Clinical Study of Tubeless Thoracoscopic Surgery via Subxiphoid Approach for Anterior Mediastinal Tumors Under the ERAS Concept
by Ran Xiong1, Hongjie Zhang2, Lei Gao1, Guangwen Xu1, Juan Li1*
1Department of Thoracic Surgery, the First Affiliated Hospital of University of Science and Technology of China, Hefei, Anhui province, China
2Department of Thoracic Surgery, People’s Hospital of Taihe County, Anhui province, China
*Corresponding author: Juan Li, Department of Thoracic Surgery, the First Affiliated Hospital of University of Science and Technology of China, Hefei, Anhui province, China
Received Date: 09 December 2024
Accepted Date: 13 December 2024
Published Date: 16 December 2024
Citation: Xiong R, Zhang H, Gao L, Xu G, Li J (2024) Clinical Study of Tubeless Thoracoscopic Surgery via Subxiphoid Approach for Anterior Mediastinal Tumors Under the ERAS Concept. J Surg 9: 11209 https://doi.org/10.29011/2575-9760.011209
Abstract
Objective: To evaluate the safety, reliability and superiority of tubeless thoracoscopic surgery via subxiphoid approach in the treatment of anterior mediastinal tumors under the guidance of Enhanced Recovery After Surgery (ERAS).
Methods: Clinical data of 51 patients who underwent subxiphoid thoracoscopic mediastinal tumor resection were collected in the Department of Thoracic Surgery of the First Affiliated Hospital of University of Science and Technology of China from September 2023 to September 2024, The study involved two groups: tubeless and control group.The intraoperative and postoperative indexes were compared between the two groups.
Results: All 51 patients were cured and discharged from hospital. Tubeless group was significantly better than control group in terms of postoperative waking time, incision pain on the first day after surgery, postoperative feeding time, postoperative hospital stay, hospitalization cost and quality of life 1 month after surgery (P<0.05). The incidence of postoperative sore throat and urinary irritation complications in tubeless group was significantly lower than that in control group (P<0.05), while anesthesia time, operative time, intraoperative blood loss, the incidence of complications of postoperative pulmonary infection, arrhythmia and delayed incision healing was not statistically significant between two groups (P>0.05).
Conclusion: Tubeless is safe and effective in the treatment of anterior mediastinal tumors via subxiphoid thoracoscopic surgery, which has unique advantages compared with traditional subxiphoid surgery, such as fewer postoperative catheter-related complications, faster recovery, and higher recent quality of life of patients.
Keywords: ERAS; Mediastinal tumor; Subxiphoid; Tubeless; Video-assisted thoracoscopic surgery
Introduction
Anterior mediastinal tumors are the most common among all mediastinal tumors. Most primary anterior mediastinal tumors are benign or low-grade malignant tumors, most of which are often accidentally found by chest CT scan, and a few of them are found by compression symptoms due to large tumors or combined with myasthenia symptoms. Once the anterior mediastinal tumor was found, surgical treatment is preferred [1]. At present, with the development of thoracoscopic technology, thoracoscopic surgery via subxiphoid approach has become the main surgical method for resection of anterior mediastinal tumors [1-3]. However, traditional subxiphoid thoracoscopic surgery usually requires general anesthesia with tracheal intubation, preoperative placement of urinary catheter, and postoperative placement of thoracic or mediastinal drainage tube, which will lead to an increase in the incidence of postoperative catheter-related complications, especially for high-risk patients with poor surgical tolerance, which will undoubtedly affect their postoperative recovery and increase their hospital stay and cost. Therefore, how to minimize the perioperative complications of patients with anterior mediastinal tumor surgery and further accelerate the recovery of patients is particularly important. Tubeless is based on the ERAS concept to reduce perioperative invasive procedures, thereby reducing complications and trauma associated with various catheters. In recent years, this technology has been gradually promoted and applied in some large medical centers. This study retrospectively analyzed the clinical data of 51 patients who underwent subxiphoid thoracoscopic resection of anterior mediastinal tumor in the First Affiliated Hospital of the University of Science and Technology of China in the past year, and discussed the clinical effect of tubeless thoracoscopic resection of anterior mediastinal tumor via subxiphoid approach.
Materials and Methods
Study Design and Patient Inclusion
Clinical data of 51 patients admitted to the Department of Thoracic Surgery, The First Affiliated Hospital of University of Science and Technology of China from September 2023 to September 2024 who underwent subxiphoid thoracoscopic resection of anterior mediastinal tumor were collected. Inclusion criteria: (1) Age ≤75 years; (2) No serious cardiopulmonary dysfunction or other serious complications; (3) Anterior mediastinal tumor was diagnosed by chest CT scan (4) No history of thoracic surgery; (5) The boundary between the tumor and peripheral blood vessels and organs was clear, and there was no obvious enlarged lymph nodes in the mediastinum; (6) Tumor length ≤6cm; (7) No urinary system dysfunction, no persistent cough and sputum, no gastroesophageal reflux disease; (8) ASA≤Ⅲ were evaluated by the American Society of Anesthesiologists, and BMI < 35 kg/m2. Exclusion criteria: (1) Tumor invasion of adjacent organs (such as innominate vein, superior vena cava, main pulmonary artery, pericardium, etc.); (2) Severe obesity (BMI≥35kg/m2); (3) Coagulation dysfunction; (4) Accompanied by urinary system dysfunction; (5) Previous surgical history of anterior mediastinal tumors. Patients were divided into tubeless group and control group according to whether they were intubated, placed urinary catheter and mediastinal drainage tube. There were 20 patients in tubeless group, 9 males and 11 females, aged (51.3±11.6) years, who were given intravenous anesthesia with laryngeal mask before surgery, no urinary catheter was placed before surgery, and no mediastinal drainage tube was retained after surgery. There were 8 cases of bronchogenic cyst, 4 cases of thymic cyst, 1 case of thymic hyperplasia, 5 cases of simple thymoma, 1 case of thymoma with myasthenia gravis, and 1 case of thymic carcinoma. The control group (31 cases), including 14 males and 17 females, aged (52.4±9.8) years, underwent general anesthesia with tracheal intubation and placed urinary catheter and mediastinal drainage tube. There were 11 cases of bronchogenic cyst, 6 cases of thymic cyst, 1 case of thymic hyperplasia, 9 cases of simple thymoma, 2 cases of thymoma with myasthenia gravis, and 2 cases of thymic carcinoma. The study was approved by institutional ethics board of the first affiliated hospital of USTC and informed consent was taken from all the patients. All patients had signed the operation consent before the surgery. Patients with thymoma and myasthenia gravis were treated with drugs before surgical treatment, and surgery was performed after symptoms were controlled and stabilized.
Anesthesia Method
Patients in both groups were placed in supine position with open venous channels, and 0.3mg penehyclidine hydrochloride was injected intravenously 15min before anesthesia. Signs such as ECG, blood oxygen saturation, blood pressure, and body temperature were continuously monitored. Tubeless group was given intravenous anesthesia with laryngeal mask ventilation (Figure 1A) : Sufentanil 0.5ug /kg and etomidate 0.3mg /kg were used for anesthesia induction. After the drug took effect, I-gel laryngeal mask was placed, oxygen flow was set to 2-3 L/min, tidal volume of anesthesia machine was set to 6-8ml /kg, and respiratory rate was 12 times/min. The anesthesia was maintained by intravenous infusion of remifentanil 5-10 ug/(kg·h), propofol 2-4 mg/(kg·h) and sevoflurane 1%-2% inhalation. Sufentanil 5ug/ time and cisatracurium 2mg/time were added as needed during the operation. The control group was given general anesthesia by tracheal intubation: Anesthesia was induced by sufentanil 0.5ug/ kg, etomidate 0.3mg/kg, and cisatracurium 0.2mg/kg. After the drug took effect, single-cavity tracheal intubation was inserted under the guidance of visual laryngoscope. The tidal volume of the anesthesia machine was set at 6-8 ml/kg, positive end-expiratory pressure (PEEP) at 3 cm H2O, respiratory rate at 12 times/min. The anesthesia was maintained by intravenous infusion of remifentanil at 5-10ug/(kg·h), propofol at 2-4 mg/(kg·h), and sevoflurane at 1%2% inhalation. Sufentanil 5ug/time and cisatracurium 4mg/time were added as needed during the operation. After the operation, the patient’s consciousness, muscle tension and spontaneous breathing recovered, the patient could complete simple actions such as tongue extension and grasping according to the instructions, and accurately answer simple questions such as name, which means that the patient was judged to be completely awake, and the laryngeal mask or tracheal intubation could be removed. The Steward score reached 4 points and reached the standard of leaving the room, and the patient was transferred to the ward.
Surgical Technique
Both groups underwent thoracoscopic resection of anterior mediastinal tumor via subxiphoid approach. Patients were supine, legs as far apart as possible in a “human” shape, urinary atheter was placed after general anesthesia in control group. After routine disinfection and drape, the surgeon was located between the patient’s legs and the assistant was located on the right side of the patient. A 2-3m straight incision was made under xiphoid process as an observation hole, and the space between the posterior sternum and the pericardium was expanded with the index finger. A 10 mm trocar was placed, and CO2 at 8-12 mmHg was injected to establish an artificial pneumothorax. A 0.5cm transverse incision was made under the costal margin of the middle line of the clavicle on both sides, respectively, and a 5 mm trocar was placed. Whether to install a single suspended sternal retractor to lift the sternum to expand the poststernal operating space was determined according to the intraoperative requirements. First, the mediastinal pleura was opened on both sides to fully expose the the tumor and operative field. The thymus and peripheral adipose tissues were completely resected from the posterior sternum to the bilateral phrenic nerves and from the lower pole of the thyroid gland to the bilateral cardiophrenic angles. Bilateral phrenic nerve and left innominate vein should be protected during resection (Figure 1B). Finally, surgical specimens were removed with a specimen bag through the subxiphoid incision, the complete specimen resembles a “butterfly” (Figure 1C). After the wound was completely hemostatic, mediastinal drainage tube was placed through the observation hole, and after the anesthesiologist was instructed to aspirate sputum and fully inflate the lung to eliminate gas, each incision was sutured (Figure 1D). The entire surgical procedure and the scope of lesion resection were consistent with those described by Chen X [4]and Mitsuteru Y [5] respectively.
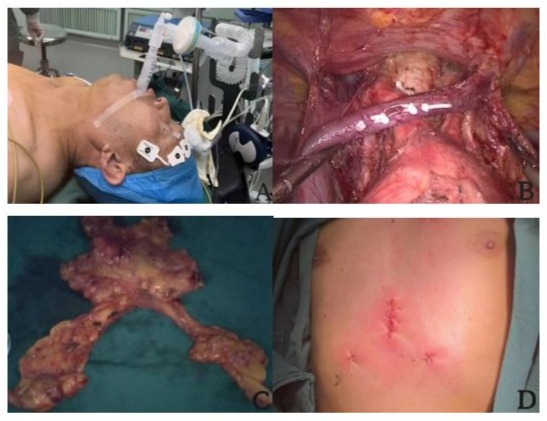
Figuer 1: A. laryngeal mask airway. B. Superior mediastinal wound presentation. C. Surgical specimen. D. Postoperative incision display.
Clinical Efficacy Evaluation Indicators
Intraoperative Observation Indicators: (1) Operative time; (2) Anesthesia time; (3) Operative blood loss.
Postoperative Observation Indicators: (1) Postoperative waking time; (2) Postoperative feeding time; (3) Incision pain on the first day after surgery: evaluation was conducted according to the visual analogue scale (VAS)[6], allowing patients to select scores according to their subjective feelings, with a scale of 0(no pain) to 10(extreme pain) for evaluation; (4) Postoperative hospital stay; (5) Total hospitalization cost; (6) Quality of life 1 month after surgery: the quality of life of patients 1 month after operation was assessed by SF-12 scale questionnaire; (7) Postoperative complications: postoperative urinary irritation symptom, postoperative sore throat, pulmonary infection, arrhythmia, delayed incision healing.
Statistical Analysis
SPSS21.0 statistical software was used for data analysis.
Measurement data were expressed as mean value with standard deviation. After normality test and variance homogeneity test, independent sample T-test was performed for measurement data with normal distribution and variance homogeneity between the two groups. The data with skewed distribution were tested by rank sum. Counting data were expressed as cases and percentage, and Chi-square test was used for comparison between groups. P<0.05 indicates that the data difference is statistically significant.
Results
Clinical Characteristics of the Patients
The clinical characteristics of the included patients are shown in Table 1. There were no deaths and serious complications in both groups during perioperative period, and all patients were discharged successfully after surgery. There was no significant difference in the general clinical data between the two groups (P > 0.05).
|
Variable |
Tubeless group (n=20) |
Contol group (n=31) |
P |
|
Age(year) |
51.3±11.6 |
52.4±9.8 |
0.701 |
|
Gender |
0.991 |
||
|
Male |
9 |
14 |
|
|
Femal |
11 |
17 |
|
|
BMI(kg/m2) |
25.2±3.3 |
25.0±3.2 |
0.902 |
|
Myasthenia gravis |
1.000 |
||
|
Yes |
1 |
2 |
|
|
No |
19 |
29 |
|
|
ASA status class |
1.000 |
||
|
I |
0 |
0 |
|
|
II |
2 |
3 |
|
|
III |
18 |
28 |
|
|
Lesion length (cm) |
3.1±1.3 |
3.6±1.2 |
0.112 |
|
Pathology |
0.988 |
||
|
Bronchogenic cyst |
8 |
11 |
|
|
Thymic cyst |
4 |
6 |
|
|
Thymic hyperplasia |
1 |
1 |
|
|
Thymoma |
6 |
11 |
|
|
Thymic carcinoma |
1 |
2 |
Data are represented as mean±SD or number. ASA: American Standards Association.
Table 1: Clinical characteristics of patients with tubeless and control group.
Intraoperative Outcomes
The intraoperative outcomes of the two groups are shown in Table 2. No conversion to thoracotomy occurred during the operation. Anesthesia time (150.6±21.7 vs. 149.0±24.3 min), operation time (106.3±19.7 vs. 112.7±21.2 min) and operative blood loss (13.0±5.2 vs. 13.2±5.7 ml) were compared between tubeless and control groups,the difference was not statistically significant (P > 0.05).
|
Variable |
Tubeless group (n=20) |
Contol group (n=31) |
P |
|
Conversion thoracotomy |
0 |
0 |
- |
|
Anesthesia time(min) |
150.6±21.7 |
149.0±24.3 |
0.816 |
|
Operative time(min) |
106.3±19.7 |
112.7±21.2 |
0.282 |
|
Operative blood loss (ml) |
13.0±5.2 |
13.2±5.7 |
0.887 |
Data are represented as mean±SD or number.
Table 2: Intraoperative index comparison between tubeless and control group.
Postoperative Outcomes
Postoperative outcomes between the two groups are shown in Table 3. Compared with the control group, In the tubeless group, postoperative waking time (12.7±2.5 vs. 26.0±6.0 min), postoperative feeding time (3.2±0.4 vs. 6.9±0.7 hour), postoperative incision pain VAS score on the first day after surgery (1.7±0.5 vs. 3.2±0.8), postoperative hospital stay (2.3±0.5 vs. 3.5±0.9 days), hospitalization cost (15654.3±1020.1 vs. 18112.3±1542.8 CNY) and quality of life 1 month after surgery (90.5±5.8 vs. 71.6±5.6) had obvious advantages, and the difference between them was statistically significant (P < 0.05).
|
Variable |
Tubeless group (n=20) |
Contol group (n=31) |
P |
|
Postoperative awaking time (min) |
12.7±2.5 |
26.0±6.0 |
0.000 |
|
Postoperative feeding time(hour) |
3.2±0.4 |
6.9±0.7 |
0.000 |
|
Postoperative incision pain score |
1.7±0.5 |
3.2±0.8 |
0.000 |
|
Postoperative hospital stay (day) |
2.3±0.5 |
3.5±0.9 |
0.000 |
|
Hospitalization cost (CNY) |
15654.3±1020.1 |
18112.3±1542.8 |
0.000 |
|
Recent quality of life score |
90.5±5.8 |
71.6±5.6 |
<0.05 |
Data are represented as mean±SD. CNY: Chinese Yuan.
Table 3: Postoperative index comparison between tubeless and control group.
Postoperative Complications
The postoperative complications of the two groups were shown in Table 4. There was no significant difference in the complications of postoperative pulmonary infection, arrhythmia and delayed incision healing(P > 0.05). Compared with the tubeless group, the postoperative sore throat (0 vs. 29.0%) and urinary irritation symptom complications (0 vs. 25.8%) in the control group were significantly increased, and the difference was statistically significant (P < 0.05).
|
Variable |
Tubeless group (n=20) |
Contol group (n=31) |
P |
|
Pulmonary infection |
0 |
2 |
0.674 |
|
Arrhythmia |
0 |
3 |
0.410 |
|
Delayed incision healing |
1 |
2 |
1.000 |
|
Sore throat |
0 |
9 |
0.023 |
|
Urinary irritation symptoms |
0 |
8 |
0.038 |
Data are represented as number.
Table 4: Comparison of postoperative complications between tubeless and control group.
Discussion
In 2012, Suda applied the subxiphoid approach for mediastinal tumor resection for the first time[7]. At present, subxiphoid thoracoscopic surgery has been widely used in anterior mediastinal tumor resection due to its advantages of clearer intraoperative visual field, wider tumor resection, avoidance of damage to intercostal nerve, and reduced impact on respiratory and circulatory function [3,8-10]. With the rapid development and popularization of ERAS concept, tubeless technique has been gradually popularized in most thoracic surgeries. In recent years, a small number of large medical centers in China have gradually promoted this technology, and we have also tried to apply tubeless technology to subxiphoid thoracoscopic anterior mediastinal tumor resection in the past two years. Tubeless is a new technology that fits the concept of ERAS. The term “Tubeless” generally refers to a thoracoscopic surgery performed under intraoperative spontaneous breathing anesthesia without the placement of urinary catheters and chest or mediastinal drainage tubes. Gonzalez-Rivas [11] believe that this concept even includes the absence of laryngeal mask. In a narrow sense, this term refers to video-assisted thoracoscopic surgery conducted under laryngeal mask anesthesia, eliminating the necessity for urinary catheters and chest or mediastinal drainage tubes.
General anesthesia with tracheal intubation can cause a variety of throat complications, and even lead to tracheal rupture and injury. In addition, tracheal intubation general anesthesia requires a large dose of anesthetic agents, which may cause an increase in postoperative nausea and vomiting, and a large amount of muscle relaxant residue may cause diaphragmatic paralysis. Anesthesia with nontracheal intubation avoided muscle relaxants, intubationrelated and mechanical ventilation-associated complications [12]. I-gel is a minimally invasive supraglottic ventilation device. Pressure controlled ventilation at moderate levels of airway pressure can be used as an appropriate alternative to tracheal intubation. Because the laryngeal mask does not enter the glottis and trachea, it can avoid the damage to vocal cords and trachea mucosa, reduce the occurrence of postoperative sore throat, laryngeal edema, vocal cord injury, laryngeal recurrent nerve paralysis, and avoid elevating the level of inflammatory factors in alveoli. Laryngeal mask intravenous anesthesia can avoid the stimulation of the sympathetic nerve around the trachea, help to maintain the stability of intraoperative hemodynamics, reduce the occurrence of cardiovascular accidents, and provide surgery opportunities for patients who can not tolerate tracheal intubation. In addition, laryngeal mask intravenous anesthesia can reduce the amount of anesthetic agents, alleviate anesthesia reaction, facilitate patients to eat as early as possible, and thus directly reduce anesthesia costs [13-16]. This study found that compared with the control group, patients in the tubeless group had significantly shorter time to wake up under anesthesia, significantly shorter time to eat in the early postoperative period, and significantly reduced the incidence of postoperative sore throat and discomfort symptoms.
Intraoperative indwelling urinary catheter may cause urethral injury, chronic infection, bladder stone and other urinary tract complications, and is not conducive to postoperative activities. Studies have found that the absence of urinary catheter before surgery can significantly reduce the incidence of postoperative hematuria and urinary tract infection, and significantly improve the comfort of patients’ first postoperative urination, which is more in line with the concept of ERAS [17]. This study found that the incidence of urinary irritation symptoms such as frequent urination, urgent urination, urodynia and urethral pruritus in the tubeless group was significantly lower than that in the control group. This is consistent with the research results of Zhou et al. [18].
The postoperative placement of chest or mediastinal drainage tubes primarily aims to facilitate the drainage of pneumothorax and pleural effusion, as well as to monitor changes within the mediastinum and thoracic cavity. However, the mechanical stimulation of the drainage tube against the skin and chest wall may intensify the patient’s pain and stress response. In addition, the friction between the drainage tube and the chest wall and pleura will increase inflammatory exudation, thus increasing the drainage flow of pleural effusion, prolonging the time of the catheter, and thus prolonging the postoperative hospital stay of patients. This study demonstrates that a tubeless approach without postoperative mediastinal drainage can significantly alleviate patient discomfort and pain, reduce pleural effusion exudate, promote earlier mobility and functional exercise post-surgery, accelerate recovery, shorten hospital stays, and lower hospitalization costs. Additionally, the reduction of postoperative discomfort symptoms can relieve psychological pressure on patients, leading to a notable improvement in their quality of life during the immediate postoperative period. The results of this study are consistent with those of Cui et al.[16,19]. An expert consensus proposed that any unnecessary use of the chest tube should be avoided [20].
This study has certain limitations and shortcomings: (1) it is a single-center retrospective analysis with a small sample size, highlighting the need for more multi-center prospective studies involving larger cohorts; (2) We did not perform propensity score matching due to the small sample size and because we wanted to present the real situations of thymectomy in our hospital; (3) Considering that the mutual interference between instruments may increase the difficulty of surgery and prolong the operation time, this study did not adopt the single-port thoracoscopic technology which may reduce traumatic. We look forward to conducting future research on tubeless subxiphoid single-port thoracoscopic resection of anterior mediastinal tumors; (4) Tubeless technology has higher requirements for surgical proficiency and comprehensive ability of the surgeon. The surgeon should perform tubeless surgery on the basis of proficiency in subxiphoid thoracoscopic mediastinal tumor resection. Therefore, tubeless subxiphoid thoracoscopic anterior mediastinal tumor resection is not available in all hospitals, which requires deep learning and repeated practice by the operator and may require a long learning curve.
Conclusions
In summary, Tubeless technique has safety, feasibility and significant advantages in the treatment of anterior mediastinal tumors by subxiphoid thoracoscopic surgery. Compared with traditional subxiphoid thoracoscopic surgery, it can further reduces the patient’s trauma and pain, accelerates the patient’s recovery speed, shortens the hospital stay, reduces hospital expenses, and is more in line with the principles of ERAS, with good clinical promotion significance. Nevertheless, the clinical efficacy requires further validation through prospective, multi-center studies.
Acknowledgments: This work was supported by grants from Anhui University Scientific Research Project Fund. Grant Number:
2024AH052055.
References
- Peng Kai-Ming, Kang Ming-qiang, Lin Ji-Hong(2018) Effect analysis of anterior mediastinal tumor treated by subxiphoid thoracoscopic surgery. Chinese Electronic Journal of Thoracic Surgery 5: 16.
- Fang W, Gu Z, Chen K (2018) Minimally invasive surgery in thymic malignances.Zhongguo FeiAi ZaZhi 21: 269-272.
- Gao L, Lu J, Shen Z, Chen H, Kang M (2021) A novel method of subxiphoid video-assisted thoracic surgery for thymectomy. Ann Transl Med 9: 1339.
- Chen X, Ma Q, Wang X(2020) Subxiphoid and subcostal thoracoscopic surgical approach for thymectomy.Surgical Endoscopy 35: 1-8.
- Yoshida M, Yuasa M, Kondo K, Tsuboi M, Kawakita N(2021) Evaluation of extended thymectomy approaches based on residual fat tissue. Interact Cardiovasc Thorac Surg 32: 250-255.
- Sung YT,Wu J (2018) The visual analogue scale for rating,ranking and Paired-Comparison(VAS-RRP):A new technique forpsychological measurement.Behav Res Methods 50: 1694-1715.
- Suda T, Sugimura H, Tochii D(2012) Single-port thymectomy through an infrasternal approach. Annals of Thoracic Surgery 93: 335.
- Numanami H, Yano M, Yamaji M(2018) Thoracoscopic Thymectomy Using a Subxiphoid Approach for Anterior Mediastinal Tumors. Ann Thorac Cardiovasc Surg 24: 65-72.
- Takashi S, Hisato I, Hiromitsu N(2020) Early outcomes in 147 consecutive cases of subxiphoid single-port thymectomy and evaluation of learning curves.European journal of cardio-thoracic surgery : official journal of the European Association for Cardiothoracic Surgery 58: i44-i49.
- Hao X, Dazhong L, Yi L (2020) The Outcomes of Subxiphoid Thoracoscopic Versus Video-Assisted Thoracic Surgery for Thymic Diseases. Journal of laparoendoscopic & advanced surgical techniques. Part 30: 508-513.
- Gonzalez-Rivas D, Yang Y, Guido W (2016) Non-intubated (tubeless) uniportal video-assisted thoracoscopic lobectomy. Ann Cardiothorac Surg 5: 15l-153.
- Pompeo E, Mineo D, Rogliani P (2004) Feasibility and Results of Awake Thoracoscopic Resection of Solitary Pulmonary Nodules.The Annals of Thoracic Surgery 78: 1761-1768.
- Liu J, Cui F, Li S (2015) Nonintubated Video-Assisted Thoracoscopic Surgery Under Epidural Anesthesia Compared With Conventional Anesthetic Option.Surgical Innovation 22: 123-130.
- Federico T, Eugenio P, Francesco S (2010) Surgical stress hormones response is reduced after awake video thoracoscopy.Interactive cardiovascular and thoracic surgery 10: 666-671.
- Boisen LM, Rao KV, Kolarczyk L (2017) The Year in Thoracic Anesthesia: Selected Highlights from 2016.Journal of Cardiothoracic and Vascular Anesthesia 31: 791-799.
- Weixue C, Danxia H, Hengrui L (2021) Tubeless video-assisted thoracoscopic surgery in mediastinal tumor resection.Gland surgery 10: 1387-1396.
- Ahmed AS, Clark BA, Joshi S(2020) Avoiding bladder catheters during atrial? fibrillation ablation. JACC Clin Electro physiol 6: 185-190.
- Zhou Junzheng, Xiao Dividend, Huai Qilin (2019) The clinical application of tubeless in the treatment of anterior mediastinal tumors with thoracoscopy through the subxiphoid approach in the elderly. Journal of Gerontology and Health Care 27: 500-504.
- Fei C, Jun L, Shuben L (2016) Tubeless video-assisted thoracoscopic surgery (VATS) under non-intubated, intravenous anesthesia with spontaneous ventilation and no placement of chest tube postoperatively.Journal of thoracic disease 8: 2226-2232.
- Jianxing H, Jun L, Chengchu Z (2019) Expert consensus on tubeless video-assisted thoracoscopic surgery (Guangzhou). Journal of thoracic disease 11: 4101-4108.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.