‘Burned Out’ Hippocampus Syndrome: A Case Report Of Surgical Outcome Without Invasive Studies
by Florian Dashi1*, Arben Rroji2, Arba Cecia3, Arsen Seferi4, Mit’hat Demneri5, Suzana Gjeci5, Mentor Petrela6
1Functional Explorations and Intraoperative Monitoring, Department of Neurosciences, University Hospital Center “Mother Teresa”, Tirana, Albania
2Department of Interventional Neuroradiology, University Hospital Center “Mother Teresa”, Tirana, Albania
3Stritch School of Medicine, Loyola University Chicago. Maywood, Illinois, USA
4Department of Neurosurgery, University Hospital Center “Mother Teresa”, Tirana, Albania
5Department of Neurology, University Hospital Center “Mother Teresa”, Tirana, Albania
6Department of Neurosurgery and Interventional Neuroradiology, American Hospital 3, Tirana, Albania
*Corresponding author: Florian Dashi, MD, Functional Explorations and Intraoperative Monitoring, Department of Neurosciences, University Hospital Center “Mother Teresa”, Rruga Kongresi i Manastirit, Tirana, Albania
Received Date: 10 December 2024
Accepted Date: 16 December 2024
Published Date: 18 December 2024
Citation: Dashi F, Rroji A, Cecia A, Seferi A, Demneri M, et al. (2024). ‘Burned Out’ Hippocampus Syndrome: A Case Report Of Surgical Outcome Without Invasive Studies. Ann Case Report. 9: 2122. https://doi.org/10.29011/2574-7754.102122
Abstract
Hippocampal sclerosis represents the most prevalent form of pharmacoresistant epilepsy encountered in clinical practice. Surgery has been demonstrated to be effective when the history of the disease, ictal semiology, interictal/ictal video EEG, neuropsychological and neuroimaging studies are concordant. However, challenges may arise when different modalities yield conflicting results regarding the lateralization of the epileptogenic zone. The pattern of unilateral hippocampal sclerosis on brain magnetic resonance imaging (MRI), with contralateral temporal scalp ictal onset in video EEG, coined as ‘burned out’ hippocampus syndrome may potentially complicate decision-making for surgical treatment especially when further invasive studies may not be possible in all medical centers dealing with epilepsy patients. We present a case report with this pattern and our approach and outcome of this rare occurrence in epilepsy surgery centers.
Keywords: Temporal lobe epilepsy; Epilepsy surgery; Burned out hippocampus syndrome; Hippocampal sclerosis
Introduction
Hippocampal sclerosis is the most common pharmacoresistant epilepsy encountered in clinical practice to date, and has been shown to be amenable to surgery when the history of the disease, ictal semiology, interictal/ictal video EEG, neuropsychological and neuroimaging studies are concordant [1]. However, challenges may arise when different modalities yield conflicting results regarding the lateralization of the epileptogenic zone. In such scenarios, invasive neurophysiological studies, PET-FDG scans, and MEG can provide valuable information to guide a more informed surgical decision, increasing the chances of a favorable outcome [2]. The pattern of unilateral hippocampal sclerosis, as observed on brain magnetic resonance imaging (MRI), with contralateral temporal scalp ictal onset in ictal video EEG, was coined by Mintzer as ‘burned out’ hippocampus syndrome [3]. Discrepancies between imaging and neurophysiological data can raise concerns about bilateral epileptogenicity, potentially complicating decisionmaking for surgical treatment. This discordant pattern has been the focus of several articles, mostly case reports and a case series [2-7]. In this context, we present our case report and the outcome involving ‘burned out’ hippocampus syndrome.
Case Presentation
A 52-year-old right-handed person was admitted to our department due to frequent epileptic seizures, experiencing 10-20 seizures per month with clusters of 4-5 seizers per day that began at the age of 26. There was no significant medical or family history. His birth and development were unremarkable, and he denied having had febrile convulsions or trauma. His neurological examination was normal. Despite being treated with polytherapy that included Levetiracetam 3000 mg/day, Carbamazepine 1200 mg/day, and Clonazepam 2 mg/day, the patient became progressively pharmacoresistant and had previously failed trials with valproate, phenobarbitone, phenytoin, topiramate and lamotrigine. Consequently, he was deemed a potential candidate for epilepsy surgery and underwent a presurgical evaluation in our department, which operates as a Basic Epilepsy Center. The patient, without any preceding aura, exhibited impaired awareness with oral and bimanual automatisms followed by incoherent vocalizations and, on some occasions, evolved to generalized tonic-clonic seizures. Interictal EEG was performed with prior sleep deprivation so that stage I and II of sleep were included in the recording. A 10-20 array of electrodes was used with additional T1 and T2 electrodes that better cover the medial temporal epileptiform activities [8]. It showed sharp wave activity of 1-2 seconds in the left anterior and inferior temporal electrodes with a phase reversal in T1 and F7 in longitudinal montage. Preoperative 3 Tesla MRI of the brain with a dedicated epilepsy protocol revealed a severely atrophied right hippocampus and dilatation of the right temporal horn of the lateral ventricle. Neuropsychological evaluation demonstrated normal verbal memory and naming with slight deficits of shortterm visuospatial memory suggesting a right, no dominant, and temporal lobe involvement. Ictal video EEG recordings were performed in a dedicated video EEG room for 96 hours with a reduction in antiepileptic drugs. The seizure onset was marked by a period staring, followed by predominantly right-arm automatisms. Approximately 10-15 seconds after the seizure onset, abrupt theta discharges were observed at the left anterior (F7) and inferior temporal electrodes (T1). These discharges evolved into a more pointed shape, displaying a combination of theta activity in the left inferior temporal electrodes and movement artifacts. Epileptiform abnormalities were not detected on the right side. Invasive studies with depth electrodes and PET scan are not possible procedures in Albania, so we could not precede with further evaluations.
Since the EEG findings indicated exclusive abnormalities in the contralateral hippocampus, contradicting the atrophied right hippocampus detected by MRI, the patient was considered a case of “burned out” hippocampus syndrome”. Due to frequent pharmacoresistant seizures, the multidisciplinary team suggested a right anterior temporal lobectomy with en-bloc resection of the hippocampus, performed by extended pterional craniotomy. The operation was uneventful. The biopsy showed normal temporal neocortices but revealed a focal disruption of the normal architecture of the hippocampus consistent with hippocampal sclerosis. The patient has remained seizure-free for the past five years following surgery.
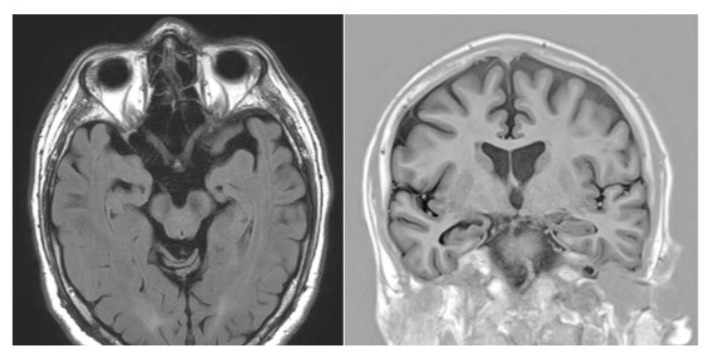
Figure 1: Preoperative MRI showing right hippocampal atrophy.
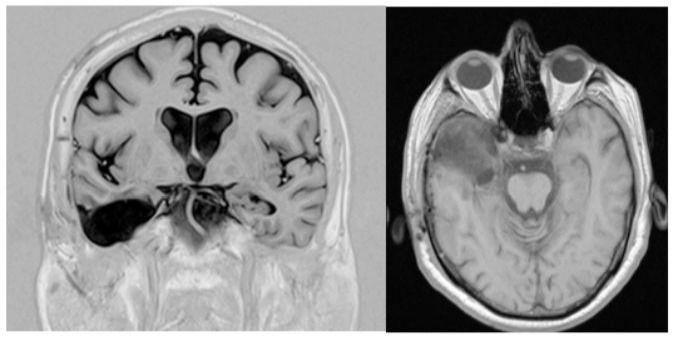
Figure 2: Postoperative MRI shows the excision of the hippocampal structures and the anterior temporal neocortex.
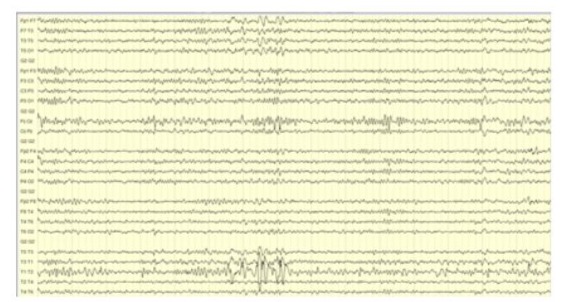
Figure 3: Interictal EEG showing phase reversal of sharp waves in the left anterior temporal (F7) and inferior temporal (T1) electrodes.
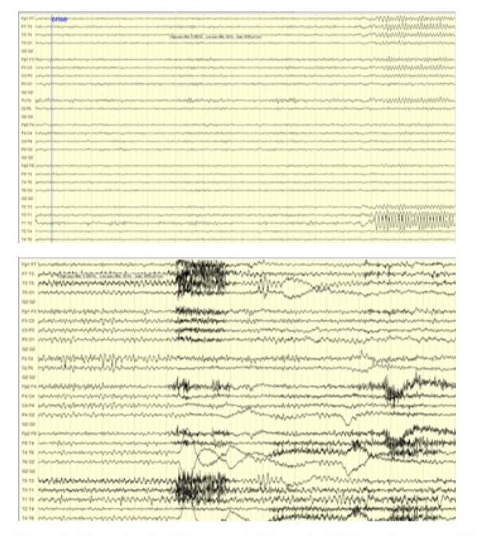
Figure 4: Ictal EEG showing focal theta discharges in the left temporal electrode chains at the onset of the seizure and still visible during the progression of the seizure on the left side.
Discussion
The syndrome of ‘burned out’ hippocampus is a rare entity in clinical practice [7]. The discrepancy between MRI and EEG may be attributed to the insufficient number of neocortical neurons in the damaged hippocampus capable of generating visible scalp discharges until the seizure propagates to the opposite temporal lobe, primarily through the anterior commissure [3, 9]. In such instances, more invasive studies using depth electrodes, when possible, may be necessary to determine the lateralization of the irritative and seizure onset zone, while other authors have successfully used foramen ovale electrodes as a semi-invasive study [7, 10]. But, in countries with only Basic Epilepsy Centers, invasive studies may not be feasible due to limited resources. However, in cases of a ‘burned out’ hippocampus documented in the literature, depth electrode recordings have indicated that the atrophied hippocampus seen on MRI scans is responsible for the onset of the seizure or have revealed bilateral abnormalities without providing clarity on the discrepancy. Several authors have suggested that in cases of the ‘burned out’ hippocampus, the epileptogenic zone probably corresponds to the side of the atrophic hippocampus in MRI [5].
Conclusion
Given the potential risks associated with persistent seizures, even in the absence of invasive studies, we recommend considering surgical removal of the atrophied hippocampus and anterior temporal structures, under the condition that the brain MRI shows an atrophy in the severe spectrum of the pattern of hippocampal sclerosis. In these cases, the imaging abnormality may be the most important factor for a favorable seizure outcome.
Declarations
Contributors: All authors contributed to planning, literature review and conduct of the review article. All authors have reviewed and agreed on the final manuscript.
Competing interests: None
Patient consent for publication: Informed consent was obtained from the patient, consent form available upon request
Ethics approval and consent to participate: Not applicable
Availability of data and materials: Not applicable
Funding: No Funding
References
- Vakharia VN, Duncan JS, Witt J, Elger CE, Staba R, et al. (2018). Getting the best outcomes from epilepsy surgery. Ann Neurol. 83: 676690.
- Gatzonis S, Siatouni A, Georgaculias N, Korfias S, Sakas DE. (2013). “Ictal Intracranial Recording from a Burned-Out Hippocampus,” Med Princ Pract. P: 4.
- Mintzer S, Cendes F, Soss J, Andermann F, Jr Engel J, et al. (2004). Unilateral Hippocampal Sclerosis with Contralateral Temporal Scalp Ictal Onset. Epilepsia. 45: 7.
- Suzgun MA, Cerci HM, Isler C, Uslu‐Besli L, Uzan M, et al. (2023). A medically refractory epilepsy case with discordant presurgical findings: The pitfall of burned‐out hippocampus. Epileptic Disord. 25: 907-910.
- Nair PP, Menon RN, Radhakrishnan A, Cherian A, Abraham M, et al. (2017). Is ‘burned-out hippocampus’ syndrome a distinct electroclinical variant of MTLE-HS syndrome? Epilepsy Behav. 69: 53-58.
- Carlier IDF, Rondon SAA, Sieger FAS, Figueroa IAF. (2020). “Síndrome de hipocampo quemado, mito o realidad. Reporte de caso,” Neurología, P: 4.
- Caboclo L, Garzon E, Miyashira FS, Carrete H (2005). “Temporal lobe epilepsy with unilateral hippocampal sclerosis and contralateral temporal scalp seizure onset: report of four patients with ‘burned-out hippocampus” J. epilepsy clin. neurophysiol. 11: 79-86.
- Silverman D. (1960). “The anterior temporal electrode and the tentwenty system,” Electroenceph. Clin. Neurophysiol. 12: 735-73.
- Gloor P, Salanova V, Olivier A, Quesney LF. (1993). “The human dorsal hippocampal commissure”. Brain. 116: 1249-1273.
- Tezer FI, Dericioglu N, Bozkurt G, Bilginer B, Akalan N, et al. (2011). “Epilepsy surgery in patients with unilateral mesial temporal sclerosis and contralateral scalp ictal onset,” Turkish Neurosurgery. 21: 549554.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.