Case Series of Severe Abdominal Sepsis, AKI with Anuria and Fulminant Guillain-Barre Syndrome (GBS) Mimicking Brain Stem Death
by Salah Essa1,2*, Mohamed Sharaf1,2, Mohamed Rabee1,2, Mohamed Hussien1,2, Ibrahim Sbieh1,2, Ahmed Esmael1,2
1SEHA Kidney care, SEHA, Abu Dhabi health service company, UAE
2Madinat Zayed Hospital Al Dhafra, SEHA, UAE
*Corresponding author: Salah Essa, SEHA Kidney Care, Nephrology department, Abu Dhabi Health Service Company, Madinat Zayed hospital Al Dhafra, SEHA, UAE; Email: issa5463@hotmail.com & salesaa@seha.ae
Received Date: 11 February 2025
Accepted Date: 14 February 2025
Published Date: 17 February 2025
Citation: Essa S, Sharaf M, Rabee M, Hussien M, Sbieh I, et al (2025) Case Series of Severe Abdominal Sepsis, AKI with Anuria and Fulminant Guillain-Barre Syndrome (GBS) Mimicking Brain Stem Death. Ann Case Report. 10: 2193. https://doi.org/10.29011/2574-7754.102193
Abstract
The presence of coma, flaccid quadriplegia without sedation and absence of most of the brain stem reflexes (fixed dilated pupil, no response to light, no corneal reflex, absent cough and gag reflexes) suggests brain stem death. However, polyneuropathy in nerve conduction studies (NCS), a high protein concentration in the CSF, and EEG suggested that the patient actually had GBS and all three patients recovered from GBS after receiving therapeutic plasma exchange (TPE) with / without IV immunoglobulin.
Brain death determination includes exclusion of reversible cerebral injury, confounding conditions, and it is vital to perform electroencephalography (EEG) when uncertainty exists about the reliability of the clinical exam to enable appropriate early diagnosis and therapeutic management.
We report these cases as an important reminder to clinicians to consider immune mediated post infectious polyneuropathy like GBS before accepting a diagnosis of brain stem death, especially in patients with sepsis. Variant GBS can be associated with Bickerstaff brainstem encephalitis and this explains coma, loss of brain stem reflexes.
Introduction
GBS is one of the most common causes of acute, acquired weakness and is often provoked by a preceding infection. In some cases, it may be complicated by respiratory failure or autonomic dysfunction. The acute polyneuropathy of GBS is often triggered by an immune response to an antecedent infection or other event where cross-reacting epitopes on peripheral nerve are similar to those of the pathogen (molecular mimicry), and all myelinated nerves (motor, sensory, cranial, sympathetic) can be affected.
Bickerstaff brainstem encephalitis (BBE) is syndrome characterized by encephalopathy with ophthalmoplegia and ataxia [1], and is associated with the presence of antibodies to particular gangliosides that are present in neuronal tissue with homologues in some pathogens (anti-GQ1b antibodies).
The exact cause of Guillain-Barre syndrome is unknown; however, two-thirds of patients report symptoms of an infection in the six weeks preceding diagnosis and such infections include COVID-19, respiratory or a gastrointestinal infection, and Zika virus.
There is no known cure for Guillain-Barre syndrome, but several treatments appear to improve symptoms and reduce the duration of the illness. Although most people recover completely occasionally severe cases can be fatal. Whilst recovery may take several years, most people are able to walk six months after symptoms. Some people may have lasting effects from it, such as weakness, numbness or fatigue. Variant GBS can be associated with Bickerstaff brainstem encephalitis which may mimic brain death [1].
Diagnosis of brain death is established by criteria first established at a conference of the Royal Colleges and Faculties of the United Kingdom in 1976, namely that all brain stem reflexes are absent (the pupils are fixed in diameter with no response to light, no corneal reflex, oculovestibular reflexes are absent, no response to painful stimulation within the cranial nerve distribution, cough and gag reflexes are absent, and there is apnea). Electroencephalography (EEG) and cerebral angiography were not initially included although they may assist in difficult cases [2,3].
Sepsis and septic shock are the causes of AKI and are known as Sepsis-Associated AKI (SA-AKI) and accounted for more than 50% of cases of AKI in the ICU, and have a poor prognosis [4].
In our rare case series three cases of abdominal sepsis with AKI and GBS mimicking brain stem death are presented.
All patients presented to Madinat Zayed Hospital (MZH), SEHAAbu Dhabi-U.A.E., this is a secondary hospital with some tertiary subspecialties and 20 ICU beds between 2009 to 2023.
Case 1:
A 38-year-old male patient from Bangladesh was admitted in 2009 with an acute abdomen. Surgical exploration revealed a sealed peptic ulcer. The patient developed severe AKI (creat 930 μmol/l, urea 20 mmol/l bic 8 mmol/l) with anuria from admission and required renal replacement therapy in the form of intermittent hemodialysis. On the day three of admission, he developed unexplained respiratory failure and required intubation and mechanical ventilation. At day 7, he developed flaccid quadriplegia without sedation, and fixed dilated pupils, together with autonomic neuropathy in the form of low blood pressure and bradycardia. On day 15 he remained unwell with flaccid quadriplegia without sedation for 10 days, and no brainstem reflexes. An apnea test was performed and he was declared brain dead.
On day 33, he passed a large amount of urine and the dialysis was ceased. He started to move his lower jaw, and his pupils began reacting to light. The diagnosis was revised and the possibility of GBS (unexplained respiratory failure, autonomic neuropathy, a high CSF protein of 4.4 G/l) was entertained and he received IV immunoglobulin. Nerve conduction studies (NCS) confirmed diffuse symmetrical mixed sensory and motor axonal polyneuropathy.
On day 48 he started to respond to verbal stimuli, moving head right and left, but still had flaccid quadriplegia. By day 56 he was fully awake and communicating, but still had a flaccid quadriplegia, and he required continued mechanical ventilation because weak respiratory muscle until day 70. On day 78 he was transferred from ICU to the rehabilitation center with good outcome but with some residual neurological disability and moderate weakness mainly lower limbs.
Case 2:
A 28-year-old male African patient without any previous history of severe or chronic illness was admitted to the ICU in 2020 with an acute abdomen, ascites, high inflammatory markers (CRP 290 mg/l,WBC 34,000 cells/mm3), high liver enzymes (ALT309 IU/l ,AST306 IU/l), severe metabolic acidosis (pH 7.2, bic 14 mmol/l) and anuria with serum creatinine 872 μmol/l and urea 19 mmol/l. A CT abdomen with contrast revealed normal sized kidneys, but with irregular medullary enhancement and hypodense nonenhancing renal cortex (reverse rim sign) [5], Figure 1, consistent with renal cortical necrosis, requiring renal replacement therapy, initially with daily intermittent hemodialysis (IHD) from day 1 until day 5. However due to increasing hemodynamic instability he started continuous renal replacement therapy (CRRT) with regional citrate anticoagulation. On day 5 he required paracentesis of his tense ascites. On day 6 he developed unexplained type 2
respiratory failure, and was intubated and ventilated. On day 10 he was noted to have flaccid quadriplegia with no gag reflex, sluggish pupillary reactions, areflexia, ophthalmoplegia, and was not responding to painful stimuli and with absent doll’s eye, corneal and gag reflexes. Nevertheless, he had a high CSF protein (of 1.7 g/l) and normal EEG. His NCS showed features consistent with acute progressive demyelinating polyneuropathy. Anti-GQ1b antibodies were requested but were unavailable in our pathology department as we suspected a GBS, Miller-Fisher, Bickerstaff brainstem encephalitis overlap syndrome and he started therapeutic plasma exchange (TPE). After five sessions of TPE followed by intravenous immunoglobulin (IVIG), he started to obey commands with improvement of the power in all limbs.
Ascitic fluid analysis revealed high white cell count with 97% lymphocytes and a high protein (of 22 g/l) and tuberculous peritonitis was suspected, and so he started antituberculosis treatment on the day 18. Given the cortical necrosis we suspected irreversible renal disease and he started intermittent hemodialysis thrice weekly from day 37. After 64 days, he was transferred from the ICU to the rehabilitation center and required maintenance hemodialysis. On day 211, he had partial recovery of AKI with sufficient renal function to become dialysis independent, with serum creatinine about 140 μmol/l without renal replacement therapy (RRT).
Case 3:
In 2023, a 44-year male patient from Bangladesh with history of hypertension, dyslipidemia was admitted with an acute abdomen, a serum creatinine of 444 µmol/l anuria, high inflammatory markers (CRP152 mg/l) and liver enzymes (AST1 50 IU/l ALT 142 IU/l) with a known previous baseline normal serum creatinine (81 µmol/l). A diagnosis of abdominal sepsis with secondary AKI was made and he required admission to ICU and started daily intermittent HD then CRRT. On day 9 the patient had an unexplained cardiac arrest, likely due to due to respiratory failure. He was successfully resuscitated but developed flaccid quadriplegia without sedation,
|
Site |
Latency (ms) |
Amplitude (mv) |
Area (mv/ms) |
Segment |
Distance (mm) |
NCS (m/s) |
|
Median L Wrist Elbow |
4.8 12.5 |
4.3 0.3 |
18 2 |
Wrist Wrist-elbow |
230 |
29.7 (RR 49–68) |
|
Median R Wrist Elbow |
4.5 9.5 |
3.3 2.9 |
12.5 12.3 |
Wrist Wrist-elbow |
220 |
44 (RR 49 –68) |
|
Ulnar L Wrist Elbow |
3.6 9.3 |
4.0 3.6 |
14.5 14.5 |
Wrist Wrist-elbow |
230 |
40 (RR51–70) |
|
Ulnar R Wrist Elbow |
3.3 8.6 |
4.7 4.3 |
15.5 16.1 |
Wrist Wrist-elbow |
240 |
45.3 (RR 51–70) |
|
Peroneal L Ankle Head of fibula |
No response (RR 36–69) |
|||||
Motor Nerve Conduction Study:
with fixed dilated pupils, loss of deep reflexes, ophthalmoplegia, no response to light, no corneal reflex, absent cough and gag reflexes and brain death was considered. On day 18, the patient started to pass more urine suggesting some renal recovery. On day 22 NCS revealed severe axonal sensory and motor polyneuropathy affecting mainly lower limbs (Table 1) and he had a normal EEG. The CSF protein was high (1 g/l), again suggesting the diagnosis of GBS with overlap syndrome. He commenced TPE on day 24 and received 10 sessions with volume of 3 liters and replacement with albumin 5%, on alternate days, followed by IVIG 26 g/d for 5 days. We noted gradual improvement in his condition and he started to open his eyes, obey commands and power improved in all limbs (upper> lower). He was weaned from ventilator support on day 93 and transferred from ICU to a medical ward on day 96. He was discharged from the hospital after 171 days walking with assistance with mild weakness in the lower limbs and serum creatinine of 120µmol/l without renal replacement therapy.
|
Peroneal R Ankle Head of fibula |
No response (RR 36–69) |
|
Tibial L Ankle Popliteal |
No response (RR 35–68) |
|
Tibial L Ankle Popliteal |
No response (RR 35–68) |
Sensory Nerve Conduction Study
|
Site |
Latency (ms) |
Amplitude (uv) |
Area (uv /ms) |
Segment |
Distance (mm) |
NCS (m/s) |
||
|
Median L Wrist |
3.0 |
8.3 |
0.7 |
Wrist |
120 |
39.5 (RR 50–74) |
||
|
Median R Wrist |
3.2 |
5.6 |
0.3 |
Wrist |
130 |
40.1 (RR (50–74) |
||
|
Ulnar L Wrist |
2.7 |
2.3 |
0.2 |
Wrist |
110 |
41 (RR 50–74) |
||
|
Ulnar L Wrist |
2.7 |
2.3 |
0.2 |
Wrist |
110 |
41 (RR 50–74) |
||
|
Ulnar R Wrist |
2.3 |
5.3 |
0.4 |
Wrist |
100 |
43.1 (RR 50–74) |
||
|
Sural Left |
No response (RR 40–70) |
|||||||
|
Sural Right |
No response (RR 40–70) |
|||||||
Table 1: NCS for case three-revealed severe axonal sensory and motor polyneuropathy affecting mainly lower limbs (RR = Reference range).
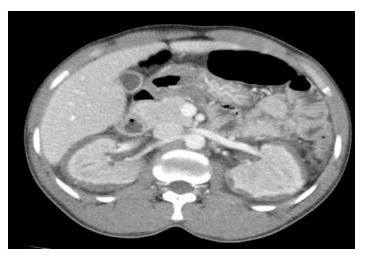
Figure 1: CT abdomen with contrast hypodense non-enhancing renal cortex, characteristics for (reverse rim sign) consistent with renal cortical necrosis.
Discussion
We present 3 patients with abdominal severe sepsis, AKI requiring renal replacement therapy who developed Guillain-Barre syndrome (GBS) mimicking brain stem death.
AKI complicating abdominal sepsis is not an uncommon finding for patients in the ICU, and sepsis is found in about 40% to 50% of patients with AKI in the ICU and amongst the critically ill, sepsis is the most common cause of AKI [4].
Cortical necrosis (CN) is a rare cause of acute kidney injury (AKI) in developed countries accounting for ~2% of all patients with AKI [6]. CN may commonly be caused by severe hemodynamic perturbation including obstetric causes like abruptio placentae, but also sepsis, burns, snake bites, pancreatitis, HUS, toxins and drugs [6]. AKI with acute anuria (urine output < 100 mL/24 h) is uncommon, and more commonly related to obstruction, shock or a catastrophic vascular event but in these cases the anuria was secondary to abdominal sepsis. Other studies have also reported that sepsis was the main cause of anuria with incidence of about 60% in hospital acquired AKI [7-10].
We report the three cases of fulminant Guillain–Barré syndrome (GBS), mimicking brain death, who were previously healthy, admitted to hospital ICU with abdominal sepsis. All patients quickly developed respiratory failure, requiring mechanical ventilation. When pharmacological sedation was withdrawn, patients were found to have a coma with absent brainstem reflexes, resembling a state of brain death.
A GBS was diagnosed, based on the NCS demonstrated motor and sensory responses unexcitable in upper and lower extremities. An EEG showed normal brain function, high CSF protein. Treatment with therapeutic plasma exchange followed by IV intravenous immunoglobulins appeared to be effective. While the flaccid quadriplegia often persisted for some time, ocular movements appeared to come back initially, along with signs of recovering consciousness. Then, after some weeks, small amplitude and long duration motor response were detectable in the upper limbs. Despite the very long ICU stays and prolonged recovery the eventual outcomes appeared favourable, after rehabilitation. Severe GBS may thus mimic brain death and it is important to stress that other testing is important when considering the possibility of Bickerstaff brainstem encephalitis (BBE). BBE is characterized by progressive ophthalmoplegia, ataxia and disturbance of consciousness, has an overlap with Miller Fisher syndrome and GBS [1-3].
Conclusion
Sepsis is the most common cause of hospital AKI rarely can associate with anuria or renal cortical necrosis (CN), with poor outcome but delayed recovery with late resumption of sufficient renal function to become dialysis independent still possible [6]. The exact cause of Guillain-Barre syndrome is unknown, but twothirds of patients report symptoms of an infection in the six weeks preceding. GBS can overlap with anti-GQ1b antibody syndromes (Miller Fisher, Bickerstaff brainstem encephalitis) which may mimic brain stem death. We strongly recommend an EEG and NCS in such cases, along with assay of anti-GQ1b antibodies where available.
Conflict of interest: The Authors declare no conflict of interest in regards this work. No funding was received for this work
Ethical approval: Ethical approval from Al Dhafra hospitals ethics committee (ADH -REC)-Abu Dhabi study no ADH REC549.
Patient consent: The patients provided written informed consent to publish their case and any associated images.
Acknowledgements: The author is grateful to Professor Stephen Geoffrey Holt, Chief executive officer SEHA kidney care for the guidance, generous academic and technical advice the author is indebted to the, Intensivists, and adult ICU staff nurses at Madinat Zayed Hospital -SEHA-Abu Dhabi -United Arab Emirates
Author statements
1-Salah Essa S, Contribution: Management of AKI, Renal replacement therapy (RRT), diagnosis of GBS, therapeutic plasma exchange (TPE) Procedure, data collection and writing the paper. Field of expertise: Critical care nephrology. Affiliations SEHA Kidney care, SEHA, Abu Dhabi health service company -Mainat Zayed Hospital Nephrology department. AL Dhafra, SEHA
2- Mohamed Sharaf, Contribution: actively engaged in the management of the patients, data collection, Madinat Zayed Hospital -internal medicine-Al Dhafra, -SEHA
3-Mohamed Rabee, Contribution: actively engaged in the management of the patients in ICU, data collection Madinat Zayed Hospital ICU-Al Dhafra, -SEHA
4-Mohamed Hussien, SEHA Kidney care, SEHA, Abu Dhabi health service company -Mainat Zayed Hospital Nephrology department. AL Dhafra, SEHA. Contribution: actively engaged in the management of the patients& Data collection.
5- Ibrahim Sbieh, Contribution: Data collection, Madinat Zayed -Hospital -ICU-Nursing -Al Dhafra, -SEHA
6-Ahmed Esmael, Contribution EEG, NCS, diagnosis and management of GBS -data collection, Neurology department Mainat Zayed Hospital Al Dhafra, SEHA.
Data availability: All data generated during cases are included in the article further inquiries to be address to the correspondence author.
References
- Arai, M, Odaka, M, Yuki, N. & Hirata, K. (2002) A patient with overlapping Bickerstaff’s brainstem encephalitis, Miller Fisher syndrome and Guillain–Barré syndrome during the clinical course. Eur. J. Neurol. 9: 115–116.
- Thomas, P. D. (2000) The Differential Diagnosis of Fixed Dilated Pupils: A Case Report and Review. Crit. Care Resusc. 2: 34–37.
- Walter U, Fernández-Torre J. L, Kirschstein T. & Laureys S. (2018) When is “brainstem death” brain death? The case for ancillary testing in primary infratentorial brain lesion. Clin. Neurophysiol. 129: 2451– 2465.
- Peerapornratana, S, Manrique-Caballero, C. L, Gómez, H. & Kellum, J. A. (2019) Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 96: 1083–1099.
- Thuysbaert, T, Standaert, C. & De Visschere, P. (2018) Reverse rim sign. Journal of the Belgian Society of Radiology 2018:102.
- Prakash, J. & Singh, V. P. (2015) Changing picture of renal cortical necrosis in acute kidney injury in developing country. World J. Nephrol. 4: 480.
- Choi, H. M. kim SC, Kim MG, Jo SK, Cho WY, et al. (2015) Etiology and outcomes of anuria in acute kidney injury: a single center study. Kidney Res. Clin. Pract. 34: 13–19.
- Willison, H. J, Jacobs, B. C. & van Doorn, P. A. (2016) Guillain-Barré syndrome. Lancet 388: 717–727.
- Coad, N. & Byrne, A. (1990) Guillain–Barré syndrome mimicking brainstem death. Anaesthesia 45: 456–457.
- Vargas, F, Hilbert G, Gruson D, Valentino R, Benissan GG, et al. (2000) Fulminant Guillain-Barré syndrome mimicking cerebral death: case report and literature review. Intensive Care Med. 26: 623–627.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.