Beyond Vaccine: Expanding the Frontiers of Community Action Network in Mitigating Mpox Outbreaks in Africa
by Simeon Cadmus1-4*, Victor Akinseye2,4,5, Samuel Ayanwale2, Eniola Cadmus6, Rashid Ansumana7, Robyn Alders8, Patrick Nguku9, Oyewale Tomori10
1Department of Veterinary Public Health and Preventive Medicine, University of Ibadan, Ibadan, Nigeria
2Damien Foundation Genomics and Mycobacteria Research and Training Centre, University of Ibadan, Nigeria
3Center for Control and Prevention of Zoonoses, Faculty of Veterinary Medicine, University of Ibadan, Ibadan, Nigeria 4Nigerian Institute of Medical Research, Yaba, Lagos, Nigeria
5Department of Chemical Sciences, Augustine University Ilara-Epe, Lagos State, Nigeria
6Department of Community Medicine, College of Medicine, University of Ibadan, Ibadan, Nigeria
7School of Community Health Sciences, Njala University, Bo, Sierra Leone
8Development Policy Centre, Australian National University, Canberra, ACT, Australia
9African Field Epidemiology Network (AFENET), Abuja, Nigeria
10African Centre of Excellence for Genomics of Infectious Diseases, Redeemer’s University, Ede, Osun State, Nigeria
*Corresponding author: Simeon Cadmus, Department of Veterinary Public Health and Preventive Medicine, University of Ibadan, Nigeria
Received Date: 19 October, 2024
Accepted Date: 28 October, 2024
Published Date: 31 October, 2024
Citation: Cadmus S, Akinseye V, Ayanwale S, Cadmus E, Ansumana R, et al. (2024) Beyond Vaccine: Expanding the Frontiers of Community Action Network in Mitigating Mpox Outbreaks in Africa. J Community Med Public Health 8: 479. https://doi.org/10.29011/2577-2228.100479
Introduction
Mpox, previously known as Monkeypox, is a viral zoonosis caused by the monkeypox virus (MPXV), a member of the Orthopoxvirus genus within the Poxviridae family. MPXV, along with variola virus, cowpox virus, and vaccinia virus, and is one of the four Orthopoxvirus species that can harm humans. It can infect many mammalian species, but its natural host remains unidentified [1]. The disease was first identified in humans in 1970 in the Democratic Republic of Congo (DRC), following an initial identification in laboratory monkeys in Demark in 1958 [1,2]. Since then, it has become an endemic disease in several Central and West African countries (DRC, Nigeria, Ghana, and Cameroon) and presently, the situation has gone from sporadic cases to an event of international concern [2-4]. The disease manifests in two distinct genetic clades: The Central African (Congo Basin, also known as Clade I and recently, Clade Ia and Ib), which is associated with a higher case fatality rate, and the West African (Clade IIa and b) which is generally less virulent and is responsible for the outbreaks in the region [5].
Mpox is reported to primarily affect rodents, primates, and other animals including rope and sun squirrels, giant-pouched rats, and African dormice [6] with humans becoming incidental hosts through contact with droppings of infected animals, body fluids, and cutaneous or mucosal lesions of infected animals [7]. However, sexual transmission of Clade I of Mpox virus (MPXV), especially among men who have sex with men (MSM) - legal (with spouse) and illegal (with sex workers) now becoming important and cases of abortion in pregnant women have been reported [8,9].
Another important source of infection is direct human-to-human contact with cases and contaminated beddings, furniture, and other fomites [7].
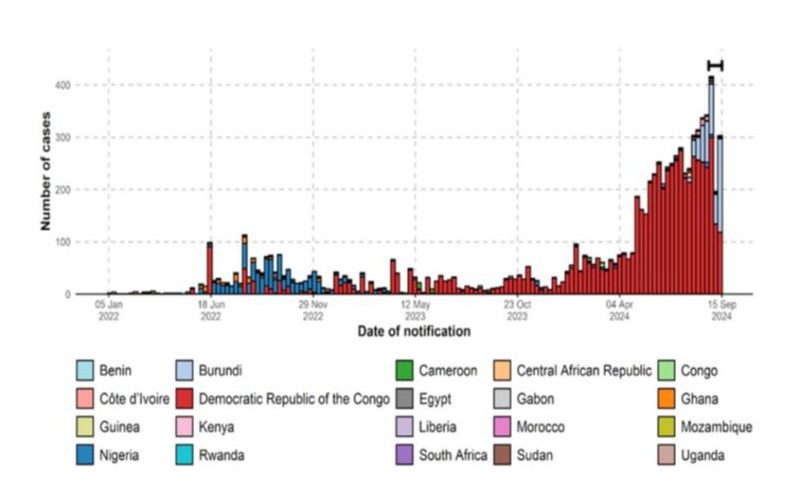
In recent times, there has been a sharp rise in Mpox cases in several countries with no clear epidemiological link to the endemic countries [10-12]. These periodic outbreaks are quite concerning and highlight the ongoing risk of zoonotic spillover, particularly in regions where human-wildlife interactions are common. Notably, the World Health Organization (WHO) declared Mpox as a Public Health Emergency of International Concern (PHEIC) in July 2022. Although this declaration was lifted in May 2023 following a significant drop in number, a total of 87,377 cases had been reported in 118 countries [13]. However, on August 14, 2024, the WHO once again declared the disease as a PHEIC following ongoing outbreaks, spread and detection of cases of MPXV in several countries, most of which are in Africa (i.e., Burundi, Kenya, Nigeria, Rwanda, and Uganda) and outside Africa, i.e. India, Philippines, Sweden, and Thailand that had never reported any case of Mpox [14]. In the endemic region of Africa, the outbreak of Mpox constitutes a huge public health concern. So far in 2024, as of 12 October 2024, 17 countries have reported 7,532 confirmed cases, including 979 deaths. The three countries with most of the most cases in 2024 are DRC, (n = 5 399), Burundi, (n = 564), and Nigeria, (n = 55) [15]. Suspected cases across the continent have surged past 17,000, a significant increase from 7,146 cases in 2022 and 14,957 cases in 2023 [14]. The African CDC reported that as of 22 October 2022, considering the geographical spread of Mpox in West Africa, Nigeria recorded the highest cases of cumulative Mpox (1824) followed by Ghana with 640 cases, and Benin Republic as well as Liberia reporting six cases each (Figure 1A).
In Nigeria in particular, it was reported that 762 and 98 confirmed cases were reported in 2022 and 2023 respectively. Also, it has been estimated that 48 Mpox cases (as of 25th of August) have been recorded this year involving 20 states and the Federal Capital Territory (FCT) [16]. In the southern region of Nigeria, specifically Enugu, Bayelsa and Cross River States are the current hotspots of the Mpox virus [16]. Furthermore, it has been observed that the disease is spreading across contiguous neighboring states like Delta, Anambra, Enugu, and Benue all having reported cases. In addition, Ebonyi, Abia and Imo states are all bordering states with reports of single cases. States in the west and north, including Lagos, Oyo, Osun, Kebbi, Zamfara, Kaduna, and Nasarawa, have recorded a single case only, while the FCT, Abuja and Plateau states have recorded two new cases. Other states like Lagos, Oyo, Osun, Kebbi, Zamfara, Kaduna, and Nasarawa, spanning western and northern regions of the country, have recorded a single case each, while the FCT and Plateau states have recorded two new cases, creating a spatial distribution of Mpox (Figure 1B).
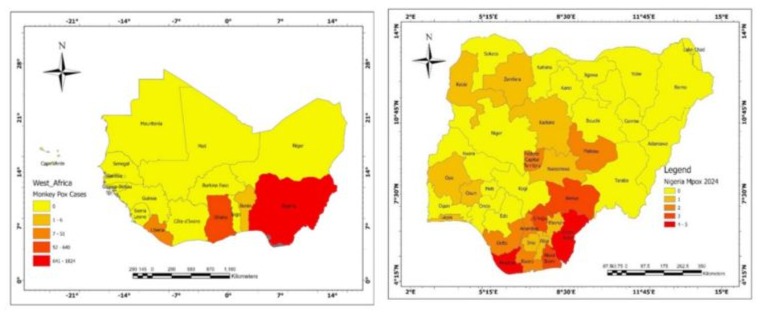
Figure 1A: West Africa Mpox. Figure 1B: Spatial distribution of Mpox 2024 in Nigeria. Source: Department of Geography, University of Ibadan; https://Grid3.org
Factors such as loss of orthopoxvirus-related herd immunity from previous smallpox vaccinations, as well as increased human contact with Mpox-related animal reservoirs due to trade, deforestation, animal husbandry, consumption of bushmeat, inadequate water, sanitation, and hygiene known as WASH (associated with poor potable water supply), inadequate infection, prevention, and control (IPC) measures, and climate change, have been implicated to contribute to the disease re-emergence and spread in Nigeria and in other affected African countries (Figure 2) [17].
Figure 2: Number of cases of Mpox in Africa from 2022 to 2024. Source: Department of Geography, University of Ibadan; https://Grid3.org
Mpox has no specific treatment, and although the smallpox vaccine offers some cross-protection against the disease, the cessation of mass vaccination and ring vaccination around cases for eradication has led to increased susceptibility in younger populations [17].
Medical interventions must be complemented by community action
Currently, one of the existing preventive measures against Mpox is the use of vaccine. In 2022, during the global health emergency for Mpox, the European Union, United States and Canada approved the use of the Bavarian Nordic’s Imvanex MVA-BN vaccine, primarily meant to protect against smallpox, for use against Mpox. In addition, a few antiviral drugs like cidofovir or tecovirimat that are classical treatments for smallpox have equally shown promise in Mpox cases, although they are not clinically approved [18]. Nevertheless, it is imperative to note that, while vaccine development and administration as well as drug discovery are critical components of the response to Mpox, they alone, are insufficient. Therefore, there is a need to develop a bottom-top holistic grassroots/local mobilization approach that, in addition to raising awareness of the disease, will enhance timely case reporting and surveillance aimed at driving the effectiveness of vaccine and drug interventions useful for mitigating against the spread and impact of the disease. One such approach is the Community Action Network (CAN), a community-based awareness creating and mobilization (for action) platform.
The CAN (Figure 3) is a group of selfless and concerned community members from various sectors of the community with equal gender representation committed to working together to improve the health and wellness of the communities at the human, animal and environment interface. Importantly, CAN is an integrated One Health team operating at the grassroots consisting of individual people and groups representing the human (such as community health workers, patent medicine vendors), animal (such as community animal health workers, pastoralist leaders, farmers, hunters), and environment (such as environment health workers, forest rangers) health. This network has emerged as a critical partner in combating the outbreak of diseases through grassroots mobilization, education, and community-driven activities. This grassroots/local mobilization approach is particularly important in Africa, where there is poor awareness and a dearth of healthcare facilities (especially in the rural settings where these cases are common and are often hard to reach), and cultural beliefs and misinformation can often hinder the adoption of health interventions like vaccination [19]. This One Health-centered initiative is important because it encompasses collaborative efforts at the grassroots community level toward developing a sustainable and equitable global plan [19] which can be easily adapted in low- and middle-income countries of Africa where Mpox and other diseases of public health importance are endemic, emerging and re-emerging.
Some of the primary contributions of CAN (Figure 3) include public education, awareness raising, and surveillance. Other advantages of this approach are that it does not necessarily require funding from external sources, it is fast and relatively easy to adopt as it involves human capacity and resource mobilization and utilization at the granular/grassroots level which can also be selfsustaining. Importantly, given the CAN platform’s human-animalenvironment membership composition and gender inclusiveness, it can play a major role in providing a better understanding of the transmission and epidemiology of Mpox and other endemic and emerging disease outbreaks including Lassa fever, yellow fever, and so on. Thus, the CAN initiative can be adopted in many other Mpox-endemic countries to support both local and international efforts at mitigating this disease and those yet to emerge.
Going forward, it is necessary to expand the frontiers of CAN to provide necessary avenues to improve the overall effectiveness of Africa’s Mpox response. This includes the integration of CAN into the surveillance and early detection of Mpox cases and the empowerment of CAN members for the provision of psychosocial support and training in basic hygiene care to those affected by the disease [20]. This will involve training CAN members in basic disease surveillance techniques and equipping them with the tools needed for contact-tracing and prompt reporting to enhance earlywarning-early-response (Figures 4 and 5). This will go a long way to optimizing resources towards mitigation and saving millions of dollars in fighting outbreaks with the aim of saving lives and reducing the disability adjusted life years (DALYs).
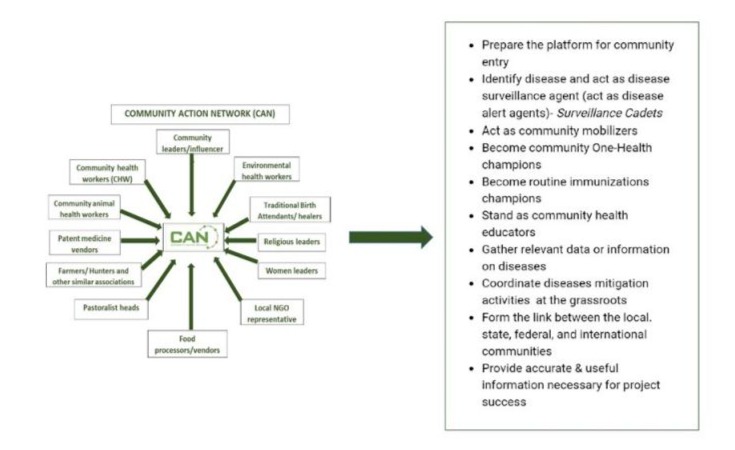
Figure 3: The CAN members and their roles in the mitigation of diseases.
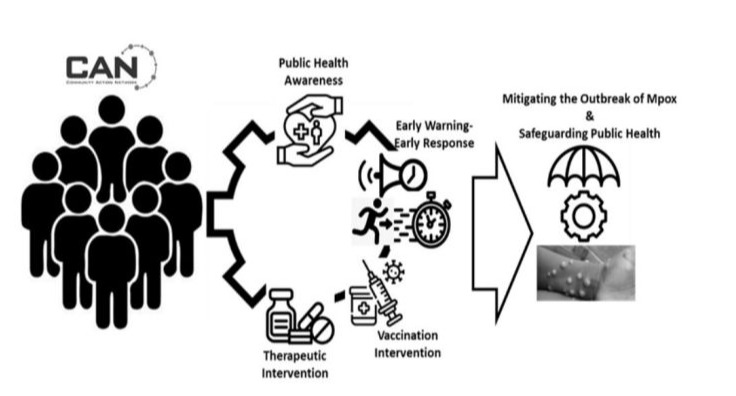
Figure 4: Mitigating capacity of CAN initiative on Mpox outbreak and management.
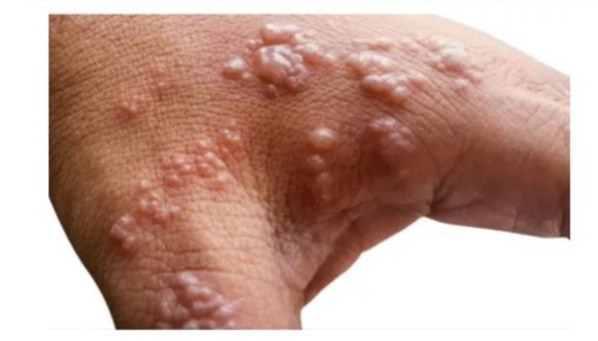
Figure 5: One of the suspected cases of Mpox discovered through the efforts of CAN members in Ebonyi State.
Conclusions
Importantly, the integration of the CAN initiative in Africa will bring about an effective way of addressing outbreaks in local communities due to the direct engagement and relationship of its members in the affected communities. While vaccinations and therapeutics are crucial tools in the fight against the current Mpox outbreaks in Africa, it is imperative to supplement these interventions with strong, community-driven efforts. Thus, the CAN initiative is in a strong position to lead these efforts because of its robust relationships with the local communities, deep understanding of socio-cultural dynamics, presence at hotspots, and influence of its members at the grassroots. Finally, through the enlargement of its scope to encompass early detection, surveillance, psychosocial support and appropriate hygiene practices in addition to public awareness, the network can play a significant role in mitigating the outbreak of Mpox and other endemic and emerging diseases as well as safeguard public health in the endemic regions of Africa.
Acknowledgments
We appreciate the support of the members of the Community Action Network (CAN) and the West African One-Health (WAOH) Consortium supported by the International Development Research Centre (IDRC).
References
- Arita I, Henderson DA (1976) Smallpox and monkeypox in non-human primates. Bull World Health Organ 39: 277-283.
- Tomori O, Ogoina D (2022) Monkeypox: The consequences of neglecting a disease, anywhere. Science 377: 1261-1263.
- Cadmus S, Akinseye V, Besong M, Olanipekun T, Fadele J, et al. (2024) Dynamics of Mpox infection in Nigeria: a systematic review and meta-analysis. Sci Rep 14: 7368.
- Yinka-Ogunleye A, Aruna O, Dalhat M, Ogoina D, McCollum A, et al. (2019) Outbreak of human monkeypox in Nigeria in 2017–18: a clinical and epidemiological report. Lancet Infect Dis 19: 872-879.
- Okwor T, Mbala P, Evans DH, Kindrachuk J (2023) A contemporary review of clade-specific virological differences in monkeypox viruses. Clin Microbiol Infect 29: 1502-1507.
- Falendysz EA, Lopera JG, Rocke TE, Osorio JE (2023) Monkeypox Virus in Animals: Current Knowledge of Viral Transmission and Pathogenesis in Wild Animal Reservoirs and Captive Animal Models. Viruses 15: 905.
- Ogoina D, Dalhat MM, Denue BA, Okowa M, Chika-Igwenyi NM, et al. (2024) Mpox Epidemiology and Risk Factors, Nigeria, 2022. Emerg Infect Dis 30: 9.
- Heskin J, Belfield A, Milne C, Brown N, Walters Y, et al. (2022) Transmission of monkeypox virus through sexual contact – A novel route of infection. J Infect 85: 334-363.
- Masirika LM, Nieuwenhuijse DF, Ndishimye P, Udahemuka JC, Steeven BK, et al. (2024). Mapping the distribution and describing the first cases from an ongoing outbreak of a New Strain of mpox in South Kivu, Eastern Democratic Republic of Congo between September 2023 to April 2024. MedRxiv (Cold Spring Harbor Laboratory).
- Huang Y, Mu L, Wang W (2022) Monkeypox: epidemiology, pathogenesis, treatment and prevention. Signal Transduct Target Ther 7: 373.
- Zumla A, Valdoleiros SR, Haider N, Asogun D, Ntoumi F, et al. (2022). Monkeypox outbreaks outside endemic regions: scientific and social priorities. Lancet Infect Dis 22: 929-931.
- Venkatesan P (2022) Global monkeypox outbreak. Lancet Infect Dis 22: 950.
- WHO (2005) Second meeting of the International Health Regulations (IHR) Emergency Committee regarding the multi-country outbreak of monkeypox.
- Africa CDC (2024) Africa CDC Declares Mpox A Public Health Emergency of Continental Security, Mobilizing Resources Across the Continent.
- WHO (2024) 2022-24 Mpox Outbreak: Global Trends.
- Nigeria Centre for Disease Control (2024) An Update of Monkeypox Outbreak in Nigeria.
- Tehranchinia Z, Robati RM, Moravvej H, Memariani M, Memariani H (2023) Monkeypox Disease with a Focus on the 2022 Outbreak; a Narrative Review. Arch Acad Emerg Med 11: e19.
- “Bavarian Nordic,” (2023) Bavarian-nordic.com.
- Tomori O, Ogoina D (2022) Monkeypox: The consequences of neglecting a disease, anywhere. Science 377: 1261-1263.
- CDC (2022) Mpox in the U.S. Centers for Disease Control and Prevention.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.