Autologous Adipose-Derived Stem Cells (Matrigen®) for Treatment of Perianal Fistula: Retrospective Single-Centre Study
by Mascali Davide1, Lo Bianco Catalin2, Basile Guido2, Calaciura Giuseppe2, Ferracane Concetta3, Piazza Luigi1, Lo Bianco Salvatore1*
1Department of Oncological Surgery, ARNAS Garibaldi, Catania, Italy
2Department of General Surgery and Medical Surgical Specialties, G. Rodolico, Catania, Italy
3Department of Gastroenterlogy, G. Rodolico, Catania, Italy
*Corresponding Author: Salvatore Lo Bianco, Department of Oncological Surgery, ARNAS Garibaldi, Catania, Italy
Received Date: 07 June 2025
Accepted Date: 12 June 2025
Published Date: 14 June 2025
Citation: Mascali D, Lo Bianco C, Basile G, Calaciura G, Ferracane C, et al. (2025) Autologous Adipose-Derived Stem Cells (Matrigen®) for Treatment of Perianal Fistula: Retrospective Single-Centre Study. J Surg 10: 11347 https://doi.org/10.29011/2575-9760.011347
Abstract
Perianal fistula is a complex and often recurrent condition with a significant impact on patients’ quality of life. Traditional surgical treatments, while effective, may have limitations in terms of complete healing and risk of recurrence. This retrospective clinical trial evaluated the efficacy of using autologous Adipose-Derived Stem Cells (Matrigen®) (ASCs) in the treatment of recurrent and/ or complex perianal fistula. Eighteen patients were divided into two groups: a control group undergoing standard surgery and an experimental group treated with ASCs. The results, obtained after 12 weeks of follow-up, showed a cure rate of 80% in the ASCs group compared to 62.5% in the control group, with a reduction in the recurrence rate. Therefore, the results of this preliminary study indicate that the use of ASCs represents a promising therapeutic option for the treatment of perianal fistula, capable of improving healing rates and reducing the risk of recurrence compared to standard surgery. However, large-scale clinical trials with long-term follow-up are needed to confirm these results and evaluate the efficacy of ASCs in different types of perianal fistulas.
Keywords: Adipose; Autologous; Fistula; Perianal; Stem Cells
Introduction
The aim of this clinical study is to determine the safety and efficacy of autologous adipose-derived stem cells for the treatment of perianal fistula. Minimally invasive surgery of perianal fistulas has become increasingly important in recent years, especially in the use of autologous stem cells. The immunomodulatory and anti-inflammatory capabilities of adipose-derived stem cells could contribute to the healing process of the fistula [1].
Materials and Methods
This study was conducted in accordance with the rules of the STROBE Statement (Strengthening the reporting of observational studies in epidemiology). The sample size included 18 patients whose data were recorded in an anonymous database. All were
admitted and treated at the Oncological Surgery Department - Garibaldi Nesima - Italy, from November 2022 to February 2023. Briefly, all patients presented with a recurrent and/or complex perianal fistula, with or without Chron’s disease. Patients provided informed consent for data recording within our database in an anonymous form. The diagnosis of perianal fistula was made by examination (MRI of the pelvis) in all patients. Treatment results were assessed by the operator at 2, 4, 6 and 12 weeks after treatment. Healing was defined as the absence of drainage through the external openings and the complete re-epithelialisation of these openings, as well as the absence of pain. A retrospective clinical study was conducted by obtaining data from the pre-filled database. Patients were enrolled from November 2022 to February 2023. During the screening visit 18 patients were divided into two groups A (control) and B (experimental). The groups were created taking into account the patients’ data to minimise bias.
Group A included 8 patients (male:female, M:F=1.6) who had received ‘Standard surgery’, consisting on excision of fistula up to the previously specillated internal orifice; the internal orifice was obliterated using mucosal flap and sutures. Group B included patients who had received ‘Standard Surgery and injection of autologous Adipose-Derived Stem Cells (ASCs)’ with 10 patients (M:F=0.66). The surgery group was the same in both groups and the same group then evaluated the patients in follow-up for 2, 4, 6 and 12 weeks after treatment. At least 4 weeks before surgery, all patients underwent drainage seton. Subsequently, during the same operating session, patients in group B underwent liposuction for ASCs.
Liposuction was performed by the same surgical team under local anaesthesia, obtaining at least 200 ml of lipoaspirate. This material was subsequently processed in a Matrigen® filtration system directly in the operating room. The final dose of ASCs was approximately 40 ml prepared in ready-to-use syringes. The adipose preparation was subsequently administered in the operating room according to a standard surgical protocol, as described in ‘A Step-By-Step Surgical Protocol for the Treatment of Perianal Fistula with Adipose-Derived Mesenchymal Stem Cells’, and with external control during the surgical procedure in all cases. Briefly, prior to the injection of ASCs (group B), the excision of all via fistula up to the previously specillated internal orifice was performed. The internal orifice was obliterated using mucosal flap and sutures. Half of each dose was injected around the inner opening and the other half through the outer opening. Finally, the external wound was sutured with detached silk stitches. Clinical evaluation of fistula healing was assessed at 2, 4, 6 and 12 weeks after treatment. The incidence of Adverse Events (AE) and Serious Adverse Events (SAE) was assessed at each follow-up visit. The operating surgeon assessed healing at weeks 2, 4, 6 and 12. Healing was defined as the absence of drainage through the external wound and its complete re-epithelialisation in the absence of pain. Considering the effective results obtained and recorded in our database, we requested the ethics committee’s permission to perform a retrospective study to perform a long-term (ongoing) follow-up of the patients included in the study, with a focus on longterm safety, recurrence of healed fistulas, and to determine the final status of healing and perianal suppuration. The data were expressed as mean ± standard deviation. The results were analyzed using Statistica® v.10 software and significance was calculated using the Student’s t and Chi-square method.
Results
This retrospective clinical study was designed to determine the efficacy of a new standardised and monitored surgical procedure using ASCs for the treatment of perianal fistula. ASCs were chosen both because the stem cells and their anti-inflammatory effects promote long-term healing and for their excellent safety profile. As shown in Figure (indicate number), 18 patients with perianal fistula were enrolled and divided into two groups: Group A underwent ‘standard surgery’ (8 patients, male:female M:F=1.6); Group B underwent ‘surgery with ASCs’ (10 patients, M:F=0.66). Group A comprised patients aged between 20-52 years; the average age was 34 years (Figure 1).
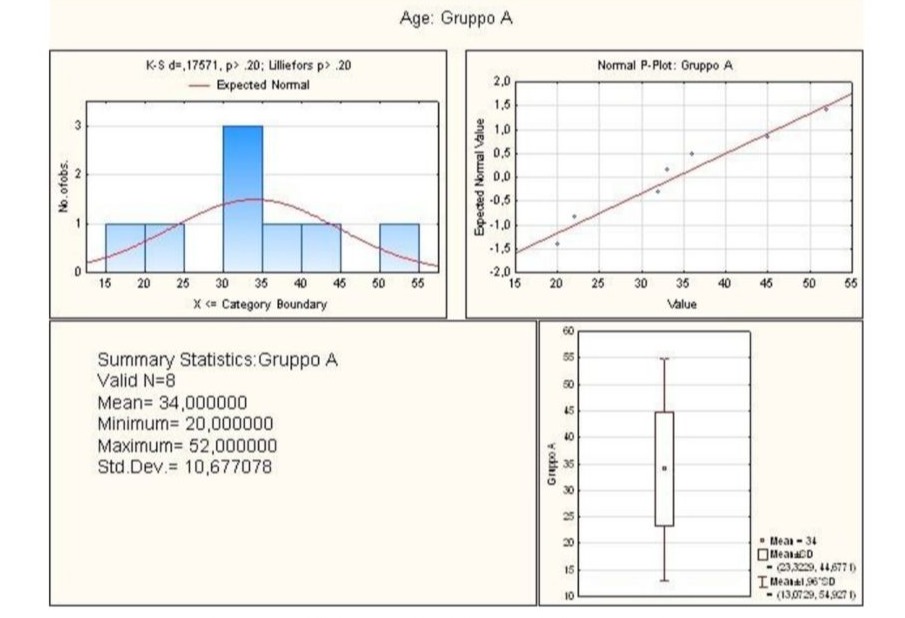
Figure 1: Graphical summary of statistical analysis of age group A patients.
Through statistical analysis, the lower and upper quartile of the age of the Group A patients was calculated. The first was 27.00 while the second was 40.50, the minimum being 20.00, the median 32.50 and the maximum 52.00. Finally, the standard deviation value was 10.68 (Figure 2).
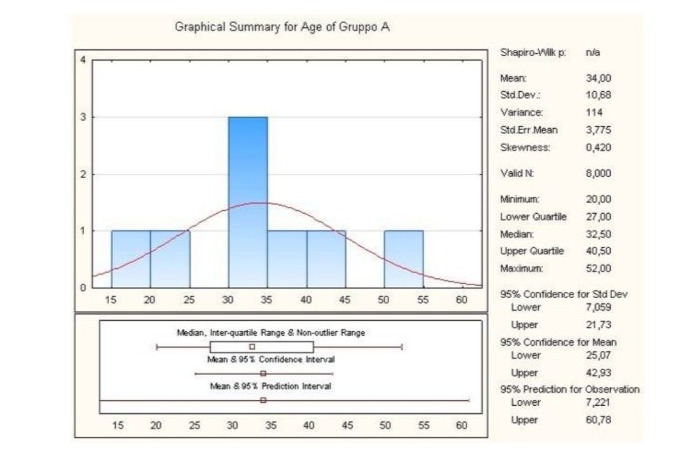
Figure 2: Graphical summary of statistical analysis of age group A patients.
As for Group B, it comprised patients aged between 18-50 years, in fact the average age was 29.2 years (Figure 3).
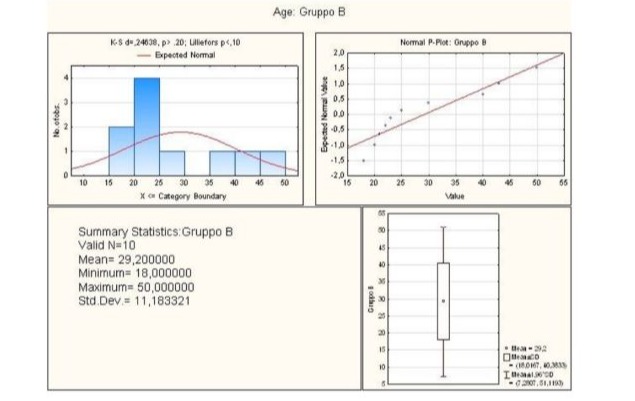
Figure 3: Graphical summary of statistical analysis of age group B patients.
Statistical analysis revealed that the lower quartile corresponded to 21.00, while the upper quartile corresponded to 40.00, the minimum being 18.00, the median 24.00 and the maximum 50.00. Finally, the standard deviation value was 11.18 (Figure 4).
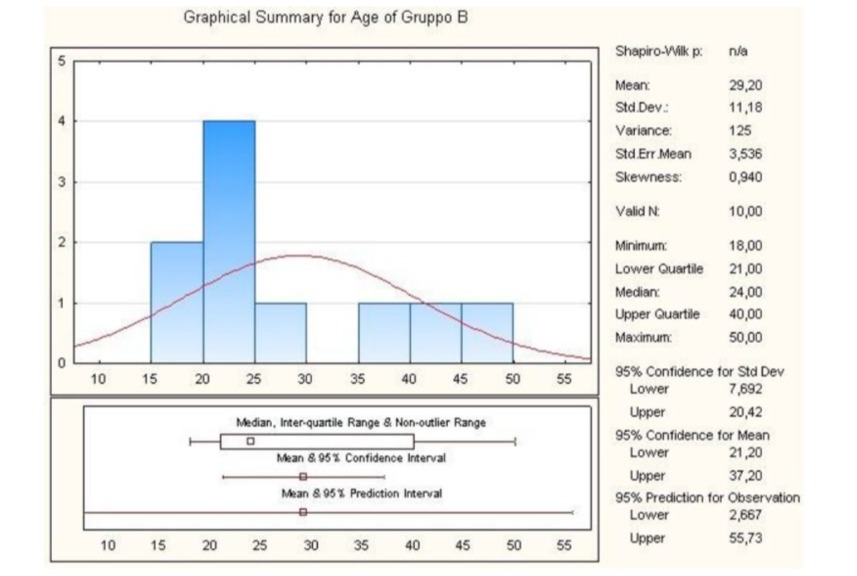
Figure 4: Graphical summary of statistical analysis of age group B patients.
All patients followed-up at 2, 4, 6 and 12 weeks after treatment. After 12 weeks significant differences were found between patients in group A and those who received the ASCs (group B). Thus, the results show that the fistula healing rate 12 weeks after the first treatment was 62.50% (95% CI; 5 out of 8 patients) for group A and 80.00% (95% CI, 21.8-65.9; 8 out of 10 patients) for group B (Figure 5). Therefore, surgical treatment with ASCs showed a significant increase of 17.5% of patients healed already after 12 weeks compared to treatment with standard surgery. However, cases of recurrence were recorded with both surgical treatments. Indeed, after 12 weeks of follow-up, a significant change was observed, as two out of eight healed patients in group A (25.00%; CI: 95%) experienced recurrence compared to only one out of 10 patients in group B (10.00%; CI: 95%) (Figure 5).
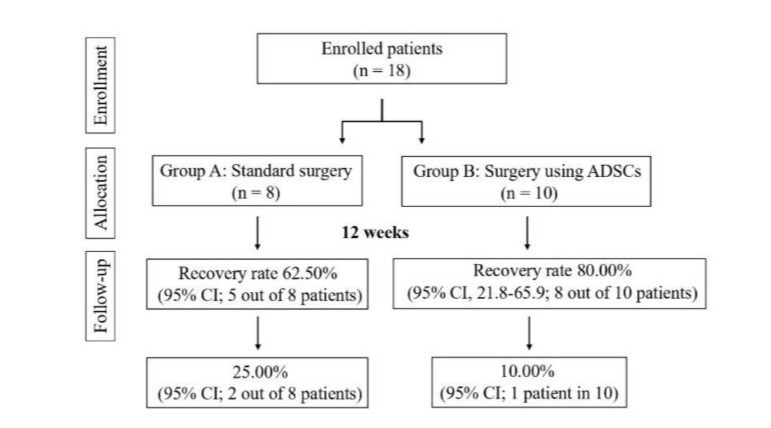
Figure 5: Flow-chart: enrollment, allocation and follow-up. ASCs: Autologous adipose-derived stem cells.
Therefore, based on the results obtained, we believe that the new surgical protocol based on surgical treatment with ASCs played an important role in reducing the number of fistula recurrence rates. Furthermore, we did not find any other differences between the groups in the clinical evolution of the patients (data not shown). Finally, no statistically significant differences were observed between the groups (P = 0.403). In this study, the placebo effect, which has been shown to have a strong influence in the field of stem cell therapy, was eliminated [1]. The main limitation of this study is the low number of patients recruited and the shortterm follow-up; in fact, both patient enrolment and follow-up were carried out in order to expand the study to a larger number of patients and to monitor the rate of healing and long- term relapse. Another limitation is that, due to the mechanism of action of ASCs, it might be advisable to use autologous or at most allogeneic ASCs from selected healthy donors where liposuction is not possible. Patients with Crohn’s disease have an inflammatory process and, due to the anti- inflammatory properties̀ of ASCs, we expect better results in patients with this disease condition. Thus, the only treatment for fistulous Crohn’s disease that has reported efficacy results in a phase III clinical trial is the use of allogeneic cells [2]. The advantage is to be able to select ASCs from the best donor and to speed up the treatment, avoiding possible inconveniences (contamination, culture problems) from the adipose tissue that is to be removed to be implanted during the process of production and/ or transport. However, it is emphasized that ASCs from patients with inflammatory illnesses have reduced anti-inflammatory capabilities and functional abnormalities [3].
Discussion
Seton placement is the primary treatment of perianal fistula surgery. More studies have shown that combination treatment of ASCs and surgery yielded better results [4,5]. Antibiotics, immunosuppressants and steroids, have little effectiveness in the treatment of Crohn’s perianal fistula, with recurrence of disease [6-8]. Present DH in his randomized trial showed that healing rates were significantly higher with infliximab than with placebo [9,10]. Transplantation of auto-ASCs for Crohn’s perianal fistula has been shown to be safe. A phase I trial in with Crohn’s perianal fistula resulted in 75% complete closure of the external opening [10]. Garcia-Olmo in his study demonstrated fistula closure in 56% of patients undergoing auto-ASC transplantation [11]. Lee WY. showed that the 1 and 2 year closure rates were 88% and 75% [12]. Given the relapse rate of Crohn’s perianal fistula in patients receiving infliximab or underwent seton placement, stem cell transplantation would be a efficacious and safe. Our study had important limitations due to its retrospective design, selection bias and small number of patients.
Conclusion
This retrospective study, although limited by a small sample size and short-term follow-up, suggests that surgical treatment of perianal fistulas with autologous Adipose Tissue-Derived Stem Cells (ASCs) may offer a clinically relevant improvement in healing rates and a reduction in recurrence compared to standard surgical procedures. Specifically, the study demonstrated a 17.5% increase in healing rates at 12 weeks in the ASCs group and a lower recurrence rate. Despite the absence of statistically significant differences between the groups, probably due to the limited number of patients, the observed trends indicate a potential benefit of ASCs in this context. However, a larger, long-term study is essential to validate these preliminary findings and to fully evaluate the long-term safety and efficacy of ASCs in the treatment of perianal fistula. Furthermore, future research should explore the use of allogeneic ASCs in specific patient populations, such as those with Crohn’s disease, and address the potential impact of inflammatory conditions on the functionality of ASCs.
References
- Thomsen GM, Gowing G, Svendsen S, Svendsen CN (2014) The past, present and future of stem cell clinical trials for ALS. Experimental neurology 262: 127-137.
- Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, et al. (2016) Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double- blind controlled trial. Lancet (London, England) 388: 1281-1290.
- Garcia-Arranz M, Garcia-Olmo D, Herreros MD, Gracia-Solana J, Guadalajara H, et al. (2020) Autologous adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistula: A randomized clinical trial with long- term follow-up. Stem cells translational medicine 9: 295-301.
- Bouguen G, Siproudhis L, Gizard E, Wallenhorst T, Billioud V, et al. (2013) Long-term outcome of perianal fistulizing Crohn’s disease treated with infliximab. Clin Gastroenterol Hepatol 11: 975-981. e974.
- Gaertner WB, Decanini A, Mellgren A, Lowry AC, Goldberg SM, et al. (2007) Does infliximab infusion impact results of operative treatment for Crohn’s perianal fistulas? Dis Colon Rectum 50: 1754-1760.
- Present DH, Lichtiger S (1994) Efficacy of cyclosporine in treatment of fistula of Crohn’s disease. Dig Dis Sci 39: 374-380.
- Hanauer SB, Smith MB (1993) Rapid closure of Crohn’s disease fistulas with continuous intravenous cyclosporin A. Am J Gastroenterol 88: 646.
- Lowry PW, Weaver AL, Tremaine WJ, Sandborn WJ (1999)Combination therapy with oral tacrolimus (FK506) and azathioprine or 6-mercaptopurine for treatment-refractory Crohn’s disease perianal fistulae. Inflamm Bowel Dis 5: 239-245.
- Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, et al. (1999) Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med 340: 1398-1405.
- García-Olmo D, García-Arranz M, Herreros D, Pascual I, Peiro C, et al. (2005) A phase I clinical trial of the treatment of Crohns fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum 48: 1416-1423.
- Garcia-Olmo D, Herreros D, Pascual I, Pascual JA, Del-Valle E, et al. (2009) Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum 52: 79-86.
- Lee WY, Park KJ, Cho YB, Yoon SN, Song KH, et al. (2013) Autologous adipose tissue‐derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn’s fistula. Stem Cells 31: 2575-2581.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.