Clinical Experience of Complete Pathological Response in MSI-H and PDL1 Positive Inoperable Gastric Cancer Treated with ICIs and Chemotherapy: Two Case Reports
by Lucchetti Jessica, Di Giacomo Emanuela*, Polito Mariam Grazia*, Nitti Daniele, Angotti Lorenzo, Lo Prinzi Federica, Barnini Giulia, Galbato Muscio Luca, Pezzola Giorgia, Di Lascio Claudia, Vincenzi Bruno, Tonini Giuseppe
Medical Oncology, University Biomedical Campus, Rome, Italy
*Corresponding author: Di Giacomo Emanuela and Polito Mariam Grazia University Biomedical Campus, Rome, Italy
Received Date: 29 August 2024
Accepted Date: 02 September 2024
Published Date: 04 September 2024
Citation: J. Lucchetti, E. Di Giacomo, MG Polito, D. Nitti, L. Angotti et al (2024) Clinical Experience of Complete Pathological Response in MSI-H and PDL1 Positive Inoperable Gastric Cancer Treated with ICIs and Chemotherapy: Two Case Reports. Ann Case Report. 9: 1960. https://doi.org/10.29011/2574-7754.101960
Abstract
Background: During last decades, Immunotherapy (IT) has become the gold standard treatment of many types of cancers. In gastrointestinal (GI) cancers, predictive factors of response to IT are high microsatellite instability (MSI-H) and programmed death ligand 1 (PDL1) expression. On the basis of the results from the phase 3 Checkmate 649, Nivolumab (anti-PD1) and chemotherapy (CT) is the new first line chemotherapy for advanced or metastatic gastric cancer (HER2-negative, PD-L1 CPS ≥5.
Case summary: Herein, we describe two clinical cases of patients affected by advanced gastric cancer (HER2-negative, PD-L1 CPS ≥5, MSI-H), treated with immune checkpoint inhibitor (ICI) and CT who reached pathological complete response.
Conclusion: MSI-H is a strong predictor of response to IT in GI cancers, also in the neoadjuvant/perioperative gastric cancer setting. We attend for confirmation by phase II infinity trial, maybe a non operative management is possible also for gastric cancer.
Keywords: Case Report; High-Microsatellite Instability; Programmed Death Ligand-1; Gastric Cancer; Immunotherapy; Gastrectomy.
Introduction
Recently, immune checkpoint inhibitors have represented an amazing revolution of standard of care for several solid tumours. Currently, immune checkpoint inhibitors in gastric cancer are limited to metastatic setting and for a restricted number of patients with programmed death ligand-1 (PD-L1) positive tumours or microsatellite instability tumours (MSI-H), that are the 22% of gastric cancers [1].
After diagnosis of metastatic gastric cancer, PD-L1 CPS score, MSI status and HER2 status are mandatory information to define drug therapy (Figure 1).
Currently, the accepted scoring system for the assessment of PDL1 expression is the combined positive score (CPS). In particular, the PD-L1 CPS score consists of the number of positive tumour cells, lymphocytes and macrophages, divided by the total number of viable tumour cells multiplied by 100. The convincing rationale of efficacy in the use of immunotherapy in these patients could be related to the presence of lymphocytes.
In a retrospective cohort study it was found that EBV positivity and high tumour mutational burden (TMB) were associated with higher PDL-1 expression, and consequently with “inflamed microenvironment”. In addition, tumours located in the fundus and cardia had more frequently higher PD-L1 expression.
The PDL-1 expression, as mentioned, is considered as a predictive biomarker for response to ICI therapy. It could be also considered as a positive prognostic biomarker that correlates with overall survival [2,3].
In consideration of the poor prognosis of patients affected by gastric cancer, introduction of new strategies is essential. In this regard, several trials have explored the efficacy and safety of the use of immunotherapy in metastatic gastric cancer.
The Checkmate 649 trial enrolled 1581 patients and studied the role of immunotherapy in combination with chemotherapy in first line setting. In this study overall survival was 13.1 months in the combination group and 11.1 months in the chemotherapy group (HR of 0.71). In particular, nivolumab led to an improvement in PFS (progression free survival) in patients with PD-L1 CPS ≥5, with a median PFS of 7.7 months versus 6.05 months in the chemotherapy group. In summary, the study demonstrated superior OS, along with PFS benefit, with an acceptable safety profile [4].
From this evidence, currently, in first line setting nivolumab is approved in combination with Platinum-fluoropyrimidine doublet chemotherapy in case of a PD-L1 CPS ≽5. While the role of immunotherapy in metastatic setting is clear, several trials investigated the efficacy of PD-1 inhibitors in other settings. The ATTRACTION-5 compared chemotherapy plus nivolumab with chemotherapy plus placebo in stage III gastric or gastroesophageal cancer after surgery. The relapse free survival (RFS) rates were 68.4% in the combination group and 65.3% in the chemotherapy group, so the addition of nivolumab failed to improve RFS [5].
A Japanese multicentre single arm phase I study evaluated Nivolumab monotherapy in patients with diagnosis of respectable gastric cancer (from stage I to stage III). 31 patients received two cycles of Nivolumab before surgery (23% of patients had MSI-H tumours and 64% had PDL-1 positive tumours). 30 patients received surgery, with 5 major partial responses and 1 complete response. 27 patient of 31 had R0 resection, and only one patient had treatment-related adverse event of grade 3-4 [6]. The recent phase II study NEONIPIGA investigated neoadjuvant approach with nivolumab plus ipilimumab (three months of therapy) in MSI-H resectable gastric cancer. 19% of patients had adverse events of grade 3-4. 29 patients of 32 underwent R0 gastrectomy, with 58.6% of complete responses. After surgery, patients received 9 cycles of adjuvant Nivolumab [7].
In a Chinese phase 2 trial standard chemotherapy was compared to a combination of camrelizumab (PD-1 inhibitor) and apatinib (VEGFR-2 tyrosine kinase inhibitor) combined plus chemotherapy (nab-paclitaxel and S-1). 106 patients were randomised to receive nab-paclitaxel and S-1 with or without camrelizumab and apatinib for three cycles. The combination group reached a significantly higher major pathological response (33% versus 17%) and RO resection rate (94.1% versus 81.1). The combination therapy was well tolerated with 33.3% of adverse events of grade 3-4 versus 26.4% in the chemotherapy alone group.
The subgroup analysis revealed a higher benefit of the combination treatment in patients with PDL-1 positive and MSI-H tumors [8].
In February 2023 a single-arm multi-cohort phase 2 trial (INFINITY) investigated the combination of tremelimumab and durvalumab in neoadjuvant setting in patients with MSI-H resectable gastric cancer. Among 15 patients recruited, 14 underwent surgery and 9 patients had a complete response (60%). 80% of patients had a major-complete pathological response and only 1 patient had disease progression. The treatment was welltolerated with grade 3 or more immune-related adverse events occurring only in 3 patients [9]. From this evidence it is clear the importance of investigating the effectiveness of immunotherapy in neoadjuvant/perioperative setting in selected patients.
|
Biomarkers in Advanced Gastric Cancer |
Molecular biology technique |
Positivity |
Frequency |
|
HER2: Human epidermal growth factor receptor 2 |
IHC - FISH |
IHC 3+ or FISH-positive |
6.0 - 29.5 % |
|
PDL- 1: a combined positive score (CPS) |
IHC |
CPS > 1-5 |
47.3–82.0% |
|
MMR: DNA mismatch repair system |
IHC – PCR - NGS |
MSI-H (high level of microsatellite instability) |
8-25% |
|
TMB: Tumor mutational burden |
NGS |
≥ 10 mut/Mb |
13-17% |
|
FGFR2: The fibroblast growth factor receptor |
NGS |
≥30 |
4-7.4% |
|
CLDN18.2: Claudin 18.2 |
IHC |
≥75% of tumor cells |
30-33% |
|
IHC: immunohistochemistry; FISH: fluorescence in situ hybridization; PCR: Polymerase Chain Reaction; NGS: Next Generation Sequencing |
|||
Figure 1: Molecular profiling for drug therapy.
Case Presentation
Herein, we discuss two clinical cases of patients affected by inoperable gastric cancer who received a combination of chemotherapy and PD-1 Inhibitor and who achieved complete pathologic response.
First clinical case
A 66-year-old female came to our observation in March 2023 with a diagnosis of unrespectable gastric cancer. In her clinical history, the following data were recorded: a papillary thyroid carcinoma operated and treated with radiotherapy in 2013, former smoker, no alcohol use/abuse, no cancer familiarity. In April 2023 she had an esophagogastroduodenoscopy. A gastric ulcer on the pyloric region and on the posterior wall of the stomach antrum was diagnosed and 12 fine-needle biopsies were taken (see Figure 2), the histological examination indicated tubular gastric adenocarcinoma, without finding of H. Pylori infection.
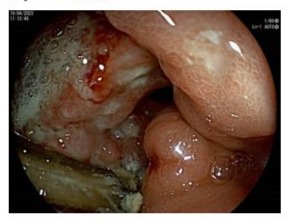
Figure 2: the gastric ulcer on the pyloric region and on the posterior wall of the stomach antrum during gastroscopy.
Immunohistochemistry (IHC) demonstrated: HER2 (-). The tumour was microsatellite unstable (MSI-H) and PD-L1-positive with a CPS score of 10 (measured with immunohistochemistry). At diagnosis, tumour markers (CEA, CA19.9, CA 125) were negative. Then she had a contrast-enhanced computed Tomography (CT) that showed gastric thickening and abdominal pathological lymphadenopathy (see Figure 3).
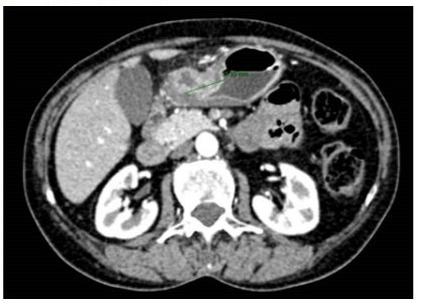
Figure 3: slice from staging CT scan.
We discussed the case during our weekly gastrointestinal Multidisciplinary Team (MDT), and we agreed with the proposal of first line therapy for advanced gastric cancer, which was defined “never radically operable” for abdominal lymph nodes. Therefore she started in June 2023 the first line of chemotherapy with Nivolumab 240 mg - mFOLFOX (Oxaliplatin 85 mg/mq, Calcio Levofolinato 200 mg/mq, 5-Fluorouracile 2800 mg/mq DT) , according to PDL1 CPS.
At the end of 7 cycles a CT scan described partial response of the gastric mass and lymph nodes. We therefore updated the case during our MDT and agreed with an exploratory laparoscopy with possible gastrectomy. In October 2023 she underwent a total gastrectomy with D2 lymphadenectomy and jejunojejunostomy, and the histological exam showed a pathological complete response and negative peritoneal washing. A postoperative CT scan performed after two months demonstrated that there was no tumour recurrence. The patient recovered well after surgery and resumed treatment with Nivolumab - mFolfox. We have planned 12 cycles of chemo-immunotherapy (7 before surgery and 5 after surgery). If no relapse will be noted, Nivolumab maintenance will be administered up to one year.
Second Clinical case
A 64-year-old female came to our observation in November 2023 with a diagnosis of unresectable gastric cancer.
In her clinical history the following data were recorded: never smoker, no alcohol use/abuse, no cancer familiarity.
Physical examination showed epigastric tenderness. Patient’s weight was 56 kg and her height was 156 cm. Also, no comorbidities were collected. A CT showed a mass of 4.5 cm of diameter indissociable from gastric antrum characterised by increased thickness and locoregional lymph node involvement (see Figure 4).
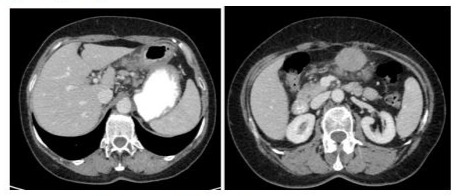
Figure 4: CT slices of the staging exam.
An upper gastrointestinal endoscopy was performed that resulted in a major macroscopic alteration in gastric antrum, 12 fine-needle biopsies were taken (see Figure 5). Histological examination described undifferentiated gastric adenocarcinoma, without finding of H. Pylori infection. Immunohistochemistry (IHC) demonstrated: HER2 (-). The tumour microenvironment (TME) of the patient was microsatellite instability (MSI-H) and PD-L1positive by IHC with a CPS score of 60.
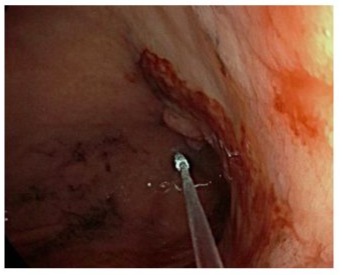
Figure 5: endoscopic view of diagnostic gastroscopy.
At diagnosis, tumour markers (CEA, CA19.9, CA 125) were negative. After diagnosis, we discussed the patient’s case during our multidisciplinary team meeting and decided to perform a diagnostic laparoscopy to exclude peritoneal involvement. The laparoscopy and peritoneal washing were negative and clinical TNM stage was defined as cT4bN3 with paracaval lymph nodes involved.
We therefore updated the case during our MDT with the laparoscopic information and agreed, all together, with the proposal of systemic therapy for advanced stomach cancer, defined by our surgery team as “never radically operable” for the paracaval lymph nodes involvement.
In December 2023, a first-line chemo-immunotherapy was started according to the CheckMate 649 regimen (Nivolumab plus oxaliplatin, fluorouracil and leucovorin), considering PDL1 CPS score, The cycles were well tolerated and no G3-4 adverse reactions were observed. Surprisingly, after the first two cycles of therapy the patient began to report less dysphagia and to gain weight. Eight cycles of treatment were performed, interspersed with a radiological check after the first 4 cycles, which showed reduction in the diameters of the gastric mass and lymph nodes (Figures 6&7).
At the end of 8 cycles, a CT scan demonstrated a major reduction of the primary tumour and lymph nodes, which pushed us to rediscuss the non-operability during our weekly multidisciplinary team: an exploratory laparoscopy with possible gastrectomy was thus scheduled. In March 2024 a laparoscopic subtotal gastrectomy with D2 lymphadenectomy and jejunojejunostomy was performed, without complications. Concurrently, the pathology review of his specimen revealed non-viable tumour cells identified in the primary tumour lesion as well as in all resected lymph nodes, that is pathological complete response (pCR).
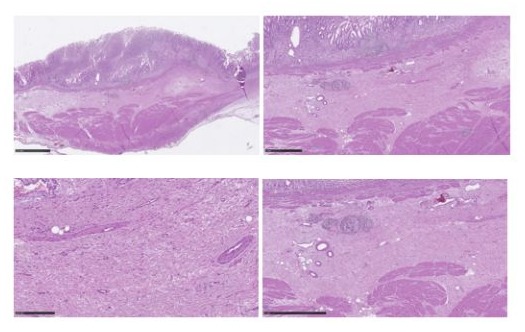
Figure 6: Mild chronic superficial inflammation with focal dysepithelialization and mucosal erosions, hyperemia, marked submucosal edema, ectasia and congestion vascular, stromal fibrosis; there is associated incomplete intestinal metaplasia and regenerative hyperplasia sometimes adenomatous glandular epithelium with low-grade dysplasia.
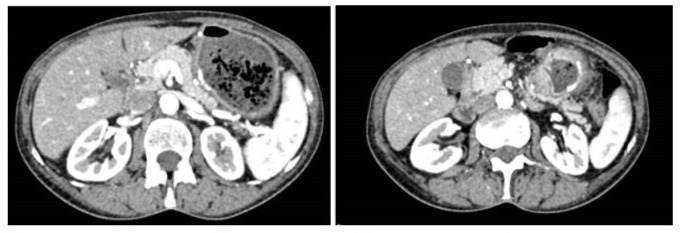
Figure 7: A postoperative CT scan performed after two months demonstrated that there was no tumour recurrence.
The patient recovered well after surgery and resumed treatment with Nivolumab 240 mg - mFOLFOX (Oxaliplatin 85 mg/mq, Calcio Levofolinato 200 mg/mq, 5-Fluorouracile 2800 mg/mq DT). We have planned 12 cycles of chemo-immunotherapy in total (8 before surgery and 4 after surgery), then, if no relapse will be registered, immunotherapy will be administered for up to one year.
Discussion
With the introduction of immunotherapy, the landscape of gastric cancer treatments has been changed. Immunotherapy has demonstrated efficacy in advanced gastric cancer, in particular in first line setting in combination with standard chemotherapy, but it has become necessary to explore the combination of chemotherapy and PD-1/PDL-1 inhibitors in other settings.
From several studies in the metastatic setting, we know that patients with positive PD-L1 expression and/or with MSI- high benefit from the addition of immunotherapy to standard chemotherapy. (CHK649)
Neoadjuvant chemotherapy has been widely used to improve R0 resection rates and event-free survival, but its efficacy is still limited to low pathological regressions. By combining immunotherapy or eventually target therapy in this setting, the outcomes could improve with an acceptable toxicity profile.
Based on preliminary data, the neoadjuvant immunotherapy obtains good responses, especially in “inflamed tumours”, characterised by MSI-H or PD-L1 positive status.
The challenge is to identify patients who may benefit from ICI in neoadjuvant setting. PD-L1 status has emerged as a potentially important predictive variable based on analysis of individual trials. The CPS score remains the strongest predictor of ICI benefit in gastric adenocarcinomas (after MSI-H status, limited by its low frequency) [10]. The current biomarkers are insufficient; the challenge is the integration of valid biomarkers.
As underlined from several studies, low tumour mutational burdens or mutations of PTEN are associated with an immunosuppressive environment [11]. Therefore, the in-depth analysis of genomic profiling could reveal the real impact of concomitant mutations in MSI-H gastric cancers and could help to select patients who benefit more from this treatment strategy. Microsatellite instability is a favourable prognostic factor and it is one of the strongest predictors of the efficacy of immunotherapy. As Infinity trial demonstrated, the activity of ICIs is even better in early stage disease and may also allow to avoid chemotherapy, radiotherapy, or even surgery.
Our little clinical experience can suggest that MSI-H and PDL-1 positive status could be predictive biomarkers for immunotherapy also in the perioperative setting, with the aim to improve response rates and event free survival in proper patients.
Another question is about the necessity of chemotherapy in combination with IC. The pre-existing “inflamed tumour environment “and the highly immunogenic profile associated with microsatellite instability could predict efficacy of immunotherapy alone. Chemotherapy has been also reported to have a negative impact in response in the case of MSI-H positive status, in particular to fluoropyrimidines, as demonstrated in stage III colorectal cancer [12]. Based on that, it is reasonable to assume that in these patients chemotherapy could play only a marginal role.
It is also important to study the possibility for patients who achieved radiological e/o clinical complete response to avoid surgery. In particular, patients who completely respond to neoadjuvant therapy might undergo non-operative management (NOM), in consideration of the short-term and long-term complications associated with gastrectomy like infections, Vitamin B12 deficiency, anaemia, reflux esophagitis or severe weight loss. In case of local recurrence salvage, surgery could be an option, but a prospective validation is imperative. From the evidence in other cancers such us rectal cancer, the strategy of NOM seems interesting but more studies are required.
Another question to investigate is the duration of adjuvant treatment in patients who undergo surgery. In other cancers like NSCLC, the validated duration of immunotherapy is 1-2 years. As demonstrated in the PACIFIC trial, a long duration of response is possible with 1 year of treatment with immunotherapy (in this particular case 1 year of durvalumab in stage III NSCLC whose disease had not progressed after chemo radiotherapy). It is reasonable to investigate a suspension of therapy after 1 year of treatment in case of stable disease, to limit adverse events connected to long-term treatment More clinical trials are needed to establish optimal duration of immunotherapy in adjuvant setting, in particular in case of no evidence of disease [13].
Conclusion
A phase II trial has demonstrated that immunotherapy is safe and can have eradicating activity in patients with MSI-high resectable gastric cancer. Immunotherapy is not yet the standard of care for this molecular subgroup of patients. Large and well-structured phase 3 studies are needed but the path is now clearer. There are many questions we still need to answer: what is the optimal combination of immune checkpoint inhibitors? How long should the treatment be? Finally, what should the objective of treatment be (neoadjuvant aim or organ preservation)?. Our small but impressive clinical experience seems to do nothing but confirm the value of microsatellite instability and PDL1 expression as major prognostic/predictive factors for immunotherapy in gastric cancer, in a perioperative setting.
Acknowledgments: No funding was received for this research.
Ethical Considerations: Patients were informed and allowed their data to be shared for scientific purposes. Informed consent for each patient was obtained.
Conflict of Interest: None.
References
- Cancer Genome Atlas Research Network. (2014) Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 513: 202-9.
- Shin M, Ahn S, Jung J, Hyung S, Kim KM, et al (2023) Impact of programmed death-ligand 1 (PD-L1) positivity on clinical and molecular features of patients with metastatic gastric cancer. Cancer Med. 12: 18633-18642.
- Liu X, Choi MG, Kim K, Kim KM, et al (2020) High PD-L1 expression in gastric cancer (GC) patients and correlation with molecular features. Pathol Res Pract. 216: 152881.
- Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, et al. (2021) First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and esophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 398: 27-40.
- Masanori Terashima et al. (2023) ATTRACTION-5: A phase 3 study of nivolumab plus chemotherapy as postoperative adjuvant treatment for pathological stage III (pStage III) gastric or gastroesophageal junction (G/GEJ) cancer. Journal of Clinical Oncology. 41:16.
- Hasegawa H, Shitara K, Takiguchi S, Takiguchi N, Ito S, et al. (2022) A multicenter, open-label, single-arm phase I trial of neoadjuvant nivolumab monotherapy for resectable gastric cancer. Gastric Cancer. 25:619-628.
- André T, Tougeron D, Piessen G, Fouchardiere CDL, Louvet C, et al. (2023) Neoadjuvant Nivolumab Plus Ipilimumab and Adjuvant Nivolumab in Localized Deficient Mismatch Repair/Microsatellite Instability-High Gastric or Esophagogastric Junction Adenocarcinoma: The GERCOR NEONIPIGA Phase II Study. J Clin Oncol. 41: 255-265.
- Lin JX, Tang YH, Zheng HL, Ye K, Cai JC, et al. (2024) Neoadjuvant camrelizumab and apatinib combined with chemotherapy versus chemotherapy alone for locally advanced gastric cancer: a multicenter randomized phase 2 trial. Nat Commun. 15:41.
- Pietrantonio F, Raimondi A, Lonardi S, Murgioni S, Cardillino GG, et al. (2023) INFINITY: A multicentre, single-arm, multi-cohort, phase II trial of tremelimumab and durvalumab as neoadjuvant treatment of patients with microsatellite instability-high (MSI) resectable gastric or gastroesophageal junction adenocarcinoma (GAC/GEJAC). Journal of Clinical Oncology. 41.
- Harry HY, Zhaohui J, Oudom K, Fonkoua L, Shitara K, et al (2022) Association of PD-L1 Expression and Other Variables with Benefit from Immune Checkpoint Inhibition in Advanced Gastroesophageal Cancer Systematic Review and Meta-analysis of 17 Phase 3 Randomized Clinical Trials. Jama Oncol. 8: 1456-1465.
- Chida K, Kawazoe A, Kawazu M, Suzuki T, Nakamura Y,et al. (2021) A Low Tumor Mutational Burden and PTEN Mutations Are Predictors of a Negative Response to PD-1 Blockade in MSI-H/dMMR Gastrointestinal Tumors. Clin Cancer Res. 27: 3714-3724.
- Cohen R, Taieb J, Fiskum J, Yothers G, Goldberg R, et al (2021) Microsatellite Instability in Patients With Stage III Colon Cancer Receiving Fluoropyrimidine With or Without Oxaliplatin: An ACCENT Pooled Analysis of 12 Adjuvant Trials. J Clin Oncol. 39: 642-651.
- Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D,et al. (2022) Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J Clin Oncol. 40: 1301-1311.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.