klein AM, et al. (1999) Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nat Med 5(3): 347-350.
- Brosnan JT, Brosnan ME (2007) Creatine: endogenous metabolite, dietary, and therapeutic supplement. Annu Rev Nutr 27: 241-261.
- Mallik B, Frank CA (2023) Is creatine a CNS neurotransmitter? eLife12: e91824.
- Bian X, Zhu J, Jia X, Liang W, Yu S, et al. (20236) Suggestion of creatine as a new neurotransmitter by approaches ranging from chemical analysis and biochemistry to electrophysiology. eLife 12: RP89317.
- Meftahi GH, Hatef B, Pirzad Jahromi G (2023) Creatine Activity as a Neuromodulator in the Central Nervous System. Arch Razi Inst 78(4): 1169-1175.
- Almeida LS, Salomons GS, Hogenboom F, Jakobs C, Schoffelmeer ANM, et al. (2006) Exocytotic release of creatine in rat brain. Synap NYN 60(2): 118-123.
- Cunha MP, Lieberknecht V, Ramos-Hryb AB, Olescowicz G, Ludka FK, et al. (2016) Creatine affords protection against glutamate-induced nitrosative and oxidative stress. Neurochem Int 95: 4-14.
- Cunha MP, Pazini FL, Ludka FK, Rosa JM, Oliveira Á, et al. (2015) The modulation of NMDA receptors and L-arginine/nitric oxide pathway is implicated in the anti-immobility effect of creatine in the tail suspension test. Amino Acids 47(4): 795-811.
- Gerbatin RR, Silva LFA, Hoffmann MS, Della-Pace ID, do Nascimento PS, et al. (2019) Delayed creatine supplementation counteracts reduction of GABAergic function and protects against seizures susceptibility after traumatic brain injury in rats. Prog Neuropsychopharmacol Biol Psychiatry 92: 328-338.
- Koga Y, Takahashi H, Oikawa D, Tachibana T, Denbow DM, et al. (2005) Brain creatine functions to attenuate acute stress responses through GABAnergic system in chicks. Neuroscience 132(1): 65-71.
- Nersesova LS, Petrosyan M, Arutjunyan AV (2025) Neuroprotective Potential of Creatine. Hidden Resources of Its Therapeutic and Preventive Use. Neurochemical Journal 16: 14-30.
- Andres RH, Huber AW, Schlattner U, Pérez-Bouza A, Krebs SH, et al. (2005) Effects of creatine treatment on the survival of dopaminergic neurons in cultured fetal ventral mesencephalic tissue. Neuroscience 133(3): 701-713.
- Leem YH, Kato M, Chang H (2018) Regular exercise and creatine supplementation prevent chronic mild stress-induced decrease in hippocampal neurogenesis via Wnt/GSK3β/β-catenin pathway. J Exerc Nutr Biochem 22(2): 1-6.
- Lapena-Luzon T, Hernandez-Bueno A, Tomas-Cobos L, MorenoPuente P, Garcia-Benlloch S, et al. (2025) Evaluation of the Effect of Clonapure® Versus Creatine Monohydrate on ATP Levels in a C2C12 Muscle Cell Line. Am J Sports Sci13(2): 50-6.
- Gohil VM, Sheth SA, Nilsson R, Wojtovich AP, Lee JH, Perocchi F, et al. (2010) Nutrient-sensitized screening for drugs that shift energy metabolism from mitochondrial respiration to glycolysis. Nat Biotechnol 28(3): 249-255.
- de Kok MJC, Schaapherder AF, Wüst RCI, Zuiderwijk M, Bakker JA, et al. (2021) Circumventing the Crabtree effect in cell culture: A systematic review. Mitochondrion 59: 83-95.
- Orlicka-Płocka M, Gurda-Wozna D, Fedoruk-Wyszomirska A, Wyszko E (2020) Circumventing the Crabtree effect: forcing oxidative phosphorylation (OXPHOS) via galactose medium increases sensitivity of HepG2 cells to the purine derivative kinetin riboside. Apoptosis 25(11): 835-852.
- Rossi A, Rigotto G, Valente G, Giorgio V, Basso E, et al. (2020) Defective Mitochondrial Pyruvate Flux Affects Cell Bioenergetics in Alzheimer’s Disease-Related Models. Cell Rep 30(7): 2332-2348.
- Swerdlow RH, Lezi E, Aires D, Lu J (2013) Glycolysis–respiration relationships in a neuroblastoma cell line. Biochim Biophys Acta BBA - Gen Subj 1830(4): 2891-2898.
- Marshall RP, Droste JN, Giessing J, Kreider RB (2022) Role of Creatine Supplementation in Conditions Involving Mitochondrial Dysfunction: A Narrative Review. Nutrients 14(3): 529.
- Meyer JN, Leuthner TC, Luz AL (2017) Mitochondrial fusion, fission, and mitochondrial toxicity. Toxicology 391: 42-53.
- Johri A, Beal MF (2012) Mitochondrial dysfunction in neurodegenerative diseases. J Pharmacol Exp Ther 342(3): 619-30.
- Reeve AK, Krishnan KJ, Elson JL, Morris CM, Bender A, et al. (2008) Nature of mitochondrial DNA deletions in substantia nigra neurons. Am J Hum Genet 82(1): 228-235.
- Coskun PE, Beal MF, Wallace DC (2004) Alzheimer’s brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc Natl Acad Sci USA 101(29): 1072610731.
- Beal MF (2011) Neuroprotective effects of creatine. Amino Acids 40(5): 1305-1313.
- Royes LFF, Busanello GL, Godinho D, Nascimento AS, Lima GC, et al. (2023) Creatine maintains mitochondrial integrity and protects against dysfunction in molecular systems involved in early epileptiform activity and cognitive impairment in young rats submitted to traumatic brain injury.
- Faulkner P, Paioni SL, Kozhuharova P, Orlov N, Lythgoe DJ, et al. (2021) Relationship between depression, prefrontal creatine and grey matter volume. J Psychopharmacol Oxf Engl 35(12): 1464-1472.
- Amital D, Vishne T, Rubinow A, Levine J (2006) Observed effects of creatine monohydrate in a patient with depression and fibromyalgia. Am J Psychiatry 163(10): 1840-1841.
- Lyoo IK, Yoon S, Kim TS, Hwang J, Kim JE, et al. (2012) A randomized, double-blind placebo-controlled trial of oral creatine monohydrate augmentation for enhanced response to a selective serotonin reuptake inhibitor in women with major depressive disorder. Am J Psychiatry 169(9): 937-945.
- Roitman S, Green T, Osher Y, Karni N, Levine J, et al. (2007) Creatine monohydrate in resistant depression: a preliminary study. Bipolar Disord 9(7): 754-758.
- Coplan JD, Mathew SJ, Mao X, Smith ELP, Hof PR, et al. (2006) Decreased choline and creatine concentrations in centrum semiovale in patients with generalized anxiety disorder: relationship to IQ and early trauma. Psychiatry Res 147(1): 27-39.
- Schuff N, Neylan TC, Fox-Bosetti S, Lenoci M, Samuelson KW, et al. (2008) Abnormal N-acetylaspartate in hippocampus and anterior cingulate in posttraumatic stress disorder. Psychiatry Res 162(2): 147157.
- Sakellaris G, Nasis G, Kotsiou M, Tamiolaki M, Charissis G, Evangeliou A, et al. (2008) Prevention of traumatic headache, dizziness and fatigue with creatine administration. A pilot study. Acta Paediatr Oslo Nor 1992 97(1): 31-34.
- Sakellaris SG, Partalis NI, Nasis GD, Kotsiou ME, Tamiolaki MD, et al. (2012) Outcome of Traumatic Dysarthria and Lingual Problems of Understanding with Creatine Administration. An Open Label Randomized Pilot Study. J Trauma Treat 01(03): 1222.1000120.
- Liberti MV, Locasale JW (2016) The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci 41(3): 211-218.
- Baker JS, McCromick MC, Robergs RA (2010) Interaction among Skeletal Muscle Metabolic Energy Systems during Intense Exercise. Journal of Nutrition and Metabolism 2010: 905612.
- Siesjö BK (1985) Acid-base homeostasis in the brain: physiology, chemistry, and neurochemical pathology. Prog Brain Res 63: 121-154.
- Chesler M (2003) Regulation and modulation of pH in the brain. Physiol Rev 83(4): 1183-1221.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.