Challenging Management of Relapsed/Refractory Angioimmunoblastic T-Cell Lymphoma: Insights from Two Complex Cases with Long-Term Response to Belinostat
by Alberto Santagostino1*, Houyou Dyhia3, Daniela Gavril1, Gaetano Lucania1, Srimanta Misra1, Kinana Almohuanna1, Ibrahim Issa1, Valentina Guarino2
1Service d’Hématologie, Troyes Hospital Center, Troyes, France
2Pharmacie Centrale, Troyes Hospital Center, Troyes, France
3Unité de Recherche Clinique, Troyes Hospital Center, Troyes, France
*Corresponding author: Alberto Santagostino, Service d’Hématologie, Troyes hospital Center, Troyes, France
Received Date: 05 November 2024
Accepted Date: 11 November 2024
Published Date: 13 November 2024
Citation: Santagostino A, Dyhia H, Gavril D, Lucania G, Misra S, et al (2024) Challenging Management of Relapsed/Refractory Angioimmunoblastic T-Cell Lymphoma: Insights from Two Complex Cases with Long-Term Response to Belinostat. Ann Case Report. 9: 2059. https://doi.org/10.29011/2574-7754.102059
Abstract
Peripheral T-cell lymphomas (PTCLs) are rare and aggressive non-Hodgkin lymphomas associated with poor prognosis, marked by a rapid progression and high relapse rates. Current treatment strategies, such as standard CHOP (cyclophosphamide, doxorubicin, vincristine and prednisolone) chemotherapy, often fail to achieve long-term remission, and allogeneic stem cell transplantation (ASCT) is rarely feasible. Recent advancements include FDA-approved histone deacetylase inhibitors (HDACis), such as belinostat, which has shown promise in treating this aggressive hematological malignancy with a favourable safety profile. We present two clinical cases of relapsed/refractory (r/r) angioimmunoblastic T-cell lymphoma (AITL) - a subtype of PTCL - treated with belinostat. These cases illustrate the challenges of managing AITL, with belinostat achieving disease control for 13 and 14 months, respectively. Despite eventual progression and its related complications, belinostat demonstrated good tolerability and the potential to prolong survival and improve quality of life. These clinical results illustrate the complexity of treating high- risk PTCL patients and highlight the importance of personalized treatment strategies. In addition, those cases underline the role of vigilant monitoring and genetic profiling in optimizing therapeutic decisions. Belinostat represents a valuable option for patients with r/r AITL, potentially improving their quality of life and disease control. Further studies are needed to refine its role within treatment protocols for this challenging hematological malignancy.
Keywords: Relapsed/Refractory PTCL; AITL; Belinostat; HDAC Inhibitor; Long-Term Response.
Introduction
Peripheral T-cell lymphomas (PTCLs) are a heterogeneous and aggressive sub-group of non- Hodgkin lymphomas originating from mature T-cells. More than 25 subtypes of PTCL are characterized [1]. These malignancies are relatively rare, constituting roughly 10-15% of all non- Hodgkin lymphomas [2]. PTCL prognosis remains poor, with a 5-year overall survival (OS) rate about 30% [3]. This low survival rate accentuates the significant burden of the disease, as patients often experience rapid disease progression, frequent relapses, and severe treatment-related complications [4– 6].
For decades, the standard of care for newly diagnosed PTCL has been CHOP protocol (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CHOP- modified combination chemotherapy regimens such as CHOEP (CHOP + etoposide) [7]. Despite its widespread use, this approach often leads to poor outcomes and high relapse rates4. For eligible patients, allogenic stem- cell transplantation (allo-HSCT) may be considered, and the intensive nature of the treatment limits its applicability to a broader patient population [8]. Additionally, the high cost and limited effectiveness of these treatments pose substantial challenges for both patients and healthcare systems [9]. In recent years, the U.S. Food and Drug Administration (FDA) has granted approval to several new targeted therapies for treating relapsed/refractory (r/r) PTCLs. including the anti-folate agent pralatrexate and the CD30targeted drug-antibody conjugate brentuximab vedotin (BV) and the histone deacetylase inhibitors (HDACi) such as belinostat, romidepsin, and chidamide [10]. The 2024 updates to the National Comprehensive Cancer Network (NCCN) guidelines for the treatment of T-cell lymphomas also reflect these advancements, reinforcing the importance of developing evidence-based treatment strategies [11].
HDACis have emerged as promising therapeutic options for patients with r/r PTCL, in particular belinostat. It inhibits HDAC enzymes, which prevents the removal of acetyl groups from histones, leading to the accumulation of acetylated proteins that induce cell cycle arrest and then apoptosis in cancer cells [12]. Approved by the FDA in 2014 based on the outcomes of the BELIEF (CLN19) clinical trial study, belinostat led to an overall response rate (ORR) of 25.8% - including a complete response (CR) rate of 10.8% and a partial response (PR) rate of 15.0%. While maintaining a highly favourable safety profile [13]. Several clinical trials have demonstrated that belinostat provides a viable treatment option, with patients reporting better tolerance of side effects compared to traditional chemotherapies [14,15]. Belinostat represents a critical addition to the treatment arsenal for r/r PTCL, offering the potential for improved patient outcomes with this difficult-to-treat malignancy.
Here we present the challenges encountered in the management of r/r PTCL in two separate clinical cases.
Case Presentations
Patient 1
Medical History
In November 2018, a 75 year-old patient presented since January 2018 a range of symptoms, including painless diffuse submandibular and neck adenopathies, generalized itching (pruritus sine materia), fever, chills, and rhinitis. The patient has a complex medical history including a vertebrobasilar stroke of vascular origin, malaria with probable recurrence in 2013 treated with chloroquine, blindness in the right eye due to vascular occlusion of the central retinal artery, an intracardiac shunt, and laser-treated glaucoma. The itching intensified over time, contributing to the patient’s overall discomfort. A tonsil biopsy led to suspected T-cell lymphoma. A nodal biopsy was requested for confirmation, followed by a thoraco-abdomin-pelvic (TAP)CT scan performed in mid-November 2018. Results revealed multiple superficial and deep adenomegalies, including a 38 mm necrotic-looking adenomegaly at the hepatic pedicle, along with an enlarged, heterogeneous spleen.
Diagnostic Procedures
In November 2018 following the appearance of multiple cervical adenopathy, a cutaneous biopsy was performed, because histological artifacts rendered a strong suspicion of lymphoma with cutaneous involvement. A week later, a subsequent lymph node biopsy - in the context of lymph node involvement above and below the diaphragm, spleen, skin, and tonsils - examined a 2 cm lymphomatous left inguinal lymph node, with strong suspicion of PTCL. The analysis of the lymph node by the LYMPHOTPATH network at the Toulouse Oncopôle (France) suggested an angioimmunoblastic T-cell lymphoma (AITL); this was supported by the molecular biology results, which revealed a weak T-clone with Biomed 2 probes (TCR Gamma A). Additionally, next- generation sequencing (NGS) identified mutations in IDH2, RHOA, DNMT3A, and TET2, consistent with the molecular profile of an AITL. A PET scan performed at the end of November 2018 (Figure 1 A/B) showed numerous hyper metabolic adenopathies above and below the diaphragm, intense splenic hyper metabolism, and small subcutaneous hyper metabolic foci, suggesting high- grade lymphomatous disease with splenic involvement. The patient was subsequently diagnosed with PTCL/AITL. He was hospitalized at the end of November 2018 for treatment with CHOP plus etoposide (CHOEP) and additionally underwent a lumbar puncture for prophylaxis of central nervous system involvement.
Treatment and Outcome
The patient’s treatment began with eight courses of a CHOEP (CHOP + etoposide) chemotherapy regimen of etoposide (170mg), doxorubicin (50mg), vincristine (1.5mg) and cyclophosphamide (1,200mg) administered every 21 days from November 2018 to May 2019. During the initial hospitalization, the patient experienced complications, including a left pleural effusion treated by an evacuative thoracic puncture, a febrile neutropenia managed with antibiotics, and a disseminated intravascular coagulation (DIC) treated with a plasma transfusion. Despite those complications, the patient showed a favourable response to the initial CHOEP cycles, with the disappearance of superficial lymph node and skin lesions. The patient underwent subsequent CHOEP courses between January and May 2019, resulting in a stable follow-up chest X-ray and a PET scan in February 2019, indicating a complete metabolic response (Figure 1C).
However, a follow-up PET scan performed in June 2019 showed a tumor progression (Deauville score 5, DS5), with the reappearance of a suspicious hyper metabolic left internal mammary adenopathy and the emergence of new hyper metabolic lung lesions (Figure 2A). An inguinal adenopathy biopsy did not reveal any tumor cells, but a biopsy of the lung lesion showed an interstitial lymphoid infiltrate, leaving the diagnosis unclear between a reactive/ inflammatory process or a T-cell lymphoma.
Following further confirmatory biopsies, treatment with DHAOx (dexamethasone [40mg], cytarabine [2,500mg] and oxaliplatin [170mg] administered every 3 weeks) was initiated in August 2019. A bone marrow biopsy conducted in July 2019 showed negative results for lymphoma infiltration. Following the fourth dose of DHAOx, a PET scan in November 2019 showed complete regression of the lymphomatous lesions but revealed new mildly to moderately hyper metabolic adenopathies and pulmonary nodules (standardized uptake value SUVmax=3). This led to suspicion of a lymphoproliferative origin; however, an infective origin was not ruled out. An antibiotic treatment was administered and the treatment with DHAOx was continued for two additional courses (6 courses in total).
Following DHAOx treatment, a PET scan conducted at the end of January 2020 indicated a significant tumor progression of pre-existing parenchymal condensations and the appearance of suspicious, new areas of bilateral intensely hyper metabolic pseudo nodular parenchymal condensations predominantly involving the right pulmonary parenchyma (Figure 2B). A malignant progression was also found on the right inferior latero-tracheal adenopathy with the appearance of a mediastinal adenopathy but no abdomin-pelvic adenopathy. Overall, the pulmonary lymphomatous involvement was confirmed (DS 5).
In February 2020, the patient was administered romidepsin - a non-pan class I HDACi – monthly, which led to a rapid disappearance of the superficial adenopathy after the first infusion. A subsequent PET scan performed after the second infusion in April 2020 indicated rapid relapse with new supra-diaphragmatic adenopathies, particularly in the right cervical, axillary and mediastinal regions (DS 5), but a complete metabolic regression of pulmonary lesions (Figure 3A).
Belinostat (1,000mg/m2) was started in May 2020 as fourth line treatment, for a total of 13 consecutive cycles of 21 days. A PET scan performed in July 2020 showed a complete metabolic response in most hyper metabolic adenopathies, but persistent, less active lymph nodes and a KI67 protein of roughly 25% (Figure 3B). In light of the favourable partial response, the treatment was continued.
A subsequent PET scan in March 2021 indicated a metabolic progression with intensely hyper metabolic bilateral cervical adenopathies (DS 5). A biopsy of skin lesions revealed a dense lympho-histiocytic infiltrate whose appearance and phenotype were not suggestive of a known AITL but a histiocytic variant of Sweet syndrome.
Despite the progression of cervical adenopathy, the patient was clinically stable with small, fluctuating adenopathies and the decision was made to continue treatment.
In July 2021, a blood work revealed a significant increase in leukocytes (73.9 G/L) with an increase in monocytes (40.6 G/L). A myelogram revealed a significant blastic infiltration (averaging 66%) in the bone marrow composed of a mix of blasts of undifferentiated and monoblastic appearance, of medium to large size (with high to very high nuclear–cytoplasmic ratio) basophilic cytoplasm, agranular or with fine granulations, and no Auer bodies. There was an absence of megakaryocytes and erythroblasts, a contingent of mature monocytes (18%), and an overall cytological appearance consistent with acute myeloid leukemia (AML) with monoblastic features.
A karyotype analysis from July 2021 showed a clone with 48 and 49 chromosomes, including tetrasomy or pentasomy 21. Therefore, belinostat was discontinue due to the development of AML. The patient developed a sepsis, leading to death in September 2021.
Patient 2
Medical History and Diagnostic Procedures
In July 2020, a 75-year old patient was suspected to have AITL and had prior complex medical history that included a diagnosis of adenocarcinoma of the middle rectum, which was treated with 50.4 Gy (Gray unit) of radiation in 28 fractions.
A PET scan performed in July 2020 indicated extensive lymphomatous involvement, prompting further diagnostic procedures (Figure 4A). An axillary lymph node biopsy showed T-cell lymphoma, characterized by CD5+ and CD20- immunohistochemistry, with residual lymphoid follicles highlighted by anti-Bcl2 and CD23 studies. An immunohistochemistry review of the axillary lymph node, conducted by the reference Center at Henri Mondor University Hospital in Paris (France), indicated a diagnosis of AITL. An NGS-analysis further identified mutations in the TET2 gene, supporting the AITL phenotype.
Treatment and Outcome
A six-cycle regime of mini-CHOP chemotherapy (doxorubicin [47mg], vincristine [1.50mg], and cyclophosphamide [650mg]) was initiated in August 2020. After the first three cycles of treatment, a reassessment PET scan performed in October 2020 showed a complete metabolic response (DS2) (Figure 4B). Upon treatment completion, a follow-up PET scan performed in February 2021 showed a complete metabolic response with a persistent DS2. An additional PET scan performed in July 2021 showed a stable response (DS2).
In January 2022, the patient came to the emergency department with abdominal pain from a strangulated hernia in linea alba and associated confusion. A computed tomography (CT) scan indicated a tumor progression according to Cheason criteria, with the appearance of sub diaphragmatic adenomegaly and hepatosplenomegaly with multiple retroperitoneal and bilateral iliac adenopathies, particularly in the inter-aortic-caval region. In February 2022, the patient received six cycles of DHAOx treatments and four weekly treatments of rituximab (375mg/m²) for an autoimmune hemolytic anemia.
In January 2023, a PET scan revealed a metabolic relapse in the supra-diaphragmatic lymph nodes, along with new findings, including a hyper metabolic pulmonary nodule, pericardial effusion and small left pleural effusion (Figure 5A). The decision was made to begin belinostat to treat the progressing lymphoma (1,000mg/ m2), which began mid-February 2023; a second, identical dose was administered in March 2023.
A surveillance PET scan conducted in March 2023 found a significant regression in lymph node involvement and complete regression of the pericardial effusion (Figure 5B). However, a suspicious pulmonary nodule persisted, along with inflammatory pulmonary condensations. In April 2023, the patient continued belinostat treatment (1,000mg/m2 for cycles 3-5). A followup PET scan in June 2023 showed mixed results. While there was a continued regression in certain areas, a progression of metabolic activity was observed in other regions, including the appearance of new adenopathies and signs of infection such as fever, dyspnea and diarrhea. Belinostat was continued; however, the patient experienced a decline in general condition, leading to a hospitalization in December 2023 for infectious complications, associated to confusion and altered consciousness. The treatment of complications due to an infection led to clinical improvement.
The patient received belinostat treatments for a total of 14 cycles, (1,000mg/m2 for cycles 6-14), with the final dose received in March 2024. In April 2024, the patient was hospitalized because of fever, dyspnea, and signs of shock. A TAP-scan, blood cultures, and a cytobacteriological examination of the urine (CBEU) were performed revealing further disease progression, severe edema (grade 4), and active candida and cytomegalovirus (CMV) infections.
A TAP scan in April 2024 revealed multiple adenopathy, predominantly in the axillary and retroperitoneal regions, and a 12 mm excavated nodule in the right upper pulmonary lobe. The scan also revealed bi-basal pleural effusion, spleen enlargement (13 cm), and mild irregularities in the liver contours without suspicious lesions with evidence of anasarca, fluid accumulation in the abdomen, widespread soft tissue edema, and signs of small bowel obstruction. The CMV infection (viral load >3,000 copies/ ml) was treated with ganciclovir (dosage 5 mg /kg).
A PET scan performed in April 2024 showed progressive lymph node involvement, with new, moderately hypermetabolic polyadenopathy both above and below the diaphragm. However, the patient developed an acute kidney injury (AKI), was subsequently transferred to palliative care, and died in April 2024.
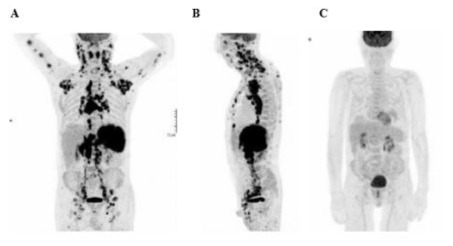
Figure 1: Patient 1: Diagnostic PET scan (A frontal/B lateral, end of November 2018) and control PET scan (C, February 2019).
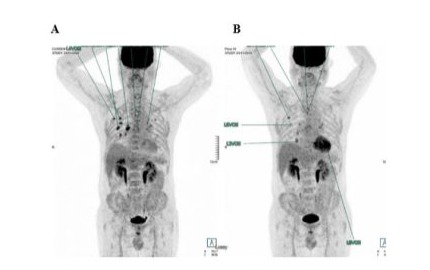
Figure 2: Patient 1: Comparison of PET scans before DHAOx (A, June 2019) and after 6 courses of DHAOx (B, January 2020).
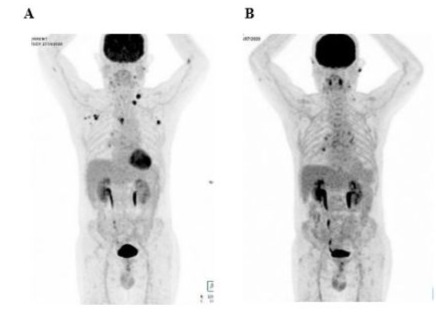
Figure 3: Patient 1: PET scans before (A, April 2020) and after (B, July 2020) belinostat treatment.
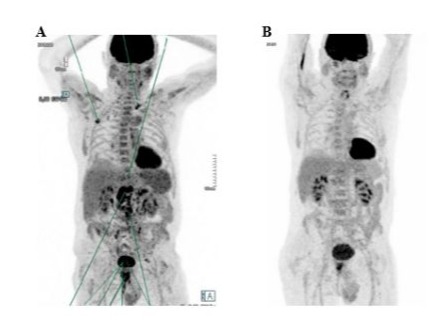
Figure 4: Patient 2: PET scans before (A, July 2020) and after (B, October 2020) CHOP chemotherapy.
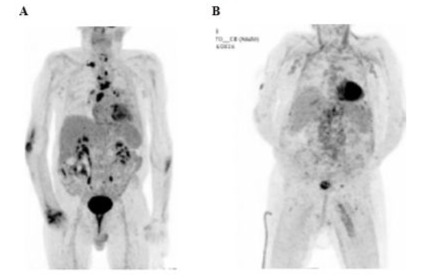
Figure 5: Patient 2: PET scan before (A, January 2023) and after (B, April 2023) belinostat start.
Discussion
We present two clinical cases of complex AITL, both treated with multiple therapeutic regimens, including belinostat as a salvage treatment. Patient 1 initially responded to CHOEP chemotherapy but relapsed multiple times, and at the last relapse developed one acute myeloid leukemia AML. Belinostat likely improved the patient’s condition for 14 months with very good tolerance and better quality of life. Patient 2, after relapsing post-mini-CHOP regimen, was treated with DHAOx followed by belinostat, resulting in 13 months of response with favourable tolerability. After periods of disease stabilization upon treatment with belinostat, both patients experienced a complication not related to the treatment, which led to their deaths.
The limited number of case reports on AITL highlights the rarity of such instances while underlining their importance [16,17]. AITL often leads to a fatal outcome in the short-term [18,19]; however, our cases suggest that belinostat, administered as third or fourth treatment line, may have prolonged life expectancy up to 14 months, with an unprecedented treatment duration and response. In a subset analysis of the BELIEF (CLN19) trial, after a median follow-up of 21.5 months, the median duration of response to belinostat was 13.6 months in 22 patients diagnosed with AITL20. To our knowledge, the only other example in the literature citing a sustained remission of AITL for one year was in a 73-year-old patient using alemtuzumab an unconjugated, nonmodulating, humanized monoclonal antibody targeting the CD52 antigen - despite its potential for highly toxic complications [19]. The poor long-term survival paired with a limited response rate of approximately 30% in r/r PTCL patients highlight the challenges of achieving durable remission with current treatment options [21].
Ghione et al. previously demonstrated that histone deacetylase inhibitors (HDACis) offer a more advantageous treatment for T follicular helper cells phenotype (TFH)-PTCL compared to nonTFH PTCL. Given the phenotypic and gene expression similarities between TFH-PTCL and AITL [22], HDACis are emerging as a preferred treatment option for AITL.
Following the withdrawal of romidepsin’s indication in May 2022 by the FDA due to the failure of its phase III trial in a first-line combination setting, belinostat remains the only approved HDAC inhibitor therapy for the treatment of r/r PTCL.
As a pan-class HDACi, belinostat offers a therapeutic potential in treating PTCL [13,23,24]. It has demonstrated effectiveness based efficacy [25-27], even in heavily pre-treated PTCL patients, as evidenced by two previous clinical cases. In fact, in two clinical cases previously published, belinostat not only provided effective treatment but also successfully bridged two heavily pre- treated AITL patients to allo-HSCT [16,17].
Of note, in newly diagnosed patients, belinostat combined with CHOP regimen has shown promising therapeutic efficacy, achieving an overall response rate (ORR) exceeding 85%; this highlights belinostat’s potential as a frontline treatment option [22].
Belinostat’s promising efficacy is complemented by a favourable safety profile. Notably, both patients in this report experienced minimal side effects and significant improvements in quality of life. Neither patient treated with belinostat experienced hematological or gastrointestinal side effects - except for one case of moderate renal toxicity - underscoring belinostat’s favourable tolerability. The progression to AML in Patient 1 and the recurrent relapses in Patient 2 emphasize the aggressive nature of certain PTCL subtypes and the increased risk of secondary malignancies, particularly in those with high-risk genetic profiles. These cases demonstrate the importance of a close monitoring and comprehensive genetic profiling which is crucial for guiding treatment decisions and anticipating complications [28,29], as evidenced by the genetic mutations identified (IDH2, RHOA, DNMT3A, and TET2) in Patient 1. Moreover, the findings reported here suggest that initiating belinostat therapy at an earlier stage of treatment, rather than in later stages, may optimize its therapeutic benefits, by improving outcomes and maximizing its potential in treating PTCL and AITL. Recent research into molecular subtypes of PTCL brings light to the need for precision medicine, which is increasingly recognized as essential in managing r/r PTCL where conventional treatments often fall short [30-32].
Conclusion
AITL is an aggressive hematological malignancy that give rise significant treatment challenges. Despite severe prognoses, belinostat treatment, as a critical early line option in both presented cases, showed considerable promise by extending survival and improving quality of life with a treatment duration and efficacy never attained before. These cases highlight once more the importance of ongoing research and tailored strategies to improve outcomes for patients with r/r PTCL.
Acknowledgments: The authors would like to thank Florence Boulmé, PhD, for writing assistance.
Disclosures
Competing interests: The authors have no conflicts of interest to report.
Informed consent: Not applicable.
Funding information: The publication of this work was sponsored by IDEOGEN AG (Switzerland).
Author contributions: All author commented on previous versions of the manuscript; subsequently, all of them read and approved the final manuscript.
References
- Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IB de O, et al. (2022) The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. 36:1720–48.
- Liu S, Liu W, Li H, Yang L, Song Y, et al. (2022) Epidemiological characteristics of peripheral T-cell lymphoma: A population-based study. Front Oncol. 12:863269.
- Savage KJ. (2007) Peripheral T-cell lymphomas. Blood Rev. 21:201– 16.
- Bellei M, Foss FM, Shustov AR, Horwitz SM, Marcheselli L, et al. (2018) The outcome of peripheral T-cell lymphoma patients failing first-line therapy: a report from the prospective, International T-Cell Project. Haematologica. 103:1191–7.
- Weiss J, Reneau J, Wilcox RA. (2023) PTCL, NOS: An update on classification, risk-stratification, and treatment. Front Oncol. 13:1101441.
- Mak V, Hamm J, Chhanabhai M, Shenkier T, Klasa R, et al. (2013) Survival of patients with peripheral T-cell lymphoma after first relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol Off J Am Soc Clin Oncol. 31:1970–6.
- Schmitz N, Trümper L, Ziepert M, Nickelsen M, Ho AD, et al. (2010) Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 116:3418-25.
- Suzuki K, Shimazu Y, Minakata D, Ikeda T, Takahashi H, et al. (2023) Efficacy of autologous stem cell transplantation for myeloma patients with suboptimal response: A multicenter retrospective analysis. Transplant Cell Ther. 29:688.e1-688.e13.
- Herrera DA, Kornblum N, Acuna-Villaorduna A, Sica RA, Shah U, et al. (2019) Barriers to allogeneic hematopoietic stem cell transplantation for human T-cell lymphotropic virus 1– associated adult T-cell lymphoma–leukemia in the United States: Experience from a large cohort in a major tertiary center. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 25:e199–203.
- Update on histone deacetylase inhibitors in peripheral T-cell lymphoma (PTCL). Clinical Epigenetics.
- Guidelines NCCN. 2024.
- Belinostat. In: LiverTox: Clinical and Research Information on DrugInduced Liver Injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
- O’Connor OA, Horwitz S, Masszi T, Van Hoof A, Brown P, et al. (2015) Belinostat in patients with relapsed or refractory peripheral T-cell lymphoma: Results of the pivotal phase II BELIEF (CLN-19) study. J Clin Oncol Off J Am Soc Clin Oncol. 33:2492–9.
- El Omari N, Bakrim S, Khalid A, Albratty M, Abdalla AN, et al. (2023) Anticancer clinical efficiency and stochastic mechanisms of belinostat. Biomed Pharmacother. 165:115212.
- Sawas A, Radeski D, O’Connor OA. (2015) Belinostat in patients with refractory or relapsed peripheral T-cell lymphoma: a perspective review. Ther Adv Hematol. 6:202.
- De Wilde S, Graux C. (2024) Complete hematologic response in a patient with multiple pretreated angioimmunoblastic T-cell lymphoma after belinostat therapy followed by allogeneic stem cell transplantation: A case report. Clin Case Rep. 12:e9159.
- Camus V, Etancelin P, Drieux F, Veresezan EL, Picquenot JM, et al. (2023) Complete hematologic response after belinostat treatment and allogeneic stem cell transplantation for multiple relapsed/refractory angioimmunoblastic T-cell lymphoma: A case report. Clin Case Rep. 11:e7623.
- Chiba S, Sakata-Yanagimoto M. (2020) Advances in understanding of angioimmunoblastic T-cell lymphoma. Leukemia. 34:2592–606.
- Halene S, Zieske A, Berliner N. (2006) Sustained remission from angioimmunoblastic T-cell lymphoma induced by alemtuzumab. Nat Clin Pract Oncol. 3:165–8.
- Sawas A, Ma H, Shustov A, Hsu P, Bhat G, Acosta M, et al. (2020) Characterizing the belinostat response in patients with relapsed or refractory angioimmunoblastic T-cell lymphoma. Leuk Lymphoma. 61:2003-7.
- Sibon D. (2022) Peripheral T-Cell Lymphomas: Therapeutic approaches. Cancers. 14:2332.
- Ghione P, Faruque P, Mehta-Shah N, Seshan V, Ozkaya N, et al. (2020) T follicular helper phenotype predicts response to histone deacetylase inhibitors in relapsed/refractory peripheral T-cell lymphoma. Blood Adv. 4:4640–7.
- Kim HJ, Bae SC. (2011) Histone deacetylase inhibitors: molecular mechanisms of action and clinical trials as anti-cancer drugs. Am J Transl Res. 3:166–79.
- Ho TCS, Chan AHY, Ganesan A. (2020) Thirty years of HDAC inhibitors: 2020 insight and hindsight. J Med Chem. 63:12460–84.
- Gimsing P, Hansen M, Knudsen LM, Knoblauch P, Christensen IJ, et al. (2008) A phase I clinical trial of the histone deacetylase inhibitor belinostat in patients with advanced hematological neoplasia. Eur J Haematol. 81:170–6.
- Plumb JA, Finn PW, Williams RJ, Bandara MJ, Romero MR, et al. (2003) Pharmacodynamic response and inhibition of growth of human tumor xenografts by the novel histone deacetylase inhibitor PXD101. Mol Cancer Ther. 2:721–8.
- Qian X, LaRochelle WJ, Ara G, Wu F, Petersen KD, et al. (2006) Activity of PXD101, a histone deacetylase inhibitor, in preclinical ovarian cancer studies. Mol Cancer Ther. 5:2086–95.
- Vega F, Amador C, Chadburn A, Hsi ED, Slack G, et al. (2022) Genetic profiling and biomarkers in peripheral T-cell lymphomas: current role in the diagnostic work-up. Mod Pathol. 35:306–18.
- Zhang Y, Lee D, Brimer T, Hussaini M, Sokol L. (2020) Genomics of peripheral T-cell lymphoma and its implications for personalized medicine. Front Oncol. 10:898.
- Huang YH, Qiu YR, Zhang QL, Cai MC, Yu H, et al. (2024) Genomic and transcriptomic profiling of peripheral T cell lymphoma reveals distinct molecular and microenvironment subtypes. Cell Rep Med. 5:101416.
- Watatani Y, Sato Y, Miyoshi H, Sakamoto K, Nishida K, et al. (2019) Molecular heterogeneity in peripheral T-cell lymphoma, not otherwise specified revealed by comprehensive genetic profiling. Leukemia. 33:2867–83.
- Misra SC, Lucania G, Guarino V, Santagostino A. (2023) The New Era for Advancements in peripheral T-cell lymphoma: A Review. J Blood Lymph. 13:1-9.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.