Central Retinal Detachment - Causes, Diagnosis and Management Based on A Case Series
by Agnieszka Kudasiewicz-Kardaszewska1*, Małgorzata Ozimek1, Karolina Bonińska2, Ferenc Kuhn1,3, Sławomir Cisiecki1,2*
1Prof. Zagórski Eye Surgery Center Nowy Sącz, Nałęczów, OCHO Medical Group, OCHO Nowy Sącz: Batorego 88, 33-300 Nowy Sącz, Poland
2Department of Ophthalmology, Jonscher Public Hospital, Milionowa 14, 93-113 Łódź, Poland
3Helen Keller Foundation, University of Alabama, Florida, USA
*Corresponding author: Agnieszka Kudasiewicz-Kardaszewska, Prof. Zagórski Eye Surgery Center Nowy Sącz, Nałęczów, OCHO
Medical Group, OCHO Nowy Sącz: Batorego 88, 33-300 Nowy Sącz, Poland; Sławomir Cisiecki, Department of Ophthalmology, Jonscher Public Hospital, Milionowa 14, 93-113 Łódź, Poland
Received Date: 14 February 2025
Accepted Date: 20 February 2025
Published Date: 22 February 2025
Citation: Kudasiewicz-Kardaszewska A, Ozimek M, Bonińska K, Kuhn F, Cisiecki S (2025) Central Retinal Detachment - Causes, Diagnosis and Management Based on A Case Series. J Surg 10: 11259 https://doi.org/10.29011/2575-9760.011259
Abstract
Introduction: Central Retinal Detachment (CRD) is a complex retinal disorder characterized by the separation of the neurosensory retina from the Retinal Pigment Epithelium (RPE) within the macular region. Unlike Rhegmatogenous Retinal Detachment (RRD), CRD occurs without a visible retinal break and is primarily caused by Vitreomacular Traction (VMT), epiretinal membrane (ERM) formation, or myopic degeneration. Myopic Tractional Maculopathy (MTM) is a major contributing factor in highly myopic eyes, particularly in those with a staphyloma, where the concave scleral contour exerts additional traction on the retina. The interplay between axial elongation, the stiffness of the Internal Limiting Membrane (ILM), and progressive retinal thinning in MTM contributes to structural instability and detachment. In cases of central posterior staphyloma, the combination of mechanical forces and reduced retinal adhesion further predisposes to detachment. However, CRD is not exclusive to myopic eyes and can also develop in non-myopic individuals due to progressive tractional forces caused by VMT and/or ERM contraction.
Material and Methods: We analysed a group of 15 patients who underwent pars plana vitrectomy due to central retinal (macular) detachment. The following examinations were conducted preoperatively and at 10 days, 1 month, 3 months, 6 months, and 3 years postoperatively:
- Best-Corrected Visual Acuity (BCVA)
- Fundoscopic examination
- Optical Coherence Tomography (OCT) imaging
Axial length was assessed in all eyes preoperatively. Additionally, staging of Myopic Tractional Maculopathy (MTM) and posterior staphyloma classification were evaluated in highly myopic cases. All patients underwent 23-gauge (23G) pars plana vitrectomy with ILM peeling and 20% Sulphur Hexafluoride (SF6) endotamponade. Follow-up assessments included BCVA measurement and evaluation of macular anatomical status.
Results: Preoperative examinations identified CRD causes as follows:
- VMT with ERM (8 eyes, 53.3%)
- Myopic degeneration with MTM and posterior staphyloma (5 eyes, 33.3%)
- VMT with Macular Neovascularisation (MNV) (2 eyes, 13.3%)
Axial length in myopic eyes ranged from 25.81 mm to 29.0 mm, whereas in non-myopic eyes, it ranged from 21.34 mm to 24.18 mm. MTM stage 3 with type 1 or 2 staphyloma was noted in the myopic subgroup. Surgical success was achieved in 13 eyes (86.7%). However, two highly myopic eyes required reoperation with silicone oil tamponade due to macular re-detachment three months after the initial CRD surgery. The macula remained attached following reoperation, even after subsequent silicone oil removal. Overall, BCVA improved in 10 eyes (66.7%), remained stable in 3 eyes (20%), and declined in 2 eyes (13.3%) over the 3-year follow-up.
Conclusions: Long-term observations suggest that Pars Plana Vitrectomy (PPV) with ILM peeling provides effective anatomical macular reattachment in CRD treatment. However, anatomical success does not always correlate with functional improvement. Cataract surgery in eyes with VMT syndrome may increase the risk of macular detachment, even in non-myopic individuals. VMT-related traction on the macula during ERM formation may cause CRD in both myopic and non-myopic eyes. A weakened RPE pump in myopic retinas is an additional factor contributing to a poor prognosis for retinal reattachment and potential complications, even after uneventful vitrectomy. While PPV with ILM peeling remains an effective treatment for CRD, it requires customised surgical approaches depending on the underlying aetiology. Highly myopic cases necessitate modified ILM peeling strategies due to retinal fragility, such as double staining or fovea-sparing peeling. Meanwhile, VMT-related cases benefit from traction release. Future studies should explore alternative surgical techniques, such as macular buckling, for advanced MTM cases.
Keywords: Central retinal detachment; Epiretinal membrane; ILM peeling; Macular Neovascularisation (MNV); Pars plana vitrectomy; Vitreomacular traction
Introduction
Central Retinal Detachment (CRD) is a complex ophthalmic condition characterised by the separation of the neurosensory retina from the Retinal Pigment Epithelium (RPE) in the macular region, while the paracentral and peripheral retina often remains attached [1]. Unlike Rhegmatogenous Retinal Detachment (RRD), where retinal breaks are one of the primary causes, CRD occurs without a visible retinal break, indicating a distinct pathophysiology and aetiology. This condition poses a serious threat to central vision and, if left untreated, can significantly impair visual function and quality of life. Therefore, early diagnosis and timely intervention are crucial in preventing permanent vision loss. CRD is primarily associated with tractional forces acting on the macula, often induced by Vitreomacular Traction (VMT) or Epiretinal Membrane (ERM) contraction [1]. An imbalance between the traction exerted by the vitreous gel on the Internal Limiting Membrane (ILM) and the opposing forces, such as the RPE pump and interphotoreceptor matrix, is more commonly observed in CRD compared to other types of retinal detachment [1]. Both anteroposterior and tangential traction from the vitreous cortex on the ILM can contribute to CRD, especially in cases involving contracting Epiretinal Membranes (ERM) or Vitreomacular Traction Syndrome (VMT); [2,3].The tractional component is minimally visible on biomicroscopic examination of eyes with a macular hole or ERM. It may be visualized in Optical Coherent Tomography (OCT), but it is revealed intraoperatively during Posterior Vitreous Detachment (PVD) in vitrectomy [3-5]. In highly myopic eyes with a staphyloma, the semidetached posterior hyaloid and the rigid internal limiting membrane are probably both responsible for CRD [6,7]. The ILM's stiffness prevents the retina from conforming properly to underlying scleral curvature, particularly in the presence of staphyloma, where the retina is excessively stretched over an extremely concave scleral bulge.
The detachment may be minimal e.g., perifoveal fluid cuffing in eyes with a macular hole, or widespread, spanning the staphyloma in highly myopic eyes [6]. In the latter case, the presence of the break (macular hole) is more likely to be secondary to the RD, not its cause [1,8]. Currently available literature describes central retinal detachment as a feature of highly myopic eyes [8-10]. In our case series CRD developed as an excessive traction over the macular region regardless the presence of high myopia, which is one of the risk factors, but apparently not the only one. The diagnosis and management of CRD require a comprehensive approach, including advanced imaging techniques such as Optical Coherence Tomography (OCT). OCT provides detailed cross-sectional images of the retina, allowing precise identification of the underlying causes of detachment, such as VMT, ERM, and myopic degeneration. OCT is indispensable for planning surgical intervention and monitoring postoperative course and outcome [11,12]. Recently published revised OCT classification of Myopic Tractional Maculopathy (MTM) supports treatment planning in the myopic but also in non-myopic cases [13]. In Myopic Tractional Maculopathy (MTM), which was present in all five myopic eyes in our study, the rigid ILM further restricts retinal mobility, contributing to schisis-like changes, foveoschisis, or full-thickness macular holes. MTM staging, as described in recent classifications, is crucial in guiding surgical decisions, particularly regarding ILM peeling and the potential need for macular buckling [14,15].
Surgical management of CRD involves Pars Plana Vitrectomy (PPV), a procedure that removes the vitreous gel and addresses the tractional forces. The addition of ILM peeling during PPV is crucial as it enhances retinal elasticity and promotes reattachment [6]. Application of blue or green dyes enhances proper visualization not only the membranes, but also of vitreous remnants attached to the retina. In our patients indocyanine green was used. With its negative staining of ERM and excellent tinting of the ILM and vitreous, ICG enables meticulous removal of all traction components from the retinal surface. Five per cent glucose as a solvent eliminates potential ICG toxicity to the retina [16]. Gas tamponade with 20% sulfur hexafluoride (SF6) and strict postoperative face-down positioning help to maintain retinal attachment during the healing process. The prognosis for CRD varies depending on the underlying cause and the timely initiation of appropriate treatment. While anatomical reattachment is often achievable, functional improvement in visual acuity may not always correlate with anatomical success. Retinal remodelling following the separation of the neuroretina from the RPE in the crucial central region influences photoreceptor function [17]. Factors such as photoreceptor and RPE atrophy, particularly in cases involving myopia and Age-Related Macular Degeneration (AMD), can limit visual recovery [18]. In summary, CRD is a multifactorial condition requiring an individualised diagnostic and therapeutic approach. Understanding the biomechanical interactions between the vitreous, ILM, and macular architecture is essential for optimising surgical strategies and improving patient outcomes. This case series presents long-term clinical outcomes in CRD management, with a specific focus on risk factors influencing final anatomical and functional results.
Aim: The aim of our case series study was to present long-term results in management of central retinal detachment together with analysis of risk factors and their influence on final anatomical outcome and visual performance.
Material and Methods
We retrospectively analysed 15 eyes from 15 patients who underwent Pars Plana Vitrectomy (PPV) for Central Retinal Detachment (CRD) confined to the macular region. Surgical procedures were performed in Prof. Zagorski Eye Surgery Centers in Nowy Sącz and Nałęczów between Jan 2021-Dec 2021. The surgeries were done by two experienced vitreoretinal surgeons. The study group included 8 females and 7 males, with a mean age of 68 years (range 52-86 years). Patients were selected based on the diagnosis of CRD confirmed through comprehensive ophthalmic examinations, including Best Corrected Visual Acuity (BCVA), fundus examination with colour fundus photograph, and optical Coherence Tomography (OCT). Inclusion criteria were a primary diagnosis of CRD and necessity for surgical intervention. Exclusion criteria included any prior retinal surgery and inadequate follow-up.
Detailed Preoperative Assessments were Performed, Which Included:
- BCVA Measurement: with the use of Snellen charts.
- Fundus Examination: Conducted with slit-lamp biomicroscopy with+90D lens to evaluate the extent and nature of the detachment.
- OCT Imaging: Used to identify the presence of Vitreomacular Traction (VMT), Epiretinal Membrane (ERM), and other relevant pathological features such as Macular Neovascularisation (MNV) and myopic degeneration. In highly myopic patients the stage of Myopic Tractional Maculopathy (MTM) was additionally assessed. In all five myopic eyes, OCT confirmed the presence of central posterior staphyloma, a hallmark feature associated with Myopic Tractional Maculopathy (MTM). These cases exhibited features such as macular schisis, foveoschisis, and varying degrees of outer retinal layer thinning, consistent with advanced myopic pathology.
To further characterize the structural changes, axial length measurements were recorded for all eyes, and for myopic eyes MTM staging was assigned according to recently published classification (Table 2) [13,14]. Type of staphyloma was also described (Table 2) [19]. These additional data points allowed for a more precise correlation between anatomical abnormalities and surgical outcomes.
All Patients Underwent 23-Gauge (23G) Pars Plana Vitrectomy. Operation Had the Following Steps:
- 1. Core Vitrectomy: Removal of the central vitreous gel to relieve traction with the use of triamcinolone acetonide crystals application to enhance vitreous visualisation(Vitreal S, Fidia Pharma)
- 2. Peripheral Vitrectomy: thorough shaving of the vitreous gel at the periphery to prevent future traction.
- 3. ILM Peeling: Performed using vital dye - indocyanine green (ICG) diluted in 5%glucose as a solvent - to stain the ILM and facilitate its visualisation and peeling. ICG was kept on the retina 30 seconds before removal with the vitrector (low flow, high cut-rate, peristaltic pump). In highly myopic eyes with posterior staphyloma, extra care was taken during ILM peeling due to increased retinal fragility and biomechanical instability. These retinas required double staining with prolonged time (1-1,5 minutes) for ICG to stay over the retina to facilitate visualisation. ICG was removed either with the use of the vitrector as above or passively by the flute-needle. Peeling encompasses 1st ERM and traction removal and after restaining removal of ILM itself. When retina was extremely thin and stretched over the staphyloma fovea sparring peeling was performed to prevent iatrogenic damage to the fovea. No macular buckling was done.
- 4. Endotamponade: 20% Sulfur hexafluoride (SF6) gas was used as an endotamponade agent to provide temporary internal pressure, supporting the reattached retina.
- 5. Face-Down Positioning: Postoperative face-down positioning was maintained for 3-5 days to ensure effective gas tamponade of the macula.
In this study, all five myopic eyes had central posterior staphyloma and developed macular detachment over the staphyloma due to myopic degeneration and tractional forces (MTM - stage 3 b or c) [13,19]. The altered retinal contour over the concave scleral bulge contributed to the complexity of surgical intervention, necessitating careful handling of the ILM and vitreous removal to minimize additional traction. The rigidity of the ILM in these cases required meticulous peeling techniques with double staining with ICG with prolonged time (1-1,5 minutes) to achieve adequate relaxation of the macular tissue and ensure retinal reattachment.
Postoperative Evaluations Were Conducted In 10 Days, 1 Month, 3 Months, 6 Months, and 3 Years (36 Months) after Surgery. These Evaluations Included:
- BCVA Measurement: To assess functional visual outcomes. Decimal values were subsequently converted to logMAR scale.
- Fundus Examination: To check for anatomical reattachment and any signs of re-detachment and additional retinal pathologies. Colour fundus photographs, captured with an ultra-wide-field fundus camera (EIDON, Centervue), were also obtained.
- OCT Imaging: To monitor the macular anatomy during the healing process and detect any residual or recurrent issues such as subretinal fluid or ERM remnants, MNV or newly formed PVR membranes.
In Cases Where Postoperative Complications Were Noted, Further Interventions Were Performed as Follows:
- Anti-VEGF Therapy: For eyes with concurrent MNV, anti-VEGF injections (aflibercept 2mg/0,1ml) were administered postoperatively to control neovascular activity and associated subretinal fluid.
- Reoperations: Two eyes developed macular re-detachment due to secondary holes formation close to the staphyloma edge. Re-detachment was noted three months after initial surgery in both instances. Patients were referred immediately to the Department of Ophthalmology of Jonscher Public Hospital in Łódź, Poland as an ophthalmic emergency. Re-vitrectomy with silicone oil tamponade was performed there. 1000 si silicone oil was applied in both instances as longer tamponade. Oil was removed after three subsequent months.
Data were analysed to assess both anatomical and functional outcomes. The primary outcome measures included the rate of anatomical reattachment of the macula and changes in BCVA. Secondary outcomes included the incidence of postoperative complications and any need for additional treatments.
Results
Patient Demographics and Preoperative Findings
A total of 15 eyes from 15 patients were included in this study, consisting of 8 females and 7 males, with a mean age of 68 years (range 52-86 years). Among these, 5 eyes (33.3%) had high myopia with posterior staphyloma and myopic tractional maculopathy (MTM). The axial length in myopic eyes ranged from 25,81mm to 29.0 mm. MTM staging was determined for all myopic cases using recently proposed classification [13], and the posterior staphyloma type was categorized according to newest OCT-based classification [20].
Preoperative Findings
Preoperative OCT Examination Revealed the Following:
- Vitreomacular Traction (VMT) and Epiretinal Membrane (ERM): In 8 eyes (53.3%), macular detachment was due to excessive vitreomacular traction (Figure 1) and contracted epiretinal membrane lifting the central retina (Figure 2). In 2 cases CRD developed shortly after uneventful cataract surgery (Figures 2,3).
- Myopic degeneration with posterior staphyloma and MTM: 5 eyes (33.3%) exhibited macular detachment over the posterior staphyloma. MTM staging revealed 1 eye with stage 3A, 2 eyes with stage 3B and 2 eyes with stage 3C (see Table 2)
- VMT and Macular Neovascularisation (MNV): 2 eyes (13.3%) had macular detachment due to VMT with concurrent active MNV (Figure 4).
The causes of CRD and the corresponding number of eyes are summarised in Table 1.
|
Cause od CRD |
Number of eyes |
|
VMT+ERM, non-myopic |
8 |
|
Myopic retinal degeneration+ERM |
5 |
|
VMT+CNV, non-myopic |
2 |
|
N=15 |
Table 1: CRD causes based on clinical and OCT examination.
|
No |
ALX (mm) |
MTM stage [13] |
Type of staphyloma [19] |
Preop BCVA |
10 days BCVA |
1 month BCVA |
3 months BCVA |
6 months BCVA |
3 years BCVA |
Cause of failure |
|
1 |
25,81 |
3A |
Type2 |
2.7 |
1 |
0.7 |
3.0*** |
1 |
1 |
reRD |
|
2 |
28,12 |
3B |
Type1 |
1 |
1.3 |
1 |
1 |
1 |
0.7 |
|
|
3 |
29,01 |
3C |
Type1 |
1 |
1.3 |
1 |
2.7*** |
0.7 |
1.3** |
reRD |
|
4 |
27,12 |
3B |
Type1 |
2.7 |
2.7 |
1.3 |
1 |
1 |
1 |
|
|
5 |
26,54 |
3B |
Type2 |
1.49 |
1.49 |
2 |
2 |
1.49 |
1.49 |
|
|
6 |
22,01 |
n/a |
n/a |
1.3 |
1.3 |
1.4 |
1.52 |
1.7 |
1.7** |
wetAMD |
|
7 |
22,38 |
n/a |
n/a |
1.3 |
1 |
1.3 |
0.7 |
0.49 |
1.3 |
wetAMD |
|
8 |
24,01 |
n/a |
n/a |
3 |
1 |
0.52 |
1.3 |
0.1 |
0.1 |
|
|
9 |
23,23 |
n/a |
n/a |
3 |
0.92 |
0.7 |
0.49 |
0.22 |
0.22 |
|
|
10 |
21,34 |
n/a |
n/a |
1.49 |
2.1 |
1.3 |
1 |
1 |
1 |
|
|
11 |
22,00 |
n/a |
n/a |
1.7 |
1 |
0.7 |
0.52 |
0.3 |
0.3 |
|
|
12 |
22,47 |
n/a |
n/a |
1.3 |
1.3 |
1 |
0.7 |
0.52 |
0.7 |
|
|
13 |
21,08 |
n/a |
n/a |
0.7 |
0.52 |
0.7 |
1 |
1.3 |
1,3** |
Dry AMD |
|
14 |
22,47 |
n/a |
n/a |
2.7 |
1 |
0.7 |
0.4 |
0.4 |
0.52 |
|
|
15 |
24,18 |
n/a |
n/a |
2 |
2 |
1.7 |
3 |
2 |
2 |
**BCVA decreased compared to preoperative values - cause of failure depicted in last column; *** BCVA temporarily worsened in 3rd month due to reRD operated subsequently with oil.
Table 2: Axial Length, Myopic Traction Maculopathy (MTM) Stage, and Best Corrected Visual Acuity (BCVA) Change Over Time Following Surgery in logMAR (values rounded to 2nd place).
Myopic eyes are depicted in blue. MTM stage acc to Parolini et al. Eyes with VMT and MNV are depicted in red font.
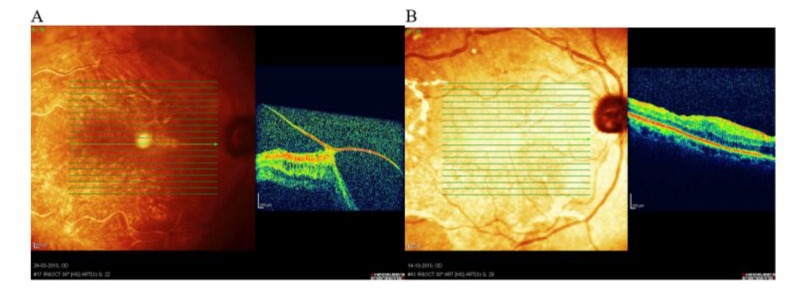
Figure 1: Patient MS, 57y, female. Emmetropic eye. Central RD with VMT. A: Preoperative image- note enormous VMT dragging the macula out of the place, BCVA = HM; B: Postoperative image - macula attached; BCVA 3 years after surgery 0,6.
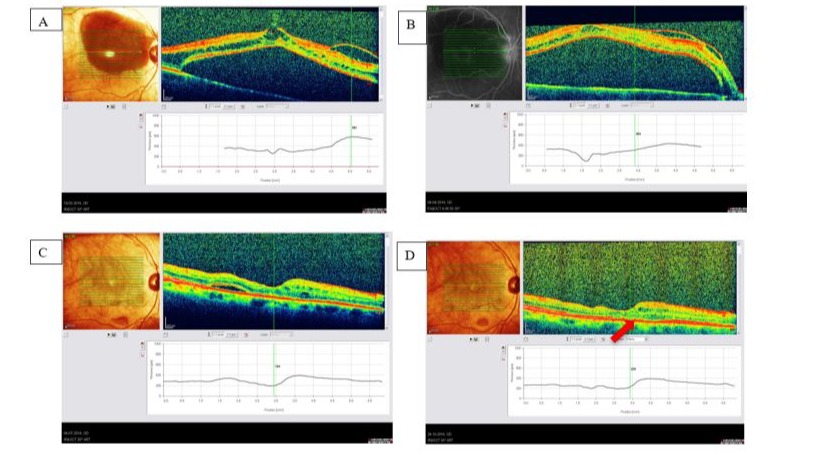
Figure 2: Patient A.S-A, female 86 y old. Central retinal detachment developed 10 days after uneventful cataract surgery (mature cataract, prior cataract extraction fundus not visible). BCVA before and after cataract surgery 0,05. A: Preoperative picture and OCT showing VMT+ERM lifting the macula; B: Preoperative picture and OCT - detached macula and contracting ERM on top; C: Postoperative picture - macula attached 3 months postop, remaining subretinal fluid still present; BCVA=0,05; D: 3 years after PPV+ILM peeling+SF6 macula still attached, but no functional improvement due to macular atrophy (arrow).
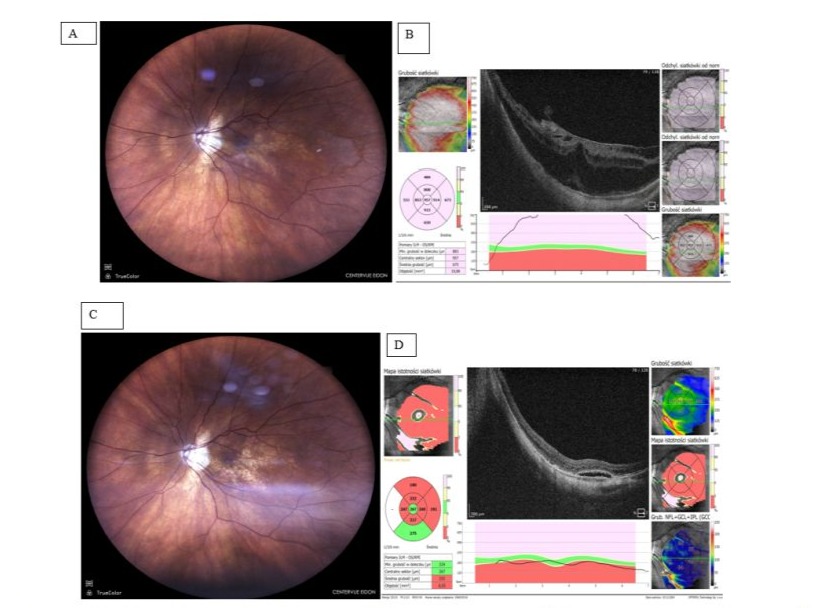
Figure 3: Patient A.B., female, 71-year-old. CRD developed due to contraction of ERM over posterior staphyloma resulting in deterioration of VA to 0,2. History of high myopia, cataract extraction 3 years earlier. This is the Patient’s better eye (BCVA prior CRD was 0,6, reading vision was full); A: Fundus photograph preop. - note hazy reflectance from central retina; B: OCT image preop. - ERM lifting central retina stretched over posterior staphyloma, stage 3 B (acc to Parolini et al.); C: Fundus Photograph postop. (12 months) - note change in macular region appearance; D: OCT image postop. (12 months) - note the remnants of subretinal fluid, retina almost completely fills in the scleral bulge, BCVA =0,5, with full reading vision.
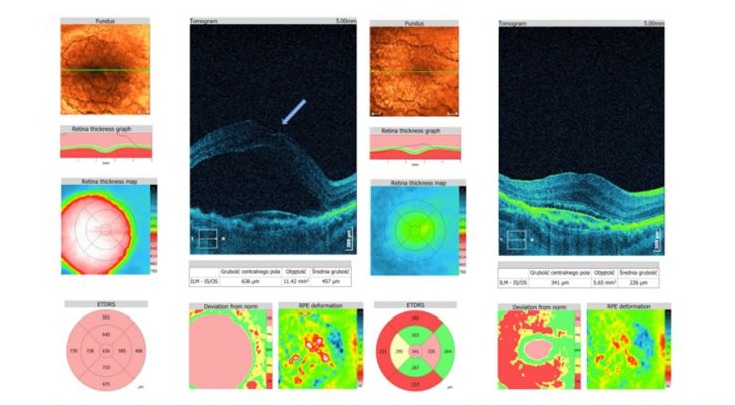
Figure 4: Patient AM, male 72y; VMT+CNV. A: Preoperatively two components lifting macula - VMT and MNV with subretinal fluid production. BCVA=0,05; patient during anti-VEGF therapy with bevacizumab. Tractional component indicated by arrow. B: 3 years and after 10 bevacizumab and 10 aflibercept injections, BCVA= 0,05 due to scarring process. Despite of remaining SRF central retina remains attached.
Surgical Outcomes
Pars Plana Vitrectomy (PPV) with ILM peeling was performed in all eyes. In two highly myopic cases with deep staphyloma and MTM stage 3B or higher, adjunct macular buckling was considered but ultimately not performed. Neovascularization in MNV cases was managed postoperatively with anti-VEGF therapy.
Intaroperative success was achieved in all eyes, with reattachment of the macula observed immediately post-surgery. The following observations were made during the follow-up period:
- Anatomical Reattachment: Maintained in 13 eyes (86.7%) without further intervention.
- Re-detachment: Two cases (13.3%) with high myopia developed macular re-detachment 3 months after initial surgery due to the formation of secondary holes. These cases were successfully managed with re-vitrectomy and 1000 si silicone oil tamponade, with stable reattachment observed after oil removal.
Overall Visual Acuity Outcomes
Visual acuity improved in 10 eyes (66.7%), in 3 eyes (20%) remained at the same level and worsened in 2 eyes (13,3%) by the 6-month follow-up. Subsequent three years (36 months) observation indicated BCVA improvement although variability across conditions was noted (see Table 2 and Table 3), which is graphically presented in Figure 5.
BCVA results are summarised below (Table 3) and depicted at Figure 5.
Table 3 Best Corrected Visual Acuity (BCVA) in log MAR scale obtained preoperatively, and postoperatively at 10 days, 1 months, 3 months, 6 months and 36 months, per condition. Mean VA values for the whole cohort are depicted in the last column; values are rounded to 2nd place.
|
Time |
Myopia |
VMT_ERM |
VMT_MNV |
Mean |
|
preop |
1.78 |
1.99 |
1.3 |
1.69 |
|
10 d |
1.56 |
1.23 |
1.15 |
1.31 |
|
1 m |
1.2 |
0.91 |
1.35 |
1.15 |
|
3 m |
1.94 |
1.05 |
1.11 |
1.37 |
|
6 m |
1.04 |
0.73 |
1.1 |
0.96 |
|
3 years (36m) |
1.1 |
0.77 |
1.5 |
1.12 |
BCVA Results in Detail Can be Described as Follows:
1. High myopia - 5 cases- Preoperative visual acuity was the poor (Log MAR ~1.78).
- Significant improvements were observed by 10 days (~1.56) and 1 month (~1.20).
- A temporary regression was noted at 3 months (~1.94), followed by recovery to the best recorded value at 6 months (~1.04); with further stabilization in 3rd year of observation ((~1.10)
- Preoperative acuity was the worst overall (Log MAR ~1.99).
- Postoperative recovery showed substantial gains by 10 days (~1.23) and 1 month (~0.91).
- Despite minor fluctuations at 3 months (~1.05), sustained improvement was observed at 6 months (~0.73), and remained good in 3rd year, indicating excellent long-term recovery.
- Preoperative acuity was moderate (Log MAR ~1.30).
- Visual acuity improved gradually at 10 days (~1.15) and stabilised around 1.10 by 3 and 6 months.
- A decline to 1.50 was observed at the 3-year follow-up, likely reflecting the influence of macular neovascularisation.
- Significant gains were observed across all conditions within the first 1-3 months post-treatment.
- Long-term improvements were sustained by 6 months, with VMT+ERM showing the most notable recovery.
- Variability was observed, particularly for Myopia and VMT+ERM, at 3 months.
- 3rd year follow-up revealed sustained improvement of BCVA, although variability was noted among the conditions. 11 eyes (73,3%) had stable or improved BCVA. Decreased BCVA was noted in 4 eyes (26,7%). Two of them had progression of neovascular AMD and 2 were myopes with RD development after initial CRD surgery.
- Across the groups the best gain was in ERM-VMT non-myopic group, the poorest in VMT-MNV group, with significant influence of AMD on the long-term result. BCVA after 3 years in myopic eyes also revealed significant improvement compared to preoperative values.
In summary the findings demonstrate the effectiveness of the surgical intervention in improving visual acuity, with case-specific recovery trajectories and good long-term outcomes (Figure 5). Due to the small sample size, subgroup analyses were limited; future studies with larger cohorts are needed.
Statistical analysis based on t-Paired test showed overall significant BCVA improvement postoperatively (p<0,05), but condition specific analysis indicated consistent improvements for VMT+ERM, variable improvements for Myopia, and mixed results for VMT+MNV.
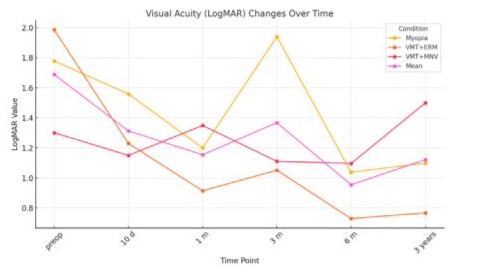
Figure 5: BCVA changes with time. (p<0,05).
Discussion
The findings from our study contribute to the growing body of evidence regarding the pathophysiology, surgical management, and outcomes of central retinal detachment. Our series of 15 cases highlights the complexity of CRD and underlines the importance of individualised management based on the aetiology and anatomical characteristics. The study identifies CRD as a feature not only myopic, but also non-myopic eyes.
Role of Myopia in CRD Development
In our study, only five out of 15 eyes were highly myopic. High myopia is a well-recognized risk factor for CRD due to structural and biomechanical features of the eye. In such eyes with posterior staphyloma the retina is stretched over the extremely concave scleral bulge and stiff ILM does not allow the retinal tissue to adhere properly to the eye wall. The rigid ILM in these eyes prevents the retina from conforming to the underlying scleral curvature [5]. Additionally, the reduced efficacy of the RPE pump in myopic eyes contributes to the accumulation of subretinal fluid and hinders retinal reattachment [18]. The incomplete Posterior Vitreous Detachment (PVD) observed intraoperatively in myopic eyes is another critical factor. Dynamic tractional forces exerted by the semidetached posterior hyaloid can initiate or worsen retinal detachment. Our findings align with those reported previously, which described similar mechanisms in myopic foveoschisis and CRD [10]. Furthermore, Pars Plana Vitrectomy (PPV) in these cases is particularly challenging due to the difficulty in visualizing the posterior vitreous. Even with the use of Triamcinolone Acetonide (TA), the poor contrast between the crystals and the hypopigmented retina and choroid complicates the removal of the posterior hyaloid [21]. One may try to use Indocyanine Green (ICG) to stain vitreous in such cases [16,22]. ILM peeling usually needs re-staining and the dye should be kept longer over the central retina to make the structures clearly visible. The myopic retina is much thinner than the healthy one and more prone to re-detachment, that is why the surgery should be done by an experience vitreo-retinal surgeon [22]. In our case series 2 eyes, which developed re-detachment were from the myopic sub-group. Intraoperatively, ILM peeling in myopic eyes posed unique challenges due to increased retinal fragility. Our findings align with reports suggesting that peeling techniques must be adapted based on MTM severity, with a greater need for re-staining in highly myopic eyes and sometimes fovea sparring method [8,23,24]. While macular buckling has been proposed as an alternative approach for advanced MTM stages, it was not performed in our series due to surgical complexity and patient considerations.
Alternative Surgical Techniques: Macular Buckling [25-27] and Posterior Scleral Contraction [28-30]
For advanced MTM cases with significant posterior staphyloma depth, macular buckling has been proposed as an alternative to ILM peeling alone. This technique supports the posterior pole by counteracting the tractional forces exerted by the concave sclera, reducing the risk of post-vitrectomy retinal re-detachment. Posterior scleral contraction techniques, including scleral shortening or hydrogel scleral implants, have also been explored to reduce axial length and improve retinal stabilization in myopic eyes. While these techniques are not yet widely adopted, they may represent future advancements in CRD management.
Epiretinal Membranes and VMT - the Role of Vitreoschisis and Dynamic Traction
It was suggested that RD development is connected to the dynamic traction exerted on the retina by incompletely detached posterior vitreous [1]. Eight eyes in our study were non-myopic and presented with tractional forces primarily due to VMT (Figure 1) or/and ERM (Figure 2). Excessive adhesion between the posterior vitreous cortex and the macula resulted in localized traction that detached the central retina. There was no break. The role of vitreoschisis in the formation of ERM and its contribution to tractional forces has been extensively documented in the literature [31-33]. Our study reinforces the importance of addressing these pathological changes during surgery to achieve successful reattachment.
The surgical elimination of VMT and ERM via ERM and ILM peeling was a critical step in all cases. Peeling the ERM and ILM not only removes the tractional component but also enhances retinal elasticity, facilitating reattachment [23]. The procedure is technically demanding, particularly in eyes with thin retinas or pre-existing macular atrophy. Our findings are consistent with previous studies that emphasize the need for meticulous surgical technique and the use of appropriate staining agents such as Indocyanine Green (ICG) to enhance visibility during ILM peeling [22].
Role of Cataract Surgery and Dynamic Traction
In two cases from the VMT/ERM group (Table 3) CRD developed shortly after cataract surgery (Figure 2). The replacement of the natural lens with a much thinner Intraocular Lens (IOL) increases the space available for vitreous movement, amplifying tractional forces on the macula [34,35]. This phenomenon highlights the importance of preoperative evaluation of the vitreoretinal interface in patients undergoing cataract surgery, particularly those with predisposing conditions such as VMT or ERM. Such patients would eventually need closer and longer follow-up with OCT examination after cataract removal [17,36].
Mixed Etiology: VMT and Macular Neovascularization
In two eyes, central RD had mixed origin-there was vitreomacular traction pulling on the central retina, but there was also MNV which, by fluid production, lifted the macula from the eye wall (Figure 3). The tractional element was eliminated by PPV and MNV was treated postoperatively with anti-VEGF agents. Such eyes unfortunately have poorer prognoses, but elimination of tractional component usually facilitates further anti-VEGF therapy [37,38]. It was suggested previously, that tractional forces may cause the pharmacological resistance to anti-VEGF treatment in these patients [38]. Removal of traction may improve the effect of anti-VEGF therapy and maintain the functional vision [39]. Additionally, the vitreo-macular traction removal may facilitate oxygenation, decrease of oedema and inflammation within the macula affected by AMD [37]. Traction removal seemed to enhance drug effectiveness in our two cases, reflected in BCVA stabilisation over time (Figure 5).
Functional Outcomes and Long-term Prognosis
PPV with ERM and ILM peeling was a method of choice in all eyes to re-attach the central retina in our case series. Removal of the vitreous and all its abnormal connections to the central retina eliminates tractional component [5,11,34]. ILM peeling makes the retina more elastic and easier to re-attach [6]. To give interphotoreceptor matrix environment to re-establish its normal connections we used gas tamponade (SF6) and position the patients strictly face-down for at least 3-5 days. Retina remained attach in 13 eyes after the procedure. In two eyes 3 months postoperatively, re-detachment developed due to secondary holes formation, but reoperation with silicone oil was done to manage it successfully. These findings underscore the need for long-term monitoring and individualized postoperative care to optimize outcomes.
It is important to address functional status after anatomical re-attachment of the central retina, which itself is crucial for visual performance [40]. Looking to the preoperative BCVA data one notice that before surgery BCVA was poor or very poor (see Table 2 and 3). VA improved after macular attachment but not in all instances. VA decreased with time due to the natural progression of AMD in 3 cases. It was comparable with previously published data, and it is worthy to stress that elimination of tractional component seemed to be helpful with further control of AMD [37,39,41]. In two myopic eyes with subsequent re-RD the VA deterioration in 3rd month was due to the detachment. However, there wasn’t much improvement of the visual performance post reattachment of the retina, probably due to structural changes related to the re-RD itself, such as retinal atrophy, tissue remodelling and apoptosis of photoreceptors [40,42]. Overall functional improvement in our material was not high but stable with time (see Table 2). Variablity was noted across the conditions (Figure 5).
Future Directions
Our findings highlight several areas for further research. The development of advanced imaging techniques to better visualize the vitreoretinal interface and guide surgical interventions is critical. Additionally, exploring adjunctive pharmacological treatments to enhance the efficacy of surgical procedures and address underlying RPE dysfunction could improve outcomes. Long-term studies investigating the natural history of CRD and the impact of emerging therapies, such as gene therapy and regenerative approaches, are also warranted.
Conclusions
- 1. Anatomical Success with Variable Functional Outcomes.
Our study demonstrates that pars plana vitrectomy (PPV) with removal of tractions and internal limiting membrane (ILM) peeling is an effective surgical approach for achieving anatomical reattachment in central retinal detachment (CRD). However, functional recovery, as measured by best-corrected visual acuity (BCVA), varies significantly depending on the underlying aetiology and retinal integrity at the time of surgery.
- 2. Key Role of Aetiology in Surgical Outcomes
The aetiology of CRD profoundly influences surgical and visual outcomes. In highly myopic eyes with posterior staphyloma, retinal reattachment is complicated by the rigid ILM and the biomechanical challenges posed by a concave scleral bulge. In non-myopic eyes, vitreomacular traction (VMT) and epiretinal membranes (ERM) are the primary contributors to CRD, emphasizing the importance of addressing these tractional elements surgically. Eyes with mixed pathology, such as VMT and macular neovascularization (MNV), tend to have poorer prognoses, highlighting the complexity of managing multifactorial CRD.
- 3. Importance of Preoperative Evaluation and Individualized Management
Preoperative evaluation of the vitreoretinal interface using optical coherence tomography (OCT) is critical for tailoring surgical strategies. In eyes with incomplete posterior vitreous detachment (PVD) or severe VMT, surgical removal of tractional components is essential to restore anatomical integrity. Special attention is required for eyes undergoing cataract surgery, as the altered dynamics of the vitreous can exacerbate tractional forces, leading to CRD.
- 4. Challenges in Myopic Eyes
In myopic eyes, the challenges of visualizing and removing the posterior hyaloid, even with the use of triamcinolone acetonide (TA) or indocyanine green (ICG), highlight the need for expertise in vitreoretinal surgery. The thin and fragile retina in these eyes is prone to re-detachment, emphasizing the importance of careful handling during surgery and diligent postoperative monitoring.
- Mixed Aetiology and Anti-VEGF Therapy
In cases where MNV contributes to CRD, surgical elimination of tractional forces facilitates subsequent anti-VEGF therapy, enhancing the management of macular oedema and reducing fluid accumulation. However, these cases often have limited visual outcomes due to underlying retinal atrophy and chronicity of the disease.
- 6. Postoperative Strategies and Long-term Monitoring
The use of gas tamponade (SF6) combined with strict face-down positioning postoperatively contributes to successful anatomical reattachment in most cases. However, the occurrence of re-detachment in two eyes underlines the need for long-term monitoring and, when necessary, reoperation with silicone oil tamponade.
- 7. Prognostic Factors for Functional Recovery
Functional outcomes are influenced by the chronicity of the detachment, the presence of concomitant conditions such as age-related macular degeneration (AMD), and the extent of photoreceptor damage, which may limit functional success.
References
- Kuhn F, Aylward B (2014) Rhegmatogenous Retinal Detachment: A Reappraisal of Its Pathophysiology and Treatment. Ophthalmic Res 51: 15-31.
- Gandorfer A, Rohleder M, Kampik A (2002) Epiretinal Pathology of Vitreomacular Traction Syndrome. British Journal of Ophthalmology 86: 902-909.
- Yamada N, Kishi S (2005) Tomographic Features and Surgical Outcomes of Vitreomacular Traction Syndrome. Am J Ophthalmol 139: 112-117.
- Duker JS, Kaiser PK, Binder S, De Smet MD, Gaudric A, et al. (2013) The International Vitreomacular Traction Study Group Classification of Vitreomacular Adhesion, Traction, and Macular Hole. Ophthalmology 120: 2611-2619.
- Sakaguchi H, Ikuno Y, Choi JS, Ohji M, Tano T (2004) Multiple Components of Epiretinal Tissues Detected by Triamcinolone and Indocyanine Green in Macular Hole and Retinal Detachment as a Result of High Myopia. Am J Ophthalmol 138: 1079-1081.
- Kuhn F (2003) Internal Limiting Membrane Removal for Macular Detachment in Highly Myopic Eyes. Am J Ophthalmol 135: 547-549.
- Philippakis E, Couturier A, Gaucher D, Gualino V, Massin P, et al. (2016) Tadayoni, R. Posterior Vitreous Detachment in Highly Myopic Eyes Undergoing Vitrectomy. In Proceedings of the Retina 36: 1070-1075.
- Ikuno Y, Sayanagi K, Soga K, Oshima Y, Ohji M, et al. (2008) Foveal Anatomical Status and Surgical Results in Vitrectomy for Myopic Foveoschisis. Jpn J Ophthalmol 52: 269-276.
- Ohsugi H, Ikuno Y, Matsuba S, Ohsugi E, Nagasato D, et al. (2019) Morphologic Characteristics of Macular Hole and Macular Hole Retinal Detachment Associated with Extreme Myopia. Retina 39: 1312-1318.
- Gaucher D, Haouchine B, Tadayoni R, Massin P, Erginay A, et al. (2007) A. Long-Term Follow-up of High Myopic Foveoschisis: Natural Course and Surgical Outcome. Am J Ophthalmol 143: 455-462.
- Sallam AB, Kuhn F, Gini G, Adelman RA, Eds, et al. (2024) Practical Manual of Vitreoretinal Surgery;.; Springer International Publishing: Cham.
- Li AL, Feng M, Wang Z, Baxter SL, Huang L, et al. (2023) Automated Detection of Posterior Vitreous Detachment on OCT Using Computer Vision and Deep Learning Algorithms. Ophthalmology Science 3: 100254.
- Parolini B, Palmieri M, Finzi A. Besozzi G, Lucente A, et al. (2021) The New Myopic Traction Maculopathy Staging System. Eur J Ophthalmol 31: 1299-1312.
- Parolini B, Palmieri M, Finzi A, Frisina R (2021) Proposal for the Management of Myopic Traction Maculopathy Based on the New MTM Staging System. Eur J Ophthalmol 31: 3265-3276.
- Frisina R, Gius I, Palmieri M, Finzi A, Tozzi L, et al. (2020) Myopic Traction Maculopathy: Diagnostic and Management Strategies. Clinical Ophthalmology 14: 3699-3708.
- Bergamo VC, Caiado RR, Maia A, Magalhães O, Moraes NSB et al. (2021) Role of Vital Dyes in Chromovitrectomy. Asia-Pacific Journal of Ophthalmology 10: 26-38.
- Goldhardt R, Rosen BS (2020) Optical Coherence Tomography: Critical Tool to Manage Expectations after Cataract Extraction. Curr Ophthalmol Rep 8: 129-135.
- Gohil R, Sivaprasad S, Han LT, Mathew R, Kiousis G, et al. (2015) Myopic Foveoschisis: A Clinical Review. Eye (Basingstoke) 29: 593-601.
- Shinohara K, Shimada N, Moriyama M, Yoshida T, Jonas JB, et al. (2017) Posterior Staphylomas in Pathologic Myopia Imaged by Widefield Optical Coherence Tomography. Invest Ophthalmol Vis Sci 58: 3750-3758.
- Ruiz-Medrano J, Montero JA, Flores-Moreno I, Arias L, García-Layana A, et al. (2019) Myopic Maculopathy: Current Status and Proposal for a New Classification and Grading System (ATN). Prog Retin Eye Res 69: 80-115.
- Fang X, Zheng X, Weng Y, Chen Z, Liu J, et al. (2009) Anatomical and Visual Outcome after Vitrectomy with Triamcinolone Acedonide-Assisted Epiretinal Membrane Removal in Highly Myopic Eyes with Retinal Detachment Due to Macular Hole. Eye 23: 248-254.
- Musat O, Stefan C, Boariu AM, Colta D, Cernat C, et al. (2016) Chromovitrectomy. Rom J Ophthalmol 60: : 59-62.
- Lamas-Francis D, Bande-Rodríguez M, Blanco-Teijeiro MJ (2023) Primary ILM Peeling during Retinal Detachment Repair: A Systematic Review and Meta-Analysis. Sci Rep 13: 3586.
- Wu J, Xu Q, Luan J (2021) Vitrectomy with Fovea-Sparing ILM Peeling versus Total ILM Peeling for Myopic Traction Maculopathy: A Meta-Analysis. Eur J Ophthalmol 31: 2596-2605.
- Mateo C, Gómez-Resa MV, Burés-Jelstrup A, Alkabes M (2013) Surgical Outcomes of Macular Buckling Techniques for Macular Retinoschisis in Highly Myopic Eyes. Saudi Journal of Ophthalmology 27: 235-239.
- Mateo C, Burés-Jelstrup A, Navarro R, Corcóstegui B (2012) Macular Buckling for Eyes with Myopic Foveoschisis Secondary to Posterior Staphyloma. Retina 32: 1121-1128.
- Xiong SQ, Jiang HB, Li FL, Li YX, Yang J, et al. (2017) Treatment of Myopic Foveoschisis via Macular Buckling and Vitrectomy. Int J Ophthalmol 10: :815-818.
- Ye J, Pan AP, Zhu S, Zheng L, Lu F, et al. (2021) Posterior Scleral Contraction to Treat Myopic Foveoschisis in Highly Myopic Eyes. Retina 41: 1047-1056.
- Zheng L, Pan A, Zhu S, Wu Y, Dong L, et al. (2019) Posterior Scleral Contraction to Treat Recurrent or Persistent Macular Detachment after Previous Vitrectomy in Highly Myopic Eyes. Retina 39: 193-201.
- Ye J, Wu Y, Zhu S, Dong L, Qu J, et al. (2021) Evaluation Of The Efficacy Of Posterior Scleral Contraction In The Treatment Of Macular Hole With Retinal Detachment In High Myopia. Retina 41: 1874-1882.
- Sebag J (2008) Vitreoschisis. Graefe’s Archive for Clinical and Experimental Ophthalmology 246: 1874-1882.
- Sebag J, Gupta P, Rosen RR, Garcia P, Sadun AA (2007) Macular Holes and Macular Pucker: The Role of Vitreoschisis as Imaged by Optical Coherence Tomography/Scanning Laser Ophthalmoscopy. Trans Am Ophthalmol Soc 105: 1874-1882.
- Gupta P, Yee KMP, Garcia P, Rosen RB, Parikh J, et al. (2011) Vitreoschisis in Macular Diseases. British Journal of Ophthalmology 95: 376-380.
- McDonald HR, Johnson RN, Schatz H (1994) Surgical Results in the Vitreomacular Traction Syndrome. Ophthalmology 101: 1397-1402.
- Carpineto P, Ciciarelli V, Borrelli E, Aharrh-Gnama A, Mastropasqua R (2019) Epiretinal Membrane In Eyes With Vitreomacular Traction. Retina 39: 1061-1065.
- Murphy G, Owasil R, Kanavati S, Ashena Z, Nanavaty MA (2023) Preoperative Fundoscopy versus Optical Coherence Tomography to Detect Occult Maculopathy during Cataract Surgery Preassessment. Eye (Basingstoke) 37: 665-669.
- Schulze S, Hoerle S, Mennel S, Kroll P (2008) Vitreomacular Traction and Exudative Age-Related Macular Degeneration. Acta Ophthalmol 86: 470-481.
- Xie P, Zheng X, Yu Y, Ye X, Hu Z, et al. (2017) Vitreomacular Adhesion or Vitreomacular Traction May Affect Antivascular Endothelium Growth Factor Treatment for Neovascular Age-Related Macular Degeneration. British Journal of Ophthalmology 101: 1003-1010.
- Robison CD, Krebs I, Binder S, Barbazetto IA, Kotsolis AI, et al. (2009) Vitreomacular Adhesion in Active and End-Stage Age-Related Macular Degeneration. Am J Ophthalmol 148: 79-82.e2.
- Fisher SK, Lewis GP,Linberg KA, Verardo MR (2005) Cellular Remodeling in Mammalian Retina: Results from Studies of Experimental Retinal Detachment. Prog Retin Eye Res 24: 395-431.
- Mojana F, Cheng L, Bartsch DUG, Silva GA, Kozak I, et al. (2008) The Role of Abnormal Vitreomacular Adhesion in Age-Related Macular Degeneration: Spectral Optical Coherence Tomography and Surgical Results. Am J Ophthalmol 146: 218-227.
- de Souza CF, Kalloniatis M, Polkinghorne PJ, McGhee CNJ, Acosta ML (2012) Functional and Anatomical Remodeling in Human Retinal Detachment. Exp Eye Res 97: 73-89.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.