Anatomical Ultrasound Study of the Cephalic Vein in the Deltopectoral Groove in Oncological Patients Eligible for a Totally Implantable Venous Access Port
by Barba A
Department of Angiology and Vascular Surgery, IMQ Zorrotzaurre University Hospital, Bilbao, Basque Country, Spain
*Corresponding author: Ángel Barba Vélez, Department of Angiology and Vascular Surgery, IMQ Zorrotzaurre University Hospital, Ballets Olaeta Kalea. 48014 Bilbao, Basque Country, Spain
Received Date: 29 November 2024
Accepted Date: 05 December 2024
Published Date: 07 December 2024
Citation: Barba A (2024) Anatomical Ultrasound Study of the Cephalic Vein in the Deltopectoral Groove in Oncological Patients Eligible for a Totally Implantable Venous Access Port. J Surg 9: 11199 https://doi.org/10.29011/2575-9760.011199
Abstract
Background: Cephalic Vein Cutdown (CVC) in the Deltopectoral Groove (DG) is a simple surgical technique used for the insertion of pacemakers or Totally Implantable Venous Access Ports (TIVAP), with few intra- or postoperative complications. Due to possible anatomical variations, its success rate has reported to be lower than percutaneous ultrasound-guided techniques. The aim of this study is to describe the anatomy of the Cephalic Vein (CV) in the DG.
Methods: A total of 2400 CVs in the DGs of 1200 patients were studied with ultrasound before the implantation of the TIVAP for chemotherapy treatment. Diameter, depth, tortuosity, CV/Axillary Vein junction (CV/AV) and AV patency were measured and related to gender and laterality.
Results: The mean age of the 1200 patients was 63.3 years, 744 (62.0%) of whom were women. The CV was absent in 155 (6.5%) cases and there were no significant differences between sexes. The mean diameter of the CVs, when present, was 3.8 mm and the mean depth was 13.6 mm (SD: 2.9). Both diameter and depth were significantly greater in men. In 91 (4.1%) cases there was stenosis of the CV/AV junction, being significantly greater on the left side. CV tortuosity and AV occlusion were observed in 32 (1.4%) and in 22 (0.9%) cases, respectively, with no statistically significant differences found between sexes. Two thousand and fifty-seven CVs (85.7%) had a diameter ≥ 3.0 mm.
Conclusions: The present study showed that the diameter and depth of the CVs were greater in men and on the right side. Stenosis of the CV/AV junction and tortuosity were more common in left-sided CVs.
Keywords: Cephalic Vein; Deltopectoral groove; Preoperative Duplex Ultrasonography; Totally Implantable Venous Access Ports
Introduction
The first available description of the Cephalic Vein (CV) was proposed in the 2nd century AD by Galen of Pergamon who transposed his observations made on monkeys onto humans and claimed that the CV (Galen’s humeral vein) “arose” from the external jugular vein and encircling the clavicle “ran towards the periphery” [1]. The CV as a term originates from the Arabic word al-kefal, which means “outer” and was first used by Muslim physician Abu Ali al-Hossein ibn Adbullah Ibn Sina (known as Avicenna in the West), when the term was translated to Latin, cephalic inaccurately was selected to replace the Arabic origin of the term [2]. Until the last decade of the 20th century, most studies of the cephalic veins were anatomical and were performed on cadavers [3] or with intraoperative venography [4], while ultrasound is the most widely used technique nowadays [5]. Since the beginning of pacemaker implants in 1959 [6] and Totally Implantable Venous Access Port (TIVAP) in 1982 via the Cephalic Vein Cutdown (CVC) approach [7], this is the access route with the fewest intra- and postoperative complications, although due to variations in caliber and depth in the deltopectoral groove (DG), as well as other possible anomalies, a success rate of 71.0% has been reported [8]. Hence the importance of identifying the exact anatomy.
Materials and Methods
In January 2008, our Department of Angiology and Vascular Surgery´s Outpatient Clinic began performing a preoperative Doppler ultrasound on all patients referred by the Oncology Department for the implantation of a TIVAP for chemotherapy treatment. Although the first choice for implantation is the Left Cephalic Vein (LCV), except in left-handed patients, those who had undergone breast surgery on the left side with/without axillary lymphadenectomy or previous TIVAP/pacemaker implanted on this side, assessment of CVs in both DGs were made. During the outpatient clinic and with the patient in supine position the CV is displayed by means of a 7.5 – 12 MHz probe of MyLab50 and MyLabX5 (Esaote, Genoa, Italy) colour Doppler ultrasound at the level of both DGs, registering its diameter, depth, course, drainage of the CV to AV junction (CV/AV), as well as the patency of the AV. The CV diameter (in millimeters) is considered as the maximum transverse measurement in its course up to the CV/AV junction. CV depth as the distance (in millimeters) between the skin and its upper edge in its closest portion to the skin.
The CV was classified by its size into absent (when not visible), small (diameter < 3.0 mm) and normal (diameter ≥ 3.0 mm). This classification is used because the TIVAP implantation system is a NuPport HP* device (PHS MEDICAL - Fuldabrück, Germany) with a single-chamber titanium port and a silicone catheter with an external diameter of 9.6 F (≈ 3.0-3.2 mm). CV stenosis is defined as a narrowing of the vessel lumen of more than 50% of the total. The absence of flow signals in the AV with intraluminal echogenic material is considered as venous thrombosis. Figure 1 shows the different parameters assessed in this study.
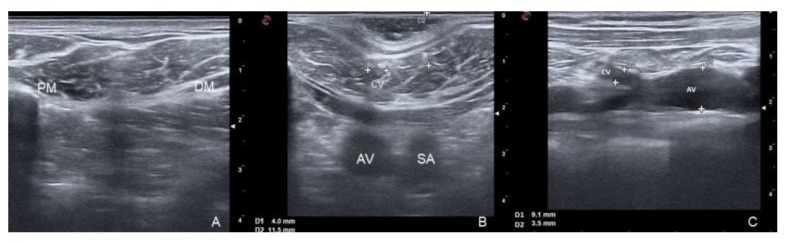
Figure 1: Ultrasound findings in the DG; 1A: Cephalic Vein (CV) absent in the DG (located between Pectoralis Major Muscle [PM] and Deltoid Muscle [DM]); 1B: CV diameter (D1) and CV depth (D2); Cross-sectional view of Axillary Vein (AV) and Subclavian Artery (SA); 1C: confluence of the CV into the AV.
The surgical procedure for the implantation of TIVAP is always performed through a 3-4 cm single incision in the DG via CVC or Infraclavicular Subclavian Vein Puncture (SVP). The CVC surgical technique is perfectly described by Jouvin [9]. If the CV is small or there is a partial stenosis of the CV/AV junction, sometimes the venous access is possible with the help of a Micropuncture Kit (AngioDynamics - Latham, USA) with a 21-Gauge needle an a 0.018′′ guidewire. When the CV is absent or not suitable, the TIVAP is placed through SVP using the Landmak technique [10]. Ultrasound studies and surgical techniques for TIVAP insertion and removal were performed by the same vascular surgeon with 35 years of experience in this field. The data obtained were recorded in a FileMaker Pro database. All patients provided signed informed consent for the procedures.
Statistical Analysis
The baseline characteristics of the study groups were described using means with their corresponding Standard Deviations (SD) for continuous variables and frequencies and percentages for categorical variables. Boxplots were plotted and medians and Interquartile Ranges (IQR) were computed in order to see differences in numerical variables distributions. Differences between sex were compared according to sociodemographic and clinical variables. If the compared variable was numerical, Wilcoxon rank-sum test was used for non-normally distributed data; if the variable was categorical, Chi-squared or Fisher’s exact test was used, as appropriate. Differences between left and right veins were compared by Wilcoxon signed-rank test for related samples or by McNemar test depending on the nature of the compared variable. Statistical significance was set at P value ≤ 0.05 and statistical analysis were performed by using RStudio® version 4.3.1.
Results
Between January 2008 and December 2023 a total of 2400 CVs in the DGs of 1200 patients were studied with ultrasound before the implantation of the TIVAP, 744 (62.0%) of whom were women. The mean age was 63.3 years (SD: 11.6). Table 1 details the general characteristics of all patients. Men were significantly older and had a higher incidence of colorectal, digestive and laryngeal cancers, while breast cancer was significantly more common in women.
Table 1: General characteristics.
|
Male (n=456) |
Female (n=744) |
p |
|
|
Age, years (mean±SD) |
65.4 ± 11.1 |
60.0 ± 11.4 |
<0.0001 |
|
Neoplasm Location n (%) Breast |
2 (0.4%) |
339 (45.6%) |
<0.0001 |
|
Lung |
60 (13.2%) |
58 (7.8%) |
0.0025 |
|
Colorectal |
185 (40.6%) |
171 (23.0%) |
<0.0001 |
|
Digestive |
118 (25.9%) |
73 (9.8%) |
<0.0001 |
|
Genitourinary |
40 (8.8%) |
67 (9.0%) |
0.8904 |
|
Larynx |
14 (3.1%) |
4 (0.5%) |
0.0005 |
|
Haematological |
16 (3.5%) |
12 (1.6%) |
0.0347 |
|
Others |
21 (4.6%) |
13 (2.7%) |
0.0760 |
SD: Standard Deviation
Of 2400 CVs scanned with ultrasound, in 30 occasions (2.5%) the CV was not visualized on either side, in 17 (1.4%) the right CV (RCV) was not found, and in 78 (6.5%) the LCV was not found. Excluding absent CVs, the mean diameter of CVs was 3.8 mm (SD: 0.5) and the mean depth was 13.6 mm (SD: 2.9). Table 2 shows the distributions of the CVs, comparing the findings between the two sides. The mean CV diameter and depth were similar on both sides. Although not reaching statistical significance, the number of CVs ≥ 3.0 mm was higher on the left side. Ninety-one (4.1%) stenosis at the CV/AV junction were observed, which were significantly more frequent in the LCVs. Tortuosity was found in 32 CVs (1.4%), all of them in CVs with diameters < 3.0 mm, with LCVs being the most significantly affected. AV thrombosis was found on 22 occasions (1.0%). Significant differences were not found between sexes for AV occlusion. Additionally, during the 2057 dissections of CVs ≥ 3.0 mm, 45 (2.2%) venous spasms occurred, 16 (2.0%) in RCVs and 29 (2.3%) in LCVs. The median ultrasound scan time per vein was 2.2 ± 1.1 minutes.
Table 2: CV distribution on both sides, except for CVs absent.
|
RCV (n=1153) |
LCV (n=1092) |
p |
|
|
Diameter (mm) (mean±SD) |
3.7 ± 0.5 |
3.9 ± 0.5 |
0.0025 |
|
Depth (mm) (mean±SD) |
13.9 ± 3.0 |
13.4 ± 2.7 |
0.0035 |
|
Diameter ≥3.0mm n (%) |
1016 (88.1%) |
1041 (95.3%) |
0.0152 |
|
Crossing stenosis CV/AV n (%) |
26 (2.3%) |
65 (6 .0%) |
<0.0001 |
|
Tortuosity n (%) |
8 (0.7%) |
24 (2.2%) |
0.0021 |
|
AV Occlusion n (%) |
8 (0.7%) |
14 (1.3%) |
0.3320 |
SD: Standard Deviation - RCV: Reigh Cephalic Vein – LCV: Left Cephalic Vein – AV: Axilar Vein
The Wilcoxon signed-rank test for related samples showed (4.0 mm), differences between both sides were observed as the differences between the diameter and depth distributions of the diameters were smaller in the RCVs than in the LCVs (Q1 3.5 mm CVs assessed (Figure 2). Although the median diameters of the versus Q1 3.7 mm). The median depth was significantly higher in RCVs and LCVs were similar (3.8 mm), as was the third quartile the RCVs (Q1 13.9 mm vs. 13.4 mm).
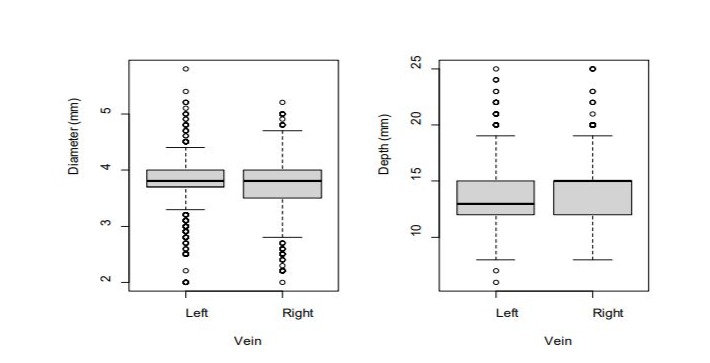
Figura 2: Box plot representation of CV diameter (mm) and depth (mm), comparing both sides.
Table 3 shows that CVR is absent in a higher percentage of women, sexes, by side of vein, no significant differences were found in the being statistically significant. CVs diameter and depth on both other variables sides were significantly greater in men. When comparing between
Table 3: CV distribution on both sides and sexes, except for CVs absent.
|
Male (n=456) |
Female (n=744) |
p |
|
|
RCV Absent (n) (%) |
8 (1.8%) |
39 (5.2%) |
0.0025 |
|
Diameter (mm) (mean ± SD) |
3.8 ± 0.5 |
3.7 ± 0.5 |
0.0002 |
|
Depth (mm) (mean ± SD) |
14.4 ± 2.8 |
13.5 ± 3.0 |
<0.0001 |
|
Diameter ≥3mm n (%) |
410 (89.9%) |
634 (85.2%) |
0.3688 |
|
CV/AV Crossing stenosis n (%) |
11 (2.4%) |
15 (2.0%) |
0.7149 |
|
Tortuosity n (%) |
3 (0.7%) |
5 (0.7%) |
1 |
|
AV occlusion n (%) |
5 (1.1%) |
4 (0.5%) |
0.3218 |
|
LCV Absent |
46 (10.1%) |
62 (8.3%) |
0.3026 |
|
Diameter (mm) (mean ± SD) |
3.9 ± 0.5 |
3.8 ± 0.5 |
0.0005 |
|
Depth (mm) (mean ± SD) |
14.0 ± 3.1 |
13.0 ± 2.8 |
<0.0001 |
|
Diameter ≥3.0mm n (%) |
383 (84.0%) |
630 (84.7%) |
0.5209 |
|
CV/AV Crossing stenosis n (%) |
22 (4.8%) |
43 (5.8%) |
0.5253 |
|
Tortuosity n (%) |
10 (2.2%) |
14 (1.9%) |
0.6733 |
|
AV Occlusion n (%) |
4 (0.9%) |
9 (1.2%) |
0.7764 |
SD: Standard Deviation; RCV: Reigh Cephalic Vein; LCV: Left Cephalic Vein; AV: Axilar Vein
In Figure 3 shows the same results: the median CVs diameter was 15 mm (IQR: 12mm – 16mm) in men versus 13 mm in women significantly higher in men (3.9 mm [IQR: 3.7 mm - 4.1 mm]) (IQR: 11 mm – 15 mm). versus women (3.8 mm [IQR: 3.6 mm - 4.0 mm]) as was the depth:
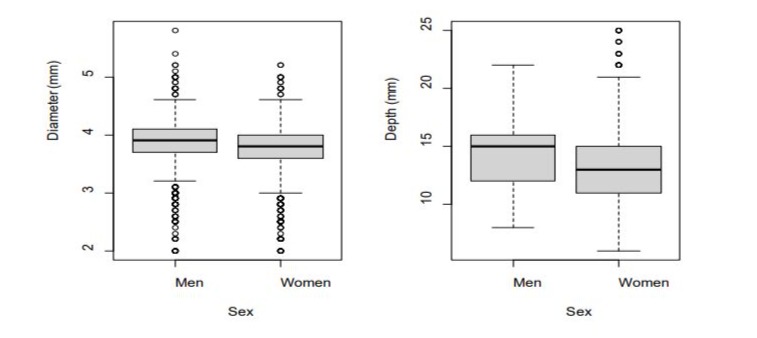
Figure 3: Box plot representation of CV diameter (mm) and depth (mm), comparing both sexes.
Discussion
The motivation for this study stems from my experience as a vascular surgeon trained in the last quarter of the 20th century, during which pacemaker implantation was taught and performed mainly via CVC and, although new approaches for pacemaker and TIVAP placement have emerged, for several authors it remains an effective and safe option [11,12]. Similarly, the decision to implant the TIVAP by CVC via the LCV as the first choice is based on three important facts: the curve traced by the catheter through this route is smaller than on the right side, not puncturing the subclavian vein as it passes through the costoclavicular space avoids possible pinch-off syndrome, and that since most of the population is righthanded, shoulder joint movement is more frequent on the right side [13]. Several methods for assessing the CV absence in the DG are reported in the literature. In autopsy studies, the percentages range between 3.3% and 14.0% [4,3] except Au [14], which found a CV in all 157 DG explored. When intraoperative venography was performed, CV absence was noted in 12.0% of cases [4]. During DG dissection in the surgical field, values range from 2.5% to 15.0% [15]. Finally, when preoperative ultrasound is used, the prevalence ranges between 5.9% and 14.3% [16,17]. In the present study, it is 6.5%.
Evidence [18,19] has shown that the mean CV diameter with ultrasound in the DG ranges between 3.1 mm and 4.6 mm, although Loukas [3] observed a mean diameter of 8.0 mm in his study on cadavers. Only Taleski [20] reported that CV diameter was statistically greater in women than in men. In the present study, as in most studies, CV diameter is higher in men (3.9 mm vs 3.8 mm). In relation to the mean CV depth at this level, the values range from 10.2 mm to 23.6 mm [15,21], not differentiating between men and women. However, as with the diameter, the present study also shows that the CV depth is higher in men (14.3 mm vs. 13.2 mm). The main factor influencing the success of the TIVAP implantation via CVC is CV diameter. Although values vary, most authors agree that a CV is small when it is < 3.0 mm [20-22], as in this study. This is because the catheters used are 9.6 F (approximately 3.0/3.2 mm external diameter). In the present study, 91.6% of all CVs were ≥ 3.0 mm.
Another factor to consider is the stenosis at the confluence of the CV and AV, which in the literature is reported in 3.5% to 7.5% of cases [20,23]. In this study it was found in 4.1% of cases and was more common in LCVs. Venous spasms during CV dissection have a prevalence ranging from 0.2% to 7.5% [24,25]; in this study, it was 2.2%. The diameter of the CV is directly related to success rates. CVC approach without using preoperative ultrasound has a success rate between 75.6% and 93.7% [26,27]. However, with its use Otsubo achieved a 97.2% success rate [15]. In this study, the success rate in CVs ≥ 3.0 mm increased to 98.7%. On the other hand, the success rates of SVP without intraoperative ultrasound range between 79.5% and 99.0% [28,29], while with its use, they increase up to 95.0% and 100.0% [30-32]. No literature has been found regarding CV tortuosity in the DG, nor for idiopathic AV occlusion. Finally, it is essential to acknowledge the limitations of this study, as it is observational, single-center, and conducted by a single operator. However, the objective was to provide information about the CV anatomy in the DG, prior to TIVAP implantation via CVC in order to improve its success rate.
Conclusion
Preoperative Doppler ultrasound of the CV in the DG is a very useful diagnostic method to determine its anatomical characteristics and possible anomalies. Both CV diameter and depth were greater in men. LCVs had a higher incidence of CV/AV junction stenosis and tortuosity than RCVs.
Declaration of conflicting interests
The author declare that they have no competing interests
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Brain P (1986) Galen on Bloodletting: A Study of the Origins, Development and Validity of His Opinions, with a Translation of the Three Works. Cambridge University Press 1986.
- Amr SS, Tbakhi A (2007) Ibn Sina (Avicenna): the prince of physicians. Ann Saudi Med 27: 134-135.
- Loukas M, Myers CS, Wartmann ChT, Tubbs RS, Judge T (2008) The clinical anatomy of the cephalic vein in the deltopectoral triangle. Folia Morphol (Warsz) 67: 72-77.
- Steckiewicz R, Świętoń E, Stolarz P, Grabowski M (2015) Clinical implications of cephalic vein morphometry in routine cardiac implantable electronic device insertion. Folia Morphol 74: 458-464.
- Otsubo R, Yano H, Matsumoto M (2021) Comparison of Central Venous Port Procedures Between Puncture vs. Cut-down and Residents vs. Senior Surgeons. In Vivo 35: 1197-1204.
- Furman S, Schwedel JB (1959) An intracardiac pacemaker for StokesAdams seizures. N Engl J Med 261: 943-948.
- Niederhuber JE, Ensminger W, Gyves JW, Liepman M, Doan K (1982) Totally implanted venous and arterial access system to replace external catheters in cancer treatment. Surgery 92: 706-712.
- Nocito A, Wildi S, Rufibach K, Clavien PA, Weber M (2009) Randomized clinical trial comparing venous cutdown with the Seldinger technique for placement of implantable venous access ports. Br J Surg 96: 11291134.
- Jouvin I, Pocard M, Najah H (2019) Cephalic vein portacath placement technique. J Vasc Surg 156: 239-243.
- Velioğlu Y, Yüksel A, Sınmaz E (2019) Complications and management strategies of totally implantable venous access port insertion through percutaneous subclavian vein. Turk Gogus Kalp Damar Cerrahisi Derg 27: 499-507.
- Klaiber U, Probst P, Hackbusch M (2021) Meta-analysis of primary open versus closed cannulation strategy for totally implantable venous access port implantation. Langenbecks Arch Surg 406: 587-596.
- Atti V, Turagam MK, Garg J (2020) Subclavian and Axillary Vein Access Versus Cephalic Vein Cutdown for Cardiac Implantable Electronic Device Implantation: A Meta-Analysis. JACC Clin Electrophysiol 6: 661-671.
- Ben Kridis W, Toumi N, Khanfir A (2020) Causas de fractura en el catéter del puerto de acceso venoso totalmente implantable: una revisión sistemática. Acta Med Irán 57: 686-689.
- Au FC (1989) The anatomy of the cephalic vein. Am Surg 55: 638-639.
- Rademakers LM, Bracke FA (2021) Cephalic vein access by modified Seldinger technique for lead implantations. Pacing Clin Electrophysiol 44: 607-613.
- Otsubo R, Hatachi T, Shibata K (2016) Evaluation of totally implantable central venous access devices with the cephalic vein cut-down approach: Usefulness of preoperative ultrasonography. J Surg Oncol 113: 114-119.
- Staszewicz W, Naiken SP, Mennet A (2019) Ultrasound-based prediction of cephalic vein cutdown success prior to totally implantable venous access device placement. J Vasc Surg Venous Lymphat Disord 7: 865-869.
- Rhu J, Jun KW, Song BJ, Sung K, Cho J (2019) Cephalic vein approach for the implantable central venous access: A retrospective review of the single institution’s experiences; Cohort Study. Medicine (Baltimore) 98: e18007.
- Yalniz A, Cam I, Bozyel S (2022) Ultrasound guided percutaneous cephalic venipuncture for implantation of cardiac implantable electronic devices. J Vasc Access 23: 416-421.
- Taleski J, Stankovik S, Risteski D (2019) Sex-related differences regarding cephalic vein lead access for CIEDs implantation. Int J Arrhythm 22.
- Chen JY, Chang KC, Lin YC, Hung JS (2005) Preprocedure duplex ultrasonography to asist cephalic veinisolation in pacemaker and defribrillator implantation.J. Interv. Card. Electrophysiol 12: 75-81.
- Mathews J, Abraham S, Philip R (2018) Cephalic vein cut down technique for chemoport implantantion and ease of chemoport Access. A cohort study. Arch Int Surg 8: 113-118.
- Chang HM, Hsieh CB, Hsieh HF (2006) An alternative technique for totally implantable central venous access devices. A retrospective study of 1311 cases. Eur J Surg Oncol 32: 90-93.
- Hashimoto S, Otsubo R, Adachi M (2019) Cephalic Vein Cut-down for Totally Implantable Central Venous Access Devices With Preoperative Ultrasonography by Surgical Residents. In Vivo 33: 2079-2085.
- Wennevold A, Christiansen I, Lindeneg O (1965) Complications in 4,413 catheterizations of the right side of the heart. American Heart Journal 1965: 173-180.
- Steckiewicz R, Świętoń EB, Bogdańska M, Stolarz P (2018) Vasoconstrictive responses of the cephalic vein during first-time cardiac implantable electronic device placement. Folia Morphol (Warsz) 77: 464-470.
- Hüttner F, Bruckner T, Hackbush et al. (2020) Primary Open Versus Closed Implantation Strategy for Totally Implantable Venous Access Ports: The Multicentre Randomized Controlled PORTAS-3 Trial (DRKS 00004900). Annals of Surgery 272: 950-960.
- Koketsu S, Samesima S, Yoneyama S (2010) Outcome of cephalic vein cut-down approach: A safe and feasible approach for totally implantable venous access device placement. Oncol Lett 1: 10291031.
- Tsai YF, Ku YH, Chen SW, Huang WT, Lu CC (2012) Right- and leftsubclavian vein port-a-cath systems: comparison of complications. Eur Surg Res 49: 66-72.
- Paprottka KJ, Voelklein J, Waggershauser T, Reiser MF, Paprottka PM (2019) Retrospective outcome analysis of rates and types of complications after 8654 minimally invasive radiological port implantations via the subclavian vein without ultrasound guidance. Radiol Med 124: 926-933.
- Mudan S, Giakoustidis A, Morrison D, Iosifidou S, Raobaikady R (2015) 1000 Port-A-Cath ® placements by subclavian vein approach: single surgeon experience. World J Surg 39: 328-34.
- Bai XM, Wang J, Zhou Y, Sun XW, Cheng L (2020) Totally implantable venous access devices: The supraclavicular percutaneous approach and early complications. J Cancer Res Ther 16: 1575-1581.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.