CLL Management: Towards an MRD Guided Therapy
by Mohammad Hassan Hodroj1*, Colette Hanna2*
1Division of Hematology and Oncology, Department of Internal Medicine, American University of Beirut Medical Center, Beirut, Lebanon
2Division of Hematology and Oncology, Faculty of Medicine Lebanese American University Medical Center Rizk Hospital Beirut Lebanon
*Corresponding Author: Mohammad Hassan Hodroj, Division of Hematology and Oncology, Department of Internal Medicine, American University of Beirut Medical Center, Beirut, Lebanon
Colette Hanna, Division of Hematology and Oncology, Faculty of Medicine Lebanese American University Medical Center Rizk Hospital Beirut Lebanon
Received Date: 03 August 2025
Accepted Date: 07 August 2025
Published Date: 11 August 2025
Citation: Hodroj MH, Hanna C (2025). CLL Management: Towards an MRD Guided Therapy. Ann Case Report. 10: 2369. https://doi.org/10.29011/2574-7754.102369
Abstract
The field of immunophenotypic and molecular diagnostics has experienced significant transformation, particularly over the past two decades, becoming a pivotal component in diagnosing and even treating various diseases. Minimal residual disease (MRD) assessment has gained substantial clinical significance in hematologic malignancies, serving as an indicator of treatment response and playing a critical role in risk stratification and management decisions conditions. In chronic lymphocytic leukemia (CLL), MRD assessment has evolved over the years, now being an integral part of evaluating treatment responses and an efficient tool for detecting disease progression. MRD status has important prognostic implications post-treatment and can influence decisions regarding treatment options, duration, and intensity. CLL patients who achieve undetectable MRD after treatment tend to have better outcomes compared to those with detectable MRD following specific treatment regimens. The shift in CLL treatment strategies from chemotherapy and chemo-immunotherapy to targeted therapies has altered the clinical significance of MRD, which varies depending on the treatment or combination of treatments used. MRD measurement generally relies on highly sensitive techniques that assess the immunophenotypic profiles of tumor cells and specific genetic alterations, such as flow cytometry, polymerase chain reaction (PCR), and genetic sequencing. Currently, multicolor flow cytometry, which detects CLL cells in peripheral blood, is the most widely used method for MRD detection. However, clinical trial data on the optimal assay for determining undetectable MRD in CLL remains limited. This review aims to provide an overview of the current techniques for MRD measurement in CLL and discuss its clinical significance in guiding personalized treatment for patients.
Keywords: Chronic Lymphoblastic Leukemia; Minimal Residual Disease; Targeted Therapy; Genetic Sequencing; Flow Cytometry; Polymerase Chain Reaction.
Introduction
The field of immunophenotypic and molecular diagnostics has dramatically changed with several advancements and developments especially in the last two decades, where it became a key player in the diagnosis and even treatment of different diseases. The depth and quality of remission were always important in assessing the response to treatment in cancer patients and were based on imaging and laboratory tests compared to the baseline at diagnosis [1]. Minimal residual disease (MRD) assessment has gained clinical importance in hematologic malignancies as a measure for therapy response and became a cornerstone in risk stratification and guidance for management of some types. The initial use of MRD was in chronic myeloid leukemia via quantification of BCR-ABL transcripts to identify the level of remission [2]. This was followed by the extensive use of MRD in acute myeloid leukemia to guide prognosis, therapeutic decisions, need for stem cell transplantation or the addition of targeted therapies to the treatment regimen [3].
Chronic lymphoid leukemia/small cell lymphoma (CLL/SLL) is the most common leukemia of the adults in western countries with small monomorphic mature B-lymphocytes involving the bone marrow, peripheral blood and secondary lymphoid tissues such as the lymph nodes and the spleen. CLL currently accounts for around 7% of all non-Hodgkin’s lymphomas [4]. The MRD approach in chronic lymphoid leukemia (CLL) is different, where it was a debatable topic over years until the emergence of effective targeted therapies due to enhanced understanding of the pathophysiology and molecular biology of this disease [5]. The standard staging methods CLL were initially based on cytology and clinical assessment, making them less accurate than the current MRD, in determining the response to treatment and the depth of complete remission (CR). Given what is mentioned formerly, the need for an accurate definition of CR and the depth of remission based on MRD, in the era of precision and personalized medicine, has become the focus of clinical trials in CLL to guide treatment options in clinical practice [6].
The treatment of CLL had dramatically changed with the use of targeted therapies and chemoimmunotherapy leading to more prominent MRD negative disease. The importance of MRD status was highlighted in a number of clinical trials that assessed the relationship between MRD status and progression free survival (PFS) after treatment showing a longer PFS in patients with undetectable MRD disease even if they achieved only partial response than that in patients with complete response but detectable MRD disease [7].
The first method to be used for accurate detection of MRD in CLL was the flow cytometry that is dependent on the leukemiaassociated immunophenotype of the cells. The abilities of flow cytometry measurement of MRD in CLL were developed with time reaching very low thresholds of detection [3]. The molecular testing was then added to the tools of MRD assessment with polymerase chain reaction (PCR) and next generation sequencing (NGS) that lowered the threshold of detection and became technically validated assays [8].
The present review aims to provide an overview about the current techniques for measurement of MRD in CLL, their history and development, together with their role in clinical practice through guiding personalized treatment for patients.
MRD Techniques
Flow Cytometry
The use of flow cytometry as an approach to detect MRD goes back to 2007 in which an automated phenotyping of cells is performed through fluorescently labelled antibodies that target specific antigens on cell surface [4]. Compared to MRD by cytology that was limited to detect the presence of less than one CLL cell in a maximum of 100 leukocytes, flow cytometry was able to detect MRD of less than one CLL cell per 10000 cells in a sample of one to two million cells known as <10-4 or MRD4, which was developed later and became an accurate detection of one CLL cell per 100000 termed as <10-5 or MRD5 [1, 9]. Performing a flow cytometry requires the preparation of the sample through whole blood lysis with or without fixative agents before the addition of the fluorescent antibodies resulting in quantitative examination of the cells [10]. The initial standardized testing was dependent on four-colors set of antibodies and with evolution of flow cytometry, these sets reached six to eight colors [10]. Specific panels of fluorescent antibodies were established for the identification of CLL phenotype by flow cytometry for diagnosis and to check for MRD. These panels include different targeted cell surface antigens such as CD5, CD19, CD20, CD22, CD23, CD43, CD45, CD79b, CD81, CD200, and monoclonal surface immunoglobulin. The characteristic immunophenotype for CLL shows a positive expression of CD5, CD19, CD23, CD43, CD200 while a dim expression of CD20, CD22, CD45, CD81, monoclonal surface immunoglobulins and dim to negative expression of CD79b [4]. The flow cytometry approach to detect MRD5 in CLL with a standardized six-markers panel was validated in 2016 by the European Research Initiative on CLL and included the following core panel: CD19, CD20, CD5, CD43, CD79b and CD81 with a one-tube method that showed confirmed reliability [9]. Several studies showed that the novel combination of the NK-cell receptor and tumor specific antigen, CD160 with the tumor associated antigen, receptor tyrosine kinase-like orphan receptor 1 (ROR1) has enhanced the sensitivity for MRD detection in CLL by flow cytometry [11]. CD160-ROR1FCA is being used in a single tube assay with a highly sensitive results, rapid application and simple gating strategy [12]. In addition, the enhanced ability of the flow to read more events in shorter period led to the reduction of the time and sample amount needed to perform a sensitive test that ca quantitatively detect residual disease in the 0.001–0.01% range [9]. The only exceptions for low sensitivity tests in CLL MRD using the flow cytometry are CLL patients with atypical phenotypes that are frequently uncommon and may lack CD5 and CD23 markers or normally express CD20, CD22 and CD79b [13-15]. It is essential in these cases to always compare to the pretreatment characteristic phenotype detected by flow cytometry at diagnosis [16]. Flow cytometry remains the most commonly used technique for diagnostic and MRD assessments due to its vast availability in laboratories, detections on peripheral blood samples, uncomplicated processing protocols and software with an acceptable cost. However, flow cytometry is still facing limitations such as the need for fresh blood to be processed within 48 hours
and the sensitivity of MDR5 only [17]. Efforts and research are currently in process to develop flow cytometry machines into next generation ones, aiming towards recording millions of events per sample and reaching the sensitivity of MDR6 [18].
(Figure 1) summarizes the steps of MRD detection by flow cytometry in conventional assay and single tube assay.
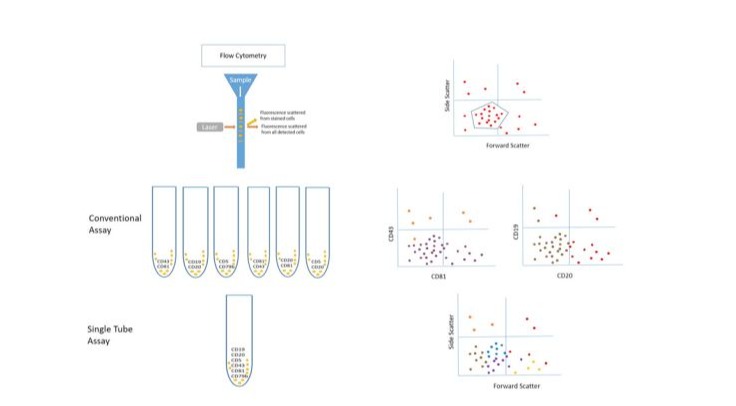
Figure 1: MRD detection using the conventional assay or the single tube assay
Polymerase Chain Reaction (PCR)
Detection by PCR and real time PCR is based on identification of specific DNA sequences to be targeted with labeled probes and amplified through fluorescent intercalation, hydrolysis or hybridization leading to quantitative results via measuring the increased fluorescence during the elongation of DNA strands [19]. This technique, specifically real time PCR, was used initially in quantification of viral load in infections such as hepatitis and human immunodeficiency virus, then its use was extended to measure MRD in some hematologic malignancies as BCR-ABL in chronic myeloid leukemia and Philadelphia positive acute lymphoid leukemia [20, 21]. In CLL unique DNA sequences were identified on the immunoglobulin gene (Ig) and specific primers were generated to target Ig heavy chain, Ig Kappa and Ig Lambda. These were followed by targeting the variable (V), diversity (D) and joining (J) segments of the Ig what is known as the VDJ fingerprint [22]. Currently, the MRD detection by PCR depends on the production of patient specific primers. This requires the sequencing of IGHV gene at diagnosis and the generation of allele specific oligonucleotide probes to target the IGHV gene and quantify the mutation [19]. Unlike the low sensitivity of consensus PCR using prepared known primers, the sensitivity of real time PCR MRD is confirmed to be 10-5 with the result being quantitative [23]. The real time PCR permits the presentation of a relative gene expression through equation and calculations. The efficiency correction method calculates the relative expression ratio from the real-time PCR efficiencies and the threshold cycle (CT). The analysis is done using the sigmoidal curve fitting methods that fit the experimental data to an empirical equation and results in the prediction of the PCR efficiency and an estimate of the initial copy number of the amplicon. The lower the CT, the greater the amount of amplicon [24]. Additionally, droplet digital PCR is a highly sensitive and reproducible technique that is still mostly used for research purposes and was recently used for the detection of MRD in CLL. The most studied gene in CLL using the digital PCR was TP53 gene were specific exons that encode for the 17p deletion were identified and tested using specific probes. The droplet digital PCR for quantitation of TP53 deletions or point mutations might replace the FISH procedure with accurate results and shorter duration of time [25]. Despite its advantages that include quantitative sensitive results and unnecessity of fresh samples, where testing can be done on frozen samples, real time PCR for MRD is still not widely used due to its requirements such as prolonged time, the need for expertise, complicated procedure and elevated cost [23]. Multiple clinical trials have been studying the increase of real time PCR sensitivity for MRD through the production of advanced targeted probes and the combination to other modalities as flow cytometry and genetic sequencing [9].
(Figure 2) summarizes the steps in Real-time PCR of IGH gene variable.
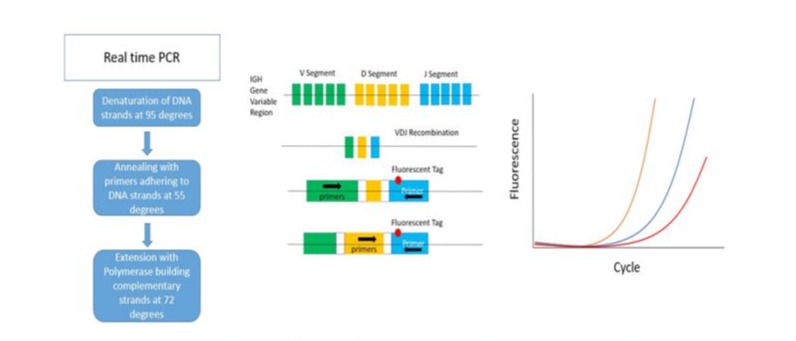
Figure 2: MRD detection of the IGH variable using Real-time PCR
Next Generation Sequencing (NGS)
Since the description of DNA structure in 1953, genetic sequencing started to be introduced and incorporated in clinical research with the use of electrophoresis followed by radioactive and fluorescent techniques [26]. Automated sequencing was established in 1990s as an efficient tool for genetic mutations identification in various diseases. Moreover, next generation sequencing, known as secondgeneration sequencing or high-throughput sequencing (HTS) was introduced in 2005 for clinical use with the ability to perform millions to billions of reads in a single run [27]. NGS has gained in the last decade an important role in accurate identification of tumor burden and MRD in different hematologic malignancies. Similarly, NGS use in CLL showed that some patients with undetectable MRD by flow cytometry and PCR at a sensitivity of MRD4, which is required by guidelines, have positive MRD by NGS at a deeper sensitivity [28]. This finding was considered as a potential explanation for the delayed relapse in patients with labeled MRD negative disease especially those with highrisk features as unmutated IGHV CLL [29]. In contrast to PCR, NGS has more sensitivity and does not require identification of primers upfront or the use of specific primers for each patient [30].
New NGS assays in CLL can reliably detect and quantify MRD beyond the level of MRD 10−5 and up to MRD 10−6 especially those IGHV based assays developed for the detection of MRD [30]. NGS depends mainly on an adequate quantity of DNA and can provide both quantitative and qualitative data that can be compared before and after treatment [31]. Recently, there is a clear evidence for the survival benefit of negative MRD in CLL patients after first line treatment over patients with positive MRD post chemoimmunotherapy or venetoclax-rituximab treatment. NGS is currently considered the most reliable tool to identify the most accurate MRD status in CLL [32]. NGS has the capability to identify clonal diversity and intraclonal dynamics through revealing the baseline rearrangements present at diagnosis, to which the results are compared post treatment to check the presence of these clones [33]. NGS role in prognostication and treatment guidance is progressing very fast. However, it is still limited to specific centers due to its high cost and the need for complicated software tools and expertise for the analysis and interpretation of the results. NGS for MRD in CLL is mainly restricted to clinical trials and needs more standardization to become available for clinical practice [34].
(Figure 3) summarizes the MRD modalities, the sensitivity and the advantages and disadvantages of each modality.
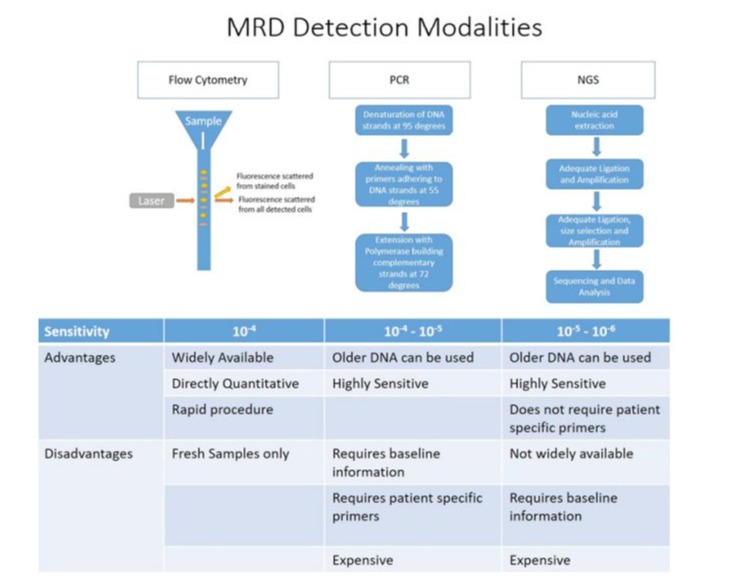
Figure 3: MRD detection modalities with advantages and disadvantages of each modality
PCR: polymerase chain reaction, NGS: next generation sequencing
Precision Medicine in CLL
As in the diagnostic field of CLL, the therapeutic field in CLL has developed in parallel in the last two decades leading to improvement in the outcome of CLL patients. The spectrum of studied and applied treatments ranged from chemoimmunotherapy to targeted therapy to immunomodulatory agents, until reaching combinations of these agents [35]. Treatment of CLL started initially with chemotherapy, mainly chlorambucil, then it was based combinations of chemotherapeutic agents until the addition of immunotherapy with the introduction of rituximab carrying high toxicity. The treatment paradigm was completely shifted in 2014 with the emergence of the novel drugs the Bruton Tyrosine Kinase inhibitors (BTKi), which switched the pattern from chemoimmunotherapy into the era of targeted therapies that are effective with less secondary adverse events [36]. Ibrutinib is a first generation BTKi that was approved initially by FDA in 2014 for refractory/relapsed (R/R) CLL and and for patients with CLL harboring the deletion of chromosome 17p (del17p) based on the result of the phase 3 RESONATE trial [37]. Ibrutinib then received the approval to be used in the frontline in the treatment of CLL after the results of phase 3 RESONATE 2 trial that compared it to chlorambucil [38]. Moreover, ibrutinib showed superiority in PFS and OS when compared to fludarabine, cyclophosphamide, and rituximab (FCR) regimen in phase 3 study E1912 [39]. Furthermore, targeting Bcl2 via the use of the Bcl2 inhibitor venetoclax, was another milestone in the era of targeted therapies. All these advancements led to survival benefit in CLL, which in turn incorporated the use of MRD to accurately assess the depth of response to treatment [40]. Moreover, the current decision for first line treatment in CLL depends on the physical fitness of the patients, molecular features of the disease, IGHV status and FISH cytogenetics including 17p deletion, and TP53 mutational status before starting treatment [34]. TP53 alterations that include both TP53 mutation and 17p deletion, indicates chemo-refractoriness and requires the use of biologics such as Bcl2 inhibitor to overcome the refractoriness [41]. In addition, the presence of IGVH mutations in the absence of TP53 disruption indicates a durable response to chemoimmunotherapy making the survival close to general population [42].
Several trials were done to investigate the role of MRD driven approach in choosing, initiating or resuming treatment for CLL patients. Despite the excellent results of ibrutinib monotherapy, it was noticed that patients treated with ibrutinib alone maintained a positive MRD [1]. The combination of ibrutinib with other agents was studied and the ILLUMINATE trial investigated its combination of ibrutinib to obinutuzumab leading to the best negative MRD results for ibrutinib reaching 30% [43]. In addition, HELIOS trial demonstrated that the MRD negativity was 26% with the combination of ibrutinib and rituximab-bendamustine, which was higher than ibrutinib monotherapy [44]. MURANO trial is a phase 3 clinical trial that compared rituximab-venetoclax combination with rituximab-bendamustine combination in patients with R/R CLL showing improved PFS and OS with rituximabvenetoclax. Venetoclax-rituximab combination showed better response in fixed duration pattern even in high-risk population with the ability to achieve undetectable MRD [45].CLARITY, a phase 2 trial, and FLAIR, a phase 3 trial, were two clinical trials reporting MRD outcomes using ibrutinib-venetoclax combination in refractory and relapsed CLL [45, 46]. The FLAIR trial showed that MRD-guided ibrutinib-venetoclax treatment was superior in PFS to FCR. Using the MRD-guided approach in the FLAIR trial led to stop therapy in ibrutinib-venetoclax group in 28.9% of patients by 2 years and in 58% by 3 years. 47.5% of patients achieved undetectable MRD with ibrutinib-venetoclax after 12 months of treatment and this percentage increased to 92.7% with continued therapy revealing that 12 months treatment duration is not sufficient to reach MRD negativity [45]. Moreover, CLL13 trial investigated different treatment modalities including chemoimmunotherapy with FCR, rituximab-bendamustine, venetoclax with rituximab, venetoclax with obinutuzumab and venetoclax combined to ibrutinib and obinutuzumab. The trial demonstrated the highest MRD negativity with the combination of venetoclax, ibrutinib and obinutuzumab exceeding 90% [47]. Furthermore, the CAPTIVATE trial is a randomized phase II international trial investigating the application of personalized medicine in CLL through tailoring the treatment based on the patient characteristics’, molecular biology and MRD status post treatment. The trial studied the combination of ibrutinib and venetoclax followed in MRD guided arm and fixed duration arm, showing comparable results in the fixed duration arm where patients achieved deep molecular response, high MRD negativity and prolonged PFS [48]. CLL2-BAAG trial is a phase 2 trial that investigated the MRD-guided triple therapy of acalabrutinib, venetoclax, and obinutuzumab in patients with R/R CLL with the addition of circulating tumor DNA (ctDNA)-based analyses to flow cytometry. The study concluded that the use of fixed MRD-guided treatment with this combination led to deep remissions in the majority of patients with improvement of early relapse detection with the addition of ctDNA [49]. CLL2-BZAG trial tested the use of obinutuzumab, venetoclax and zanubrutinib combination after bendamustine debulking in patients with R/R CLL with the treatment duration being based on MRD detection. The study revealed that fixed-duration MRD-guided treatment with this combination resulted in deep remissions in R/R CLL patients [50].
The concept of MRD at the end of treatment in CLL known as (uMRD) has gained a substantial importance due to its strong correlation with PFS. The German CLL8 trial has shown that uMRD is an independent predictor of PFS in CLL. Achieving an MRD-negative status at the conclusion of therapy is increasingly recognized as a critical endpoint, as it indicates the absence of detectable leukemic cells and is associated with improved PFS, reflecting a lower likelihood of disease relapse. Studies have demonstrated that patients with sustained uMRD negativity experience significantly longer durations of remission compared to those with detectable MRD levels after treatment with chemoimmunotherapy or venetoclax with rituximab [47]. In addition, patients with uMRD and a partial response due to residual splenomegaly were found to have similar outcomes of patients with uMRD and a complete response in a comprehensive analysis of two-phase III studies of the German CLL study group [7].
Conclusion
Diagnostic tools have shown a dramatic change during the last decade leading to improvement in detection of MRD in CLL. Currently flow cytometry remains the most common used technique for MRD assessment. However, the field in CLL is shifting towards incorporating PCR and NGS or combination of technique for a more accurate MRD assessment post-treatment.
Based on all mentioned advancements in the field, it is expected that improvements in technologies like NGS and enhanced flow cytometry will likely lead to more accurate and sensitive MRD detection. Furthermore, the integration of artificial intelligence and machine learning into data analysis may enhance the interpretation of results, facilitating better patient stratification and personalized treatment strategies. Continuous research into the biological implications of MRD levels will also play a crucial role in shaping future methodologies, ultimately influencing therapeutic decisions towards MRD-tailored treatments and long-term monitoring approaches for patients.
Author contributions
MH and CH were both responsible for the citation research, providing the topic of discussion, referencing, writing of the manuscript and final approval for submission. Both authors contributed to the article and approved the submitted version.
Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Benintende G, Pozzo F, Innocenti I, Autore F, Fresa A, et al. (2023) Measurable residual disease in chronic lymphocytic leukemia. Front Oncol. 13: 1112616
- Cumbo C, Anelli L, Specchia G, Albano F. (2020) Monitoring of Minimal Residual Disease (MRD) in Chronic Myeloid Leukemia: Recent Advances. Cancer Manag Res. 12: 3175-3189
- Heuser M, Freeman SD, Ossenkoppele GJ, Buccisano F, Hourigan CS, et al. (2021) 2021 Update on MRD in acute myeloid leukemia: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 138: 2753-2767
- Salem DA, Stetler-Stevenson M (2019) Clinical Flow-Cytometric Testing in Chronic Lymphocytic Leukemia. Methods Mol Biol. 2032: 311-321
- Al-Sawaf O (2023) Importance of Minimal Residual Disease in the Era of Targeted Therapies in Chronic Lymphocytic Leukemia. Acta Haematologica. 147: 22-32.
- Thompson PA, Wierda WG (2016) Eliminating minimal residual disease as a therapeutic end point: working toward cure for patients with CLL. Blood. 127: 279-286.
- Kovacs G, Robrecht S, Fink AM, Bahlo J, Cramer P, et al. (2016) Minimal Residual Disease Assessment Improves Prediction of Outcome in Patients With Chronic Lymphocytic Leukemia (CLL) Who Achieve Partial Response: Comprehensive Analysis of Two Phase III Studies of the German CLL Study Group. J Clin Oncol. 34: 3758-3765.
- Dagher G, Becker KF, Bonin S, Foy C, Gelmini S, et al. (2019) Pre-analytical processes in medical diagnostics: New regulatory requirements and standards. N Biotechnol. 52: 121-125.
- Rawstron AC, Fazi C, Agathangelidis A, Villamor N, Letestu R, et al. (2016) A complementary role of multiparameter flow cytometry and high-throughput sequencing for minimal residual disease detection in chronic lymphocytic leukemia: an European Research Initiative on CLL study. Leukemia. 30: 929-936.
- Rawstron AC, Villamor N, Ritgen M, Böttcher S, Ghia P, et al. (2007) International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia. 21: 956-964.
- Farren TW, Liu F, Macey MG, Kipps TJ, Warner N, et al. (2013) Combined ROR1 and CD160 Detection for Improved Minimal Residual Disease in Patients With Chronic Lymphocytic Leukemia (CLL). Blood. 122: 2572.
- Farren TW, Sadanand KS, Agrawal SG. (2020) Highly Sensitive and Accurate Assessment of Minimal Residual Disease in Chronic Lymphocytic Leukemia Using the Novel CD160-ROR1 Assay. Front Oncol. 10: 597730.
- Ahmad E, Steinberg SM, Goldin L, Hess CJ, Caporaso N, et al. (2008) Immunophenotypic Features Distinguishing Familial Chronic Lymphocytic Leukemia From Sporadic Chronic Lymphocytic Leukemia. Cytometry B Clin Cytom. 74: 221-226.
- Kampalath B, Barcos MP, Stewart C. (2003) Phenotypic Heterogeneity of B Cells in Patients with Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. Am J Clin Pathol. 119: 824-832.
- Rawstron AC, Böttcher S, Letestu R, Villamor N, Fazi C, et al. (2013) Improving Efficiency and Sensitivity: European Research Initiative in CLL (ERIC) Update on the International Harmonised Approach for Flow Cytometric Residual Disease Monitoring in CLL. Leukemia. 27: 142-149.
- Ghia P, Hallek M. (2014) Management of Chronic Lymphocytic Leukemia. Haematologica. 99: 965-972.
- Rawstron AC, Kreuzer KA, Soosapilla A, Spacek M, Stehlikova O, et al. (2018) Reproducible Diagnosis of Chronic Lymphocytic Leukemia by Flow Cytometry: An European Research Initiative on CLL (ERIC) & European Society for Clinical Cell Analysis (ESCCA) Harmonisation Project. Cytometry B Clin Cytom. 94: 121-128.
- Flores-Montero J, Sanoja-Flores L, Paiva B, Puig N, GarcíaSánchez O, et al. (2017) Next Generation Flow for Highly Sensitive and Standardized Detection of Minimal Residual Disease in Multiple Myeloma. Leukemia. 31: 2094-2103.
- Van der Velden VHJ, Hochhaus A, Cazzaniga G, Szczepanski T, Gabert J, et al. (2003) Detection of Minimal Residual Disease in Hematologic Malignancies by Real-Time Quantitative PCR: Principles, Approaches, and Laboratory Aspects. Leukemia. 17: 1013-1034.
- Scrideli CA, Assumpção JG, Ganazza MA, Araújo M, Toledo SR, et al. (2009) A Simplified Minimal Residual Disease Polymerase Chain Reaction Method at Early Treatment Points Can Stratify Children With Acute Lymphoblastic Leukemia Into Good and Poor Outcome Groups. Haematologica. 94: 781-789.
- Berger A, Braner J, Doerr HW, Weber B. (1998) Quantification of Viral Load: Clinical Relevance for Human Immunodeficiency Virus, Hepatitis B Virus and Hepatitis C Virus Infection. Intervirology. 41: 24-34.
- Uhrmacher S, Erdfelder F, Kreuzer KA. (2010) Flow Cytometry and Polymerase Chain Reaction-Based Analyses of Minimal Residual Disease in Chronic Lymphocytic Leukemia. Adv Hematol. 2010: 1-10.
- Fürstenau M, De Silva N, Eichhorst B, Hallek M. (2019) Minimal Residual Disease Assessment in CLL: Ready for Use in Clinical Routine? HemaSphere. 3: e287.
- Schmittgen TD, Livak KJ. (2008) Analyzing Real-Time PCR Data by the Comparative CT Method. Nature Protocols. 3: 1101-1108.
- Frazzi R, Bizzarri V, Albertazzi L, Cusenza VY, Coppolecchia L, et al. (2020) Droplet Digital PCR is a Sensitive Tool for the Detection of TP53 Deletions and Point Mutations in Chronic Lymphocytic Leukaemia. British Journal of Haematology. 189: e49-e52.
- Heather JM, Chain B. (2016) The Sequence of Sequencers: The History of Sequencing DNA. Genomics. 107: 1-8.
- Akintunde O, Tucker T, Carabetta VJ. (2023) The Evolution of NextGeneration Sequencing Technologies. ArXiv.
- Wu D, Emerson RO, Sherwood A, Loh ML, Angiolillo A, et al. (2014) Detection of Minimal Residual Disease in B Lymphoblastic Leukemia by High-Throughput Sequencing of IGH. Clin Cancer Res. 20: 45404548.
- Thompson PA, Srivastava J, Peterson C, Strati P, Jorgensen JL, et al. (2019) Minimal Residual Disease Undetectable by NextGeneration Sequencing Predicts Improved Outcome in CLL After Chemoimmunotherapy. Blood. 134: 1951-1959.
- Hengeveld PJ, van der Klift MY, Kolijn PM, Davi F, Kavelaars FG, et al. (2023) Detecting Measurable Residual Disease Beyond 10⁻⁴ by an IGHV Leader-Based NGS Approach Improves Prognostic Stratification in CLL. Blood. 141: 519-528.
- Molica S, Giannarelli D, Montserrat E. (2019) Minimal Residual Disease and Survival Outcomes in Patients With Chronic Lymphocytic Leukemia: A Systematic Review and Meta-Analysis. Clin Lymphoma Myeloma Leuk. 19: 423-430.
- Santacruz R, Villamor N, Aymerich M, Martínez-Trillos A, López C, et al. (2014) The Prognostic Impact of Minimal Residual Disease in Patients With Chronic Lymphocytic Leukemia Requiring First-Line Therapy. Haematologica. 99: 873-880.
- Rodríguez-Vicente AE, Bikos V, Hernández-Sánchez M, Malcikova J, Hernández-Rivas JM, et al. (2017) Next-Generation Sequencing in Chronic Lymphocytic Leukemia: Recent Findings and New Horizons. Oncotarget. 8: 71234-71248.
- Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, et al. (2018) iwCLL Guidelines for Diagnosis, Indications for Treatment, Response Assessment, and Supportive Management of CLL. Blood. 131: 2745-2760.
- Iyer P, Wang L. (2023) Emerging Therapies in CLL in the Era of Precision Medicine. Cancers. 15: 1583.
- Karr M, Roeker L. (2023) A History of Targeted Therapy Development and Progress in Novel-Novel Combinations for Chronic Lymphocytic Leukemia (CLL). Cancers (Basel). 15: 1-13.
- Munir T, Brown JR, O’Brien S, Barrientos JC, Barr PM, et al. (2019) Final Analysis from RESONATE: Up to Six Years of Follow-Up on Ibrutinib in Patients With Previously Treated Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma. Am J Hematol. 94: 13531363.
- Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, et al. (2015) Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med. 373: 2425-2437.
- Shanafelt TD, Wang XV, Hanson CA, Paietta EM, O’Brien S, et al. (2022) Long-Term Outcomes for Ibrutinib-Rituximab and Chemoimmunotherapy in CLL: Updated Results of the E1912 Trial. Blood. 140: 112-120.
- Heltai S, Ghia P, Scarfò L. (2019) Relevance of Minimal Residual Disease in the Era of Targeted Agents. Cancer J. 25: 410-417.
- Moia R, Patriarca A, Schipani M, Ferri V, Favini C, et al. (2020) Precision Medicine Management of Chronic Lymphocytic Leukemia. Cancers (Basel). 12
- Thompson PA, Tam CS, O’Brien SM, Wierda WG, Stingo F, et al. (2016) Fludarabine, Cyclophosphamide, and Rituximab Treatment Achieves Long-Term Disease-Free Survival in IGHV-Mutated Chronic Lymphocytic Leukemia. Blood. 127: 303-309.
- Shanafelt TD, Wang XV, Kay NE, Hanson CA, O’Brien S, et al. (2019) Ibrutinib-Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N Engl J Med. 381: 432-443.
- Fraser G, Cramer P, Demirkan F, Santucci Silva R, Grosicki S, et al. (2019) Updated Results from the Phase 3 HELIOS Study of Ibrutinib, Bendamustine, and Rituximab in Relapsed Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. Leukemia. 33: 969-980.
- Eichhorst B, Niemann CU, Kater AP, Fürstenau M, von Tresckow J, et al. (2023) First-Line Venetoclax Combinations in Chronic Lymphocytic Leukemia. N Engl J Med. 388: 1739-1754.
- Hillmen P, Rawstron AC, Brock K, Muñoz-Vicente S, Yates FJ, et al. (2019) Ibrutinib Plus Venetoclax in Relapsed/Refractory Chronic Lymphocytic Leukemia: The CLARITY Study. J Clin Oncol. 37: 27222729.
- Eichhorst B, Niemann CU, Kater AP, Fürstenau M. (2021) A Randomized Phase III Study of Venetoclax-Based Time-Limited Combination Treatments (RVe, GVe, GIVe) Vs Standard Chemoimmunotherapy (CIT: FCR/BR) in Frontline Chronic Lymphocytic Leukemia (CLL) of Fit Patients: First Co-Primary Endpoint Analysis of the International Intergroup GAIA (CLL13) Trial. Blood. 138: 71.
- Tam CS, Allan JN, Siddiqi T, Kipps TJ, Jacobs R, et al. (2022) FixedDuration Ibrutinib Plus Venetoclax for First-Line Treatment of CLL: Primary Analysis of the CAPTIVATE FD Cohort. Blood. 139: 32783289.
- Fürstenau M, Giza A, Weiss J, Kleinert F, Robrecht S, et al. (2024) Acalabrutinib, Venetoclax, and Obinutuzumab in Relapsed/Refractory CLL: Final Efficacy and ctDNA Analysis of the CLL2-BAAG Trial. Blood. 144: 272-282.
- Fürstenau M, Robrecht S, Schneider C, Tausch E, Giza A, et al. (2023) MRD-Guided Zanubrutinib, Venetoclax and Obinutuzumab After an Optional Debulking With Bendamustine in Patients With Relapsed/ Refractory Chronic Lymphocytic Leukemia: Primary Endpoint Analysis of the Phase 2 CLL2-Bzag Study. Blood. 142: 1897.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.