Anatomic Study of the Uterosacral Ligaments: Impact on Endometriosis Diagnosis
by Nabil Louafi1, Elie Zerbib1*, Yohann Dabi1, Clément Ferrier1, Adrien Crestani1, Kamila Kolanska1, Amélia Favier1, Isabelle Thomassin-Naggara2, Marc Bazot2, Cyril Touboul1, Emile Daraï1
1Department of Gynaecology, Obstetrics and Reproductive Medicine, Sorbonne University, Hôpital Tenon, 4 Rue de la Chine, 75020 Paris, France
2Département d’Imageries Radiologiques et Interventionnelles Spécialisées (IRIS), Hôpital Tenon, Assistance Publique-Hôpitaux de Paris, France; Sorbonne Université, INSERM U938 Équipe Biologie et Thérapeutiques du Cancer, France
Corresponding author: Elie Zerbib, Department of Gynaecology, Obstetrics and Reproductive Medicine, Sorbonne University, Hôpital Tenon, 4 Rue de la Chine, 75020 Paris, France
Received Date: 07 July 2025
Accepted Date: 14 July 2025
Published Date: 16 July 2025
Citation: Louafi N, Zerbib E, Dabi Y, Ferrier C, Adrien Crestani A, et al. (2025) Anatomic Study of the Uterosacral Ligaments: Impact on Endometriosis Diagnosis J Surg 10: 11380 https://doi.org/10.29011/2575-9760.011380
Abstract
Background: Uterosacral Ligament (USL) is the most frequent location of deep infiltrating endometriosis. Despite an abundant literature on USL anatomy in genital prolapse, few data are available on normal USL anatomy. This explains the absence of consensus on criteria to diagnose by imaging techniques this specific endometriosis location. Therefore, the aim of the present study was to assess the normal anatomy of USL.
Material and methods: Five fresh and five embalmed cadavers of adult female were dissected. The anatomic characteristics of USL at the insertion to the torus uterinum were assessed by measuring the distance between the inner edges of the USLs at the uterine insertion and the distance between the torus uterinum and the upper edge of the posterior vaginal cuff. For the 20 hemipelvis, the length and thickness of the USLs were evaluated every centimeter from the uterus to the sacral insertions. Comparisons of measures between the right and left USL and between fresh and embalmed cadavers were performed.
Results: Distance between the inner edges of the USLs at uterine insertion was 1.4±0.3cm. Distance between the torus uterinum and the upper edge of the vaginal cuff was 1.0±0.4cm. For the 20 hemipelvis, the mean length of the USL was 7±0.95cm. USL transverse thickness varied according to the distance from uterine insertion and was steady between 2 and 5cm from uterine insertion with a mean value of 0.5±0.2cm. The length of the right USL was longer (7.1 vs. 6.8cm) (p<0.01). No difference in the transverse thickness was found between right and left USL. No difference in USLs measures were found between fresh and embalmed cadavers.
Conclusion: Our results allow to determine the portion of USL where the measures are reproducible. Moreover, measures of normal USL were evaluated contributing to define cut-off of abnormal USL applicable to imaging techniques in women with suspicion of endometriosis.
Keywords: Anatomy; Cranio-caudal thickness; Endometriosis; Transverse thickness; Uterosacral ligament
Introduction
Endometriosis, defined histologically by the presence of endometrial-like tissue outside the uterus, affects 5-10% of symptomatic women of reproductive age thus representing at least 190 million worldwide [1,2]. Endometriosis diagnosis is mainly based on symptoms including severe chronic pelvic pain, dysmenorrhea, dyspareunia, dyschezia, and infertility as well as non-specific symptoms such as fatigue [3,4]. However, no single or combined signs are sufficiently specific to allow the diagnosis [5,6]. Moreover, except for vaginal endometriosis, clinical examination does not reach sufficient accuracy to assess the diagnosis [7] thus imposing further investigation [2,8,9].Three phenotypes of endometriosis, often associated, are distinguished; the Superficial Peritoneal Endometriosis (SPE), Ovarian Endometriosis (also called endometrioma) and Deep Infiltrative Endometriosis (DIE). Despite advances in imaging techniques, SPE, representing the most frequent phenotype observed in up to 80% of cases, is often ignored by both Transvaginal Ultrasonography (TVUS) and MRI [1,10,11]. Endometrioma, observed in about one third of women, is well diagnosed by both TVUS and MRI with an accuracy over 90% [2]. Another shortcoming of the diagnosis algorithms is linked to patients with a suspicion of DIE on imaging observed in 20% of patients with endometriosis. Even using data from expert centres, Nisenblat et al. demonstrated the low accuracy of imaging to accurately determine all locations of deep endometriosis especially the Uterosacral Ligament (USL) location that is the most frequent DIE lesion [2,12,13]. The difficulties to assess the diagnosis of USL endometriosis is partially linked to the absence of referent measures to define normal USL. In contrast to numerous anatomic studies on USL morphology in genital prolapse, few data are available on normal anatomy of USL using serial sectioning [14-16]. Therefore, the goal of the present study was to analyze macroscopic characteristics of normal USL to determine cut-off of normality to help radiologists to diagnose USL endometriosis.
Material and Methods
Anatomic consideration on USL
The USLs arise from the posterolateral surfaces of the supravaginal part of the cervix and the vaginal fornix, running along the recto-uterine cul-de-sac, and ending in the pre-sacral vertebrae fascia S2 to S4. In their medial part, USL lies the inferior hypogastric nerve plexus [17]. Rouvière et al. [17] defined USL as “smooth connective and muscular bundles arising from the posterior surface of the cervix, near its lateral edges and in the immediate vicinity of the isthmus, run cranially and dorsally, around the lateral surfaces of the rectum and terminate on the anterior surface of the sacrum. They lift the peritoneum to form a curved fold, concave medially, which laterally limits the culde-sac of Douglas. The name USL refers to both the serous fold and the conjunctive-muscular elements that define and support it. The thickness of these ligaments contains mixed with the smooth connective and muscular fibers, and part of the hypogastric nerve plexus, which in fact constitutes the truly resistant component of the ligament on each side. The USL on one side is joined to the ligament on the opposite side, behind the cervix, by a transverse fold known as the torus uterinum or J.L. Petit ligament. Taken together, the two USLs form a horseshoe shape.
Material
The study involved 20 hemipelvis from 10 menopausal female cadavers (five fresh and five embalmed) dissected in the anatomy laboratory of the Saint Pères and at the Fer à Moulin surgical school Paris, France.
Methods
The two USLs of each subject were resected in their entirety. The cadaver was placed on a dissecting table in dorsal decubitus position. The peritoneal cavity was opened by a large cross incision, and the uterus was fixed by a wire to the wall opposite the pubis to ensure maximum anteversion. The digestive tract was pushed upwards to ensure better exposure. The first measures (to the nearest 0.1cm) were the distance separating the medial edges of the USLs at their uterine insertion and the distance separating the lower limit of the torus uterinum from the upper limit of the posterior vaginal fornix. This measurement was performed after locating the upper limit of the posterior vaginal fornix by a vaginal digital examination. Then each USL was resected. Once the USLs resected, they were refined from the peritoneum using transillumination.Each USL was oriented and various measurements (to the nearest 0.1cm) were performed including the total length (from uterine insertion to sacral insertion), cranio-caudal and transverse thicknesses at the level of the uterine insertion. Then transverse thickness of USL was measured every centimeter in the ventro-dorsal direction (from uterine insertion to sacral insertion) (Figure 1).
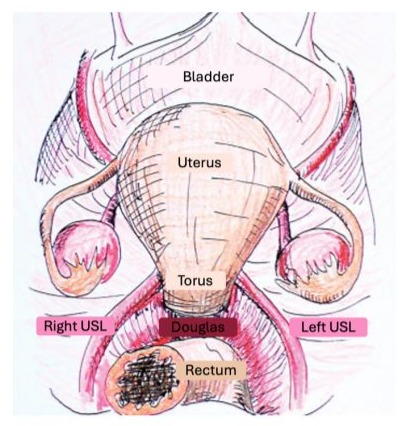
Figure 1: schematic axial view of the female pelvis.
Statistical Analysis
To compare measures between left and right USL and between fresh and embalmed cadavers, Stat View 5.0 software was used.
Results
Evaluation of USL at the Uterine Insertion
The distance between the internal edges of the USLs at their uterine insertion and the distance between the torus uterinum and posterior vagina fornix for the 10 cadavers were evaluated. The mean distance between the internal edges of the USLs at their insertion to the torus uterinum level was 1.4±0.3cm (range: 1-1.8cm). The mean distance between the torus uterinum and the posterior vagina fornix was 1.06±0.38cm (range: 0.5-1.8cm).
Evaluation of the USL Anatomic Measures
For the 20 hemipelvis, the total length, the transverse and the cranio-caudal thicknesses of USLs were evaluatedFor the 20 hemipelvis, the mean length of USL was 7±0.95cm (range: 5.58.5cm). For the 20 hemipelvis, the transverse thickness was not uniform throughout its length allowing to differentiate three segments (Figure 1), the first segment from the uterine insertion to the first centimeter with a decrease in the transverse thickness, the second segment from 2 to 5cm from uterine insertion with a steady transverse thickness, and the last segment from 6cm to the sacral insertion with a progressive increase in the transverse thickness. In contrast to the transverse thickness, the cranio-caudal thickness evaluation was evaluable only for the first centimeters of USLs from the uterine insertion. The mean cranio-caudal thickness at uterine insertion for the right and left USL were 0.7±0.2 cm and 0.7±0.1cm, respectively (not significant). In contrast to the transverse thickness that was adequately delimited, the craniocaudal thickness of USL according to the three segments was not adequately delimited especially their two-thirds dorsal segments of USL not permitting an adequate evaluation.
Comparison of Right and Left USL Measures
The comparison of right and left USL measures is given in Table 1.
|
Left USL |
Right USL |
P value |
|
|
TT at uterine insertion (cm) |
0.8 ± 0.2 |
0.7 ± 0.1 |
NS |
|
TT at 1 cm from uterine insertion (cm) |
0.6 ± 0.2 |
0.6 ± 0.2 |
NS |
|
TT at 2 cm from uterine insertion (cm) |
0.5 ± 0.2 |
0.5 ± 0.1 |
NS |
|
TT at 3 cm from uterine insertion (cm) |
0.5 ± 0.2 |
0.5 ± 0.1 |
NS |
|
TT at 4 cm from uterine insertion (cm) |
0.5 ± 0.2 |
0.5 ± 0.1 |
NS |
|
TT at 5 cm from uterine insertion (cm) |
0.6 ± 0.1 |
0.6 ± 0.1 |
NS |
|
TT at 6 cm from uterine insertion (cm) |
0.7 ± 0.2 |
0.7 ± 0.2 |
NS |
|
TT at 7 cm from uterine insertion (cm) |
0.7 ± 0.1 |
0.7 ± 0.1 |
NS |
|
TT at 8 cm from uterine insertion (cm) |
0.9 ± 0.1 |
0.6 ± 0.1 |
NS |
Table 1: Evaluation of the transverse thickness (TT) of the uterosacral ligament (USL) according to the distance from uterine insertion.
The mean total length of the right USL was longer than the left USL, 7.1cm and 6.8cm respectively (p<0.01). No differences in the transverse thicknesses according to the distance from uterine insertion were observed between left and right USL. No comparison of the cranio-caudal thickness according to sides was possible.
Comparison of USL Measures Between Fresh And Embalmed Cadavers.
The measures of the USLs according to the fresh or embalmed are summarized in the Table 2.
Embalmed cadavers (n=5) | Fresh cadavers (n=5) | P value | |
Distance between the internal edges of the USLs (cm) | 1.40 ± 0.27 | 1.38 ± 0.35 | NS |
Distance between the torus uterinum and the posterior vagina fornix (cm) | 0.98 ± 0.14 | 1.14 ± 0.54 | NS |
Mean length of the left USL (cm) | 6.64 ± 0.83 | 6.98 ± 1.20 | 0.07 |
Mean length of the right USL (cm) | 7.04 ± 0.76 | 7.18 ± 1.15 | NS |
Table 2 : Comparison of the uterosacral ligament (USL) measures between fresh and embalmed cadavers.
No differences in the distance between the internal edges of the USLs at their uterine insertion, the distance between torus uterinum and posterior vaginal fornix, and the total length of USL, were found between fresh and embalmed cadavers. Except for a trend for a higher length for the right USL in fresh cadavers, no difference in the transverse thickness values was observed between fresh and embalmed cadavers.
Discussion
The present anatomic analysis contributes to determine cutoff measure of normal USL. Among the various measures, the transverse thickness values between 2 and 5cm from uterine insertion of the USL was reproducible and steady supporting its potential use as criterion of abnormal USL on imaging techniques for endometriosis diagnosis. In the present study, we observed that the length of USL was significantly longer for the right USL (p<0.01). In a review including 13 anatomic studies, Ramanah et al. [18] underlined differences in USL length according to series. Indeed, Siddique et al. reported an USL length of 8.7cm (95 % confidence interval (CI); 7.5-10.0) [19] while Vu et al. [20] measured the USL length to 12-14cm. However, as in the present study, Campbell et al. [21] and Blaisdell et al. [15] observed that the length of USL was greater on the right side. For the 20 hemi-pelvis, when focusing on the transverse thickness of USL according to the distance from the uterine insertion, that is often analyzed on TVUS and MRI to diagnose endometriosis involvement, its measure in the current study remained steady between 2 and 5cm of 0.5±0.2cm without difference between the right and left USL supporting that this segment should be the most adequate portion for reproducibility of the measures. In contrast to the evaluation of USL length, to our knowledges, few data are available on the serial analysis of the USL transverse thickness according to the distance from uterine insertion. Vu et al. reported that the transverse thickness of the distal section and at uterine insertion of USLs varied between 5 and 20mm [20] while Ramanah et al. noted that the mean thickness of USL were 5.2±0.9cm, 2.7±1.0, and 2.0±0.5cm at the sacral, intermediate, and cervical portions, respectively [18]. However, as previously mentioned [18], this discrepancy can be explained by controversies on terminology, definition, composition, dissection artifacts, and even the existence of USL. Concerning the craniocaudal thickness, the measures were higher at the uterine and sacral insertions without difference between right and left USL but were not adequately evaluable throughout the USL length. This is probably linked to the intricating of muscle fibers as well as the inferior hypogastric nerves fibers, and to anatomic dissection artifacts. Consequently, in the absence of other criteria of endometriosis such as nodular or spicular features, and hot spot component corresponding to hemorrhagic lesion, a USL transverse thickness per se inferior or equal to 5mm at its intermediate segment (2-5cm from uterine insertion) on imaging cannot support the diagnosis of endometriosis. Moreover, as aforementioned, USL transverse thickness was measured after refining USL from the peritoneum while the measure on imaging includes the peritoneal component. This consideration is particularly relevant as among 344 DIE lesions, Chapron et al. [22] reported that USL was the most frequent location (69.2%). Moreover, they reported that the proportion of isolated lesions differed according to the DIE location with a highest rate of 83.2% for USL.
National and international guidelines recommend for the diagnosis of endometriosis an algorithm based on ultrasonography (US) or MRI [3,4]. Although these algorithms appear logical to detect ovarian endometriosis, observed in about one-third of patients, thanks to the high accuracy of both US and MRI, a debate exists on the accuracy of imaging techniques to detect USL endometriosis [2]. When considering the contribution of imaging techniques to diagnose USL endometriosis, it is important to differentiate US from MRI. In a retrospective multicenter study involving 878 patients between 15 and 45 years-old undergoing Laparoscopy (LPS) for suspected endometriosis, Abrao et al. evaluated transvaginal and transabdominal ultrasonography (US) (index test) to assess sites of endometriosis according to the 2021 AAGL Endometriosis Classification [23]. The AAGL-US and AAGL-LPS stages were concordant in 586 cases (66.7%) (weighted kappa (WK) 0.759; intraclass correlation 0.906), with the highest agreement observed in patients with no endometriosis (75.3% concordance). However, for retrocervical/uterosacral ligament the WK was only 0.656. Another prospective study of 172 patients of Ros et al. evaluated the accuracy of TVUS to diagnose DIE involving USL, torus uterinum (TU) or posterior vaginal fornix (PVF) compared to laparoscopy [24]. The global sensitivity and specificity of TVUS in diagnosing USL, TU and/or PVF endometriosis were 92% and 87%, respectively. In a meta-analysis, Zhou et al. have evaluated the accuracy of TVUS for USL endometriosis [25]. The respective pooled sensitivity, specificity, positive probability ratio (LR+) and negative probability ratio (LR-) of TVUS for detecting USL endometriosis were 65% (95% CI:43-83), 92 % (95% CI:8496), 7.80 (95% CI:4.7-13.0) and 0.38 (95% CI:0.22-0.66) with a significant heterogeneity in both sensitivity and specificity reported. Finally, in a review including 22 studies, focusing on TVUS for USL endometriosis, Maple et al. found that most (20/22) studies described abnormal criteria but only five defined the appearance of normal USL underlining the lack of data and consensus for this specific endometriosis location [26].
When considering MRI to assess USL endometriosis, using the RAND-UCLA Appropriateness Method to attain consensus guidelines, Rousset et al. elaborated a lexicon of image interpretation, and a standardized region-based reporting of DIE with MRI [27]. A consensus regarding pelvic compartment delineation and DIE reporting was thereby attained. A consensus was reached for the most frequent locations of DIE, but no consensus was reached to define the normal presentation of USL. In a meta-analysis including 10 prospective studies, Gerges et al. evaluated USL endometriosis by TVUS and MRI compared to surgery [28]. For USL endometriosis, for all TVUS and MRI the respective pooled sensitivity and specificity techniques were 60% (95%CI 32–82%) and 95% (95%CI 90–98%), and 81% (95%CI 66–90%) and 83% (95%CI 62–94%). Despite some limits of the included studies, MRI outperformed TVUS for the diagnosis of USL with higher sensitivities but similar specificities. Finally, whatever imaging techniques, a publication bias exists as studies primarily emerge from expert centers, thereby not reflecting the full scope of routine practice [2]. Moreover, Leonardi et al. underlined the difficulties for patients to find an expert sonographer [29]. Some limits of the present study deserve to be underlined. First, the limited sample size cannot exclude all bias even if homogenous evaluation of USL length and transverse thickness measures was observed. Second, the use of fresh and embalmed cadavers might be a potential bias, but our results support the absence of difference in USL characteristics between these two groups. Third, all cadavers were from menopausal women with a possible underestimation of USL measures linked to hormonal deficiency. However, none of the cadavers had a genital prolapse that is known as a cause of USL alterations. Indeed, Kökçü et al. showed a decreased cellularity in connective tissue in patients with genital prolapse compared with patients without prolapse [30]. Finally, the present study underlined the difficulties to evaluate the cranio-caudal thickness of USL due to the absence of clear delimitation of the connective tissue from adjacent muscle and inferior hypogastric nerves. In conclusion, despite some limits of the present study, our results underlined the need to consider data of anatomic analysis to define normal anatomy of USL. Further studies evaluating the morphology and dimensions of USL by imaging techniques in patients with and without symptoms suggestive of endometriosis are required to determine the characteristics of both normal and abnormal pelvic structures.
References
- Zondervan KT, Becker CM, Missmer SA (2020) Endometriosis. New England Journal of Medicine. 26 mars 382: 1244‑1256.
- Nisenblat V, Bossuyt PMM, Farquhar C, Johnson N, Hull ML (2016) Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 26 févr 2: CD009591.
- Collinet P, Fritel X, Revel-Delhom C, Ballester M, Bolze PA, et al. (2018) Management of endometriosis: CNGOF/HAS clinical practice guidelines – Short version. Journal of Gynecology Obstetrics and Human Reproduction. 1 sept 47: 265‑274.
- Becker CM, Bokor A, Heikinheimo O, Horne A, Jansen F, et al. (2022) ESHRE guideline: endometriosis. Hum Reprod Open. 2022: hoac009.
- Bendifallah S, Dabi Y, Suisse S, Jornea L, Bouteiller D, et al. (2022) MicroRNome analysis generates a blood-based signature for endometriosis. Sci Rep. 8 mars 12: 4051.
- Grover SR, Joseph K (2021) Endometriosis and pelvic pain: Time to treat the symptoms not the assumptions? Aust N Z J Obstet Gynaecol. août 61: 625‑627.
- Roditis A, Florin M, Rousset P, Touboul C, Bendifallah S, et al. (2023) Accuracy of combined physical examination, transvaginal ultrasonography, and magnetic resonance imaging to diagnose deep endometriosis. Fertil Steril. avr 119: 634‑643.
- Nisenblat V, Bossuyt PMM, Shaikh R, Farquhar C, Jordan V, et al. (2016) Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 1 mai 2016: CD012179.
- Nisenblat V, Prentice L, Bossuyt PMM, Farquhar C, Hull ML, et al. (2016) Combination of the non-invasive tests for the diagnosis of endometriosis. Cochrane Database Syst Rev. 13 juill 7: CD012281.
- Horne AW, Missmer SA (2022) Pathophysiology, diagnosis, and management of endometriosis. BMJ. 14 nov 379: e070750.
- Koninckx PR (1994) Is mild endometriosis a condition occurring intermittently in all women? Hum Reprod. déc 9: 2202‑2205.
- Koninckx PR, Ussia A, Adamyan L, Wattiez A, Donnez J (2012) Deep endometriosis: definition, diagnosis, and treatment. Fertil Steril. sept 98: 564‑571.
- Bazot M, Jarboui L, Ballester M, Touboul C, Thomassin-Naggara I, et al. (2012) The value of MRI in assessing parametrial involvement in endometriosis. Hum Reprod. août 27: 2352‑2358.
- Umek WH, Morgan DM, Ashton-Miller JA, DeLancey JOL (2004) Quantitative analysis of uterosacral ligament origin and insertion points by magnetic resonance imaging. Obstet Gynecol. mars 103: 447‑451.
- Blaisdell: The anatomy of the sacro-uterine ligaments.
- Gabriel B, Denschlag D, Göbel H, Fittkow C, Werner M, et al. (2005) Uterosacral ligament in postmenopausal women with or without pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 16: 475‑479.
- Rouvière: Anatomía humana.
- Ramanah R, Berger MB, Parratte BM, DeLancey JOL (2012) Anatomy and histology of apical support: a literature review concerning cardinal and uterosacral ligaments. Int Urogynecol J. nov 23: 1483‑1494.
- Siddique SA, Gutman RE, Schön Ybarra MA, Rojas F, Handa VL (2006) Relationship of the uterosacral ligament to the sacral plexus and to the pudendal nerve. Int Urogynecol J Pelvic Floor Dysfunct. nov 17: 642‑645.
- Vu D, Haylen BT, Tse K, Farnsworth A (2010) Surgical anatomy of the uterosacral ligament. Int Urogynecol J. sept 21: 1123‑1128.
- Campbell RM (1950) The anatomy and histology of the sacrouterine ligaments. Am J Obstet Gynecol. janv 59: 1‑12, illust.
- Chapron C, Fauconnier A, Vieira M, Barakat H, Dousset B (2003) Anatomical distribution of deeply infiltrating endometriosis: surgical implications and proposition for a classification. Hum Reprod. janv 18: 157‑161.
- Abrao MS, Andres MP, Gingold JA, Rius M, Neto JS, et al. (2023) Preoperative Ultrasound Scoring of Endometriosis by AAGL 2021 Endometriosis Classification Is Concordant with Laparoscopic Surgical Findings and Distinguishes Early from Advanced Stages. J Minim Invasive Gynecol. mai 30: 363‑373.
- Ros C, de Guirior C, Mension E, Rius M, Valdés-Bango M, et al. (2021) Transvaginal ultrasound for diagnosis of deep endometriosis involving uterosacral ligaments, torus uterinus and posterior vaginal fornix: prospective study. Ultrasound Obstet Gynecol. déc 58: 926‑932.
- Zhou Y, Su Y, Liu H, Wu H, Xu J, et al. (2021) Accuracy of transvaginal ultrasound for diagnosis of deep infiltrating endometriosis in the uterosacral ligaments: Systematic review and meta-analysis. J Gynecol Obstet Hum Reprod. mars 50: 101953.
- Maple S, Chalmers KJ, Bezak E, Henry K, Parange N (2023) Ultrasound Characteristics and Scanning Techniques of Uterosacral Ligaments for the Diagnosis of Endometriosis: A Systematic Review. J Ultrasound Med. juin 42: 1193‑1209.
- Rousset P, Florin M, Bharwani N, Touboul C, Monroc M, et al. (2023) Deep pelvic infiltrating endometriosis: MRI consensus lexicon and compartment-based approach from the ENDOVALIRM group. Diagn Interv Imaging. mars 104: 95‑112.
- Gerges B, Li W, Leonardi M, Mol BW, Condous G (2021) Meta-analysis and systematic review to determine the optimal imaging modality for the detection of uterosacral ligaments/torus uterinus, rectovaginal septum and vaginal deep endometriosis. Hum Reprod Open. 4 nov 2021: hoab041.
- Leonardi M, Uzuner C, Mestdagh W, Lu C, Guerriero S, et al. (2022) Diagnostic accuracy of transvaginal ultrasound for detection of endometriosis using International Deep Endometriosis Analysis (IDEA) approach: prospective international pilot study. Ultrasound in Obstetrics & Gynecology. 60: 404‑413.
- Kökçü A, Yanik F, Cetinkaya M, Alper T, Kandemir B, et al. (2002) Histopathological evaluation of the connective tissue of the vaginal fascia and the uterine ligaments in women with and without pelvic relaxation. Arch Gynecol Obstet. avr 266: 75‑78.
Introduction
Endometriosis, defined histologically by the presence of endometrial-like tissue outside the uterus, affects 5-10% of symptomatic women of reproductive age thus representing at least 190 million worldwide [1,2]. Endometriosis diagnosis is mainly based on symptoms including severe chronic pelvic pain, dysmenorrhea, dyspareunia, dyschezia, and infertility as well as non-specific symptoms such as fatigue [3,4]. However, no single or combined signs are sufficiently specific to allow the diagnosis [5,6]. Moreover, except for vaginal endometriosis, clinical examination does not reach sufficient accuracy to assess the diagnosis [7] thus imposing further investigation [2,8,9].Three phenotypes of endometriosis, often associated, are distinguished; the Superficial Peritoneal Endometriosis (SPE), Ovarian Endometriosis (also called endometrioma) and Deep Infiltrative Endometriosis (DIE). Despite advances in imaging techniques, SPE, representing the most frequent phenotype observed in up to 80% of cases, is often ignored by both Transvaginal Ultrasonography (TVUS) and MRI [1,10,11]. Endometrioma, observed in about one third of women, is well diagnosed by both TVUS and MRI with an accuracy over 90% [2]. Another shortcoming of the diagnosis algorithms is linked to patients with a suspicion of DIE on imaging observed in 20% of patients with endometriosis. Even using data from expert centres, Nisenblat et al. demonstrated the low accuracy of imaging to accurately determine all locations of deep endometriosis especially the Uterosacral Ligament (USL) location that is the most frequent DIE lesion [2,12,13]. The difficulties to assess the diagnosis of USL endometriosis is partially linked to the absence of referent measures to define normal USL. In contrast to numerous anatomic studies on USL morphology in genital prolapse, few data are available on normal anatomy of USL using serial sectioning [14-16]. Therefore, the goal of the present study was to analyze macroscopic characteristics of normal USL to determine cut-off of normality to help radiologists to diagnose USL endometriosis.
Material and Methods
Anatomic consideration on USL
The USLs arise from the posterolateral surfaces of the supravaginal part of the cervix and the vaginal fornix, running along the recto-uterine cul-de-sac, and ending in the pre-sacral vertebrae fascia S2 to S4. In their medial part, USL lies the inferior hypogastric nerve plexus [17]. Rouvière et al. [17] defined USL as “smooth connective and muscular bundles arising from the posterior surface of the cervix, near its lateral edges and in the immediate vicinity of the isthmus, run cranially and dorsally, around the lateral surfaces of the rectum and terminate on the anterior surface of the sacrum. They lift the peritoneum to form a curved fold, concave medially, which laterally limits the culde-sac of Douglas. The name USL refers to both the serous fold and the conjunctive-muscular elements that define and support it. The thickness of these ligaments contains mixed with the smooth connective and muscular fibers, and part of the hypogastric nerve plexus, which in fact constitutes the truly resistant component of the ligament on each side. The USL on one side is joined to the ligament on the opposite side, behind the cervix, by a transverse fold known as the torus uterinum or J.L. Petit ligament. Taken together, the two USLs form a horseshoe shape.
Material
The study involved 20 hemipelvis from 10 menopausal female cadavers (five fresh and five embalmed) dissected in the anatomy laboratory of the Saint Pères and at the Fer à Moulin surgical school Paris, France.
Methods
The two USLs of each subject were resected in their entirety. The cadaver was placed on a dissecting table in dorsal decubitus position. The peritoneal cavity was opened by a large cross incision, and the uterus was fixed by a wire to the wall opposite the pubis to ensure maximum anteversion. The digestive tract was pushed upwards to ensure better exposure. The first measures (to the nearest 0.1cm) were the distance separating the medial edges of the USLs at their uterine insertion and the distance separating the lower limit of the torus uterinum from the upper limit of the posterior vaginal fornix. This measurement was performed after locating the upper limit of the posterior vaginal fornix by a vaginal digital examination. Then each USL was resected. Once the USLs resected, they were refined from the peritoneum using transillumination.Each USL was oriented and various measurements (to the nearest 0.1cm) were performed including the total length (from uterine insertion to sacral insertion), cranio-caudal and transverse thicknesses at the level of the uterine insertion. Then transverse thickness of USL was measured every centimeter in the ventro-dorsal direction (from uterine insertion to sacral insertion) (Figure 1).
Figure 1: schematic axial view of the female pelvis.
Statistical Analysis
To compare measures between left and right USL and between fresh and embalmed cadavers, Stat View 5.0 software was used.
Results
Evaluation of USL at the Uterine Insertion
The distance between the internal edges of the USLs at their uterine insertion and the distance between the torus uterinum and posterior vagina fornix for the 10 cadavers were evaluated. The mean distance between the internal edges of the USLs at their insertion to the torus uterinum level was 1.4±0.3cm (range: 1-1.8cm). The mean distance between the torus uterinum and the posterior vagina fornix was 1.06±0.38cm (range: 0.5-1.8cm). Evaluation of the USL Anatomic Measures
For the 20 hemipelvis, the total length, the transverse and the cranio-caudal thicknesses of USLs were evaluatedFor the 20 hemipelvis, the mean length of USL was 7±0.95cm (range: 5.58.5cm). For the 20 hemipelvis, the transverse thickness was not uniform throughout its length allowing to differentiate three segments (Figure 1), the first segment from the uterine insertion to the first centimeter with a decrease in the transverse thickness, the second segment from 2 to 5cm from uterine insertion with a steady transverse thickness, and the last segment from 6cm to the sacral insertion with a progressive increase in the transverse thickness. In contrast to the transverse thickness, the cranio-caudal thickness evaluation was evaluable only for the first centimeters of USLs from the uterine insertion. The mean cranio-caudal thickness at uterine insertion for the right and left USL were 0.7±0.2 cm and 0.7±0.1cm, respectively (not significant). In contrast to the transverse thickness that was adequately delimited, the craniocaudal thickness of USL according to the three segments was not adequately delimited especially their two-thirds dorsal segments of USL not permitting an adequate evaluation.
Comparison of Right and Left USL Measures
The comparison of right and left USL measures is given in Table 1.
|
|
Left USL |
Right USL |
P value |
|
TT at uterine insertion (cm) |
0.8 ± 0.2 |
0.7 ± 0.1 |
NS |
|
TT at 1 cm from uterine insertion (cm) |
0.6 ± 0.2 |
0.6 ± 0.2 |
NS |
|
TT at 2 cm from uterine insertion (cm) |
0.5 ± 0.2 |
0.5 ± 0.1 |
NS |
|
TT at 3 cm from uterine insertion (cm) |
0.5 ± 0.2 |
0.5 ± 0.1 |
NS |
|
TT at 4 cm from uterine insertion (cm) |
0.5 ± 0.2 |
0.5 ± 0.1 |
NS |
|
TT at 5 cm from uterine insertion (cm) |
0.6 ± 0.1 |
0.6 ± 0.1 |
NS |
|
TT at 6 cm from uterine insertion (cm) |
0.7 ± 0.2 |
0.7 ± 0.2 |
NS |
|
TT at 7 cm from uterine insertion (cm) |
0.7 ± 0.1 |
0.7 ± 0.1 |
NS |
|
TT at 8 cm from uterine insertion (cm) |
0.9 ± 0.1 |
0.6 ± 0.1 |
NS |
Table 1: Evaluation of the transverse thickness (TT) of the uterosacral ligament (USL) according to the distance from uterine insertion.
|
insertion were observed between left and right USL. No comparison of the cranio-caudal thickness according to sides was possible. Comparison of USL Measures Between Fresh And Embalmed Cadavers. The measures of the USLs according to the fresh or embalmed are summarized in the Table 2.
Table 2 : Comparison of the uterosacral ligament (USL) measures between fresh and embalmed cadavers. |
The mean total length of the right USL was longer than the left USL, 7.1cm and 6.8cm respectively (p<0.01). No differences in the transverse thicknesses according to the distance from uterine No differences in the distance between the internal edges of the USLs at their uterine insertion, the distance between torus uterinum and posterior vaginal fornix, and the total length of USL, were found between fresh and embalmed cadavers. Except for a trend for a higher length for the right USL in fresh cadavers, no difference in the transverse thickness values was observed between fresh and embalmed cadavers.
Discussion
The present anatomic analysis contributes to determine cutoff measure of normal USL. Among the various measures, the transverse thickness values between 2 and 5cm from uterine insertion of the USL was reproducible and steady supporting its potential use as criterion of abnormal USL on imaging techniques for endometriosis diagnosis. In the present study, we observed that the length of USL was significantly longer for the right USL (p<0.01). In a review including 13 anatomic studies, Ramanah et al. [18] underlined differences in USL length according to series. Indeed, Siddique et al. reported an USL length of 8.7cm (95 % confidence interval (CI); 7.5-10.0) [19] while Vu et al. [20] measured the USL length to 12-14cm. However, as in the present study, Campbell et al. [21] and Blaisdell et al. [15] observed that the length of USL was greater on the right side. For the 20 hemi-pelvis, when focusing on the transverse thickness of USL according to the distance from the uterine insertion, that is often analyzed on TVUS and MRI to diagnose endometriosis involvement, its measure in the current study remained steady between 2 and 5cm of 0.5±0.2cm without difference between the right and left USL supporting that this segment should be the most adequate portion for reproducibility of the measures. In contrast to the evaluation of USL length, to our knowledges, few data are available on the serial analysis of the USL transverse thickness according to the distance from uterine insertion. Vu et al. reported that the transverse thickness of the distal section and at uterine insertion of USLs varied between 5 and 20mm [20] while Ramanah et al. noted that the mean thickness of USL were 5.2±0.9cm, 2.7±1.0, and 2.0±0.5cm at the sacral, intermediate, and cervical portions, respectively [18]. However, as previously mentioned [18], this discrepancy can be explained by controversies on terminology, definition, composition, dissection artifacts, and even the existence of USL. Concerning the craniocaudal thickness, the measures were higher at the uterine and sacral insertions without difference between right and left USL but were not adequately evaluable throughout the USL length. This is probably linked to the intricating of muscle fibers as well as the inferior hypogastric nerves fibers, and to anatomic dissection artifacts. Consequently, in the absence of other criteria of endometriosis such as nodular or spicular features, and hot spot component corresponding to hemorrhagic lesion, a USL transverse thickness per se inferior or equal to 5mm at its intermediate segment (2-5cm from uterine insertion) on imaging cannot support the diagnosis of endometriosis. Moreover, as aforementioned, USL transverse thickness was measured after refining USL from the peritoneum while the measure on imaging includes the peritoneal component. This consideration is particularly relevant as among 344 DIE lesions, Chapron et al. [22] reported that USL was the most frequent location (69.2%). Moreover, they reported that the proportion of isolated lesions differed according to the DIE location with a highest rate of 83.2% for USL.
National and international guidelines recommend for the diagnosis of endometriosis an algorithm based on ultrasonography (US) or MRI [3,4]. Although these algorithms appear logical to detect ovarian endometriosis, observed in about one-third of patients, thanks to the high accuracy of both US and MRI, a debate exists on the accuracy of imaging techniques to detect USL endometriosis [2]. When considering the contribution of imaging techniques to diagnose USL endometriosis, it is important to differentiate US from MRI. In a retrospective multicenter study involving 878 patients between 15 and 45 years-old undergoing Laparoscopy (LPS) for suspected endometriosis, Abrao et al. evaluated transvaginal and transabdominal ultrasonography (US) (index test) to assess sites of endometriosis according to the 2021 AAGL Endometriosis Classification [23]. The AAGL-US and AAGL-LPS stages were concordant in 586 cases (66.7%) (weighted kappa (WK) 0.759; intraclass correlation 0.906), with the highest agreement observed in patients with no endometriosis (75.3% concordance). However, for retrocervical/uterosacral ligament the WK was only 0.656. Another prospective study of 172 patients of Ros et al. evaluated the accuracy of TVUS to diagnose DIE involving USL, torus uterinum (TU) or posterior vaginal fornix (PVF) compared to laparoscopy [24]. The global sensitivity and specificity of TVUS in diagnosing USL, TU and/or PVF endometriosis were 92% and 87%, respectively. In a meta-analysis, Zhou et al. have evaluated the accuracy of TVUS for USL endometriosis [25]. The respective pooled sensitivity, specificity, positive probability ratio (LR+) and negative probability ratio (LR-) of TVUS for detecting USL endometriosis were 65% (95% CI:43-83), 92 % (95% CI:8496), 7.80 (95% CI:4.7-13.0) and 0.38 (95% CI:0.22-0.66) with a significant heterogeneity in both sensitivity and specificity reported. Finally, in a review including 22 studies, focusing on TVUS for USL endometriosis, Maple et al. found that most (20/22) studies described abnormal criteria but only five defined the appearance of normal USL underlining the lack of data and consensus for this specific endometriosis location [26].
When considering MRI to assess USL endometriosis, using the RAND-UCLA Appropriateness Method to attain consensus guidelines, Rousset et al. elaborated a lexicon of image interpretation, and a standardized region-based reporting of DIE with MRI [27]. A consensus regarding pelvic compartment delineation and DIE reporting was thereby attained. A consensus was reached for the most frequent locations of DIE, but no consensus was reached to define the normal presentation of USL. In a meta-analysis including 10 prospective studies, Gerges et al. evaluated USL endometriosis by TVUS and MRI compared to surgery [28]. For USL endometriosis, for all TVUS and MRI the respective pooled sensitivity and specificity techniques were 60% (95%CI 32–82%) and 95% (95%CI 90–98%), and 81% (95%CI 66–90%) and 83% (95%CI 62–94%). Despite some limits of the included studies, MRI outperformed TVUS for the diagnosis of USL with higher sensitivities but similar specificities. Finally, whatever imaging techniques, a publication bias exists as studies primarily emerge from expert centers, thereby not reflecting the full scope of routine practice [2]. Moreover, Leonardi et al. underlined the difficulties for patients to find an expert sonographer [29]. Some limits of the present study deserve to be underlined. First, the limited sample size cannot exclude all bias even if homogenous evaluation of USL length and transverse thickness measures was observed. Second, the use of fresh and embalmed cadavers might be a potential bias, but our results support the absence of difference in USL characteristics between these two groups. Third, all cadavers were from menopausal women with a possible underestimation of USL measures linked to hormonal deficiency. However, none of the cadavers had a genital prolapse that is known as a cause of USL alterations. Indeed, Kökçü et al. showed a decreased cellularity in connective tissue in patients with genital prolapse compared with patients without prolapse [30]. Finally, the present study underlined the difficulties to evaluate the cranio-caudal thickness of USL due to the absence of clear delimitation of the connective tissue from adjacent muscle and inferior hypogastric nerves. In conclusion, despite some limits of the present study, our results underlined the need to consider data of anatomic analysis to define normal anatomy of USL. Further studies evaluating the morphology and dimensions of USL by imaging techniques in patients with and without symptoms suggestive of endometriosis are required to determine the characteristics of both normal and abnormal pelvic structures.
References
1. Zondervan KT, Becker CM, Missmer SA (2020) Endometriosis. New England Journal of Medicine. 26 mars 382: 1244‑1256. 2. Nisenblat V, Bossuyt PMM, Farquhar C, Johnson N, Hull ML (2016) Imaging modalities for the non‑invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 26 févr 2: CD009591. 3. Collinet P, Fritel X, Revel‑Delhom C, Ballester M, Bolze PA, et al. (2018) Management of endometriosis: CNGOF/HAS clinical practice guidelines – Short version. Journal of Gynecology Obstetrics and Human Reproduction. 1 sept 47: 265‑274.
- 4. Becker CM, Bokor A, Heikinheimo O, Horne A, Jansen F, et al. (2022) ESHRE guideline: endometriosis. Hum Reprod Open. 2022: hoac009.
- 5. Bendifallah S, Dabi Y, Suisse S, Jornea L, Bouteiller D, et al. (2022) MicroRNome analysis generates a blood‑based signature for endometriosis. Sci Rep. 8 mars 12: 4051.
- 6. Grover SR, Joseph K (2021) Endometriosis and pelvic pain: Time to treat the symptoms not the assumptions? Aust N Z J Obstet Gynaecol.
août 61: 625‑627. 7. Roditis A, Florin M, Rousset P, Touboul C, Bendifallah S, et al. (2023) Accuracy of combined physical examination, transvaginal ultrasonography, and magnetic resonance imaging to diagnose deep endometriosis. Fertil Steril. avr 119: 634‑643.
8. Nisenblat V, Bossuyt PMM, Shaikh R, Farquhar C, Jordan V, et al. (2016) Blood biomarkers for the non‑invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 1 mai 2016: CD012179. 9. Nisenblat V, Prentice L, Bossuyt PMM, Farquhar C, Hull ML, et al. (2016) Combination of the non‑invasive tests for the diagnosis of endometriosis. Cochrane Database Syst Rev. 13 juill 7: CD012281. 10. Horne AW, Missmer SA (2022) Pathophysiology, diagnosis, and management of endometriosis. BMJ. 14 nov 379: e070750.
11. Koninckx PR (1994) Is mild endometriosis a condition occurring intermittently in all women? Hum Reprod. déc 9: 2202‑2205. 12. Koninckx PR, Ussia A, Adamyan L, Wattiez A, Donnez J (2012) Deep endometriosis: definition, diagnosis, and treatment. Fertil Steril. sept 98: 564‑571.
13. Bazot M, Jarboui L, Ballester M, Touboul C, Thomassin‑Naggara I, et al. (2012) The value of MRI in assessing parametrial involvement in endometriosis. Hum Reprod. août 27: 2352‑2358. 14. Umek WH, Morgan DM, Ashton‑Miller JA, DeLancey JOL (2004)
Quantitative analysis of uterosacral ligament origin and insertion points by magnetic resonance imaging. Obstet Gynecol. mars 103: 447‑451. 15. Blaisdell: The anatomy of the sacro‑uterine ligaments.
- 16. Gabriel B, Denschlag D, Göbel H, Fittkow C, Werner M, et al. (2005) Uterosacral ligament in postmenopausal women with or without pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 16: 475‑479.
- 17. Rouvière: Anatomía humana.
- 18. Ramanah R, Berger MB, Parratte BM, DeLancey JOL (2012) Anatomy and histology of apical support: a literature review concerning cardinal and uterosacral ligaments. Int Urogynecol J. nov 23: 1483‑1494. 19. Siddique SA, Gutman RE, Schön Ybarra MA, Rojas F, Handa VL (2006) Relationship of the uterosacral ligament to the sacral plexus and to the pudendal nerve. Int Urogynecol J Pelvic Floor Dysfunct. nov 17: 642‑645.
20. Vu D, Haylen BT, Tse K, Farnsworth A (2010) Surgical anatomy of the uterosacral ligament. Int Urogynecol J. sept 21: 1123‑1128. 21. Campbell RM (1950) The anatomy and histology of the sacrouterine ligaments. Am J Obstet Gynecol. janv 59: 1‑12, illust. 22. Chapron C, Fauconnier A, Vieira M, Barakat H, Dousset B (2003) Anatomical distribution of deeply infiltrating endometriosis: surgical implications and proposition for a classification. Hum Reprod. janv 18: 157‑161.
- 23. Abrao MS, Andres MP, Gingold JA, Rius M, Neto JS, et al. (2023) Preoperative Ultrasound Scoring of Endometriosis by AAGL 2021 Endometriosis Classification Is Concordant with Laparoscopic Surgical Findings and Distinguishes Early from Advanced Stages. J Minim Invasive Gynecol. mai 30: 363‑373.
- 24. Ros C, de Guirior C, Mension E, Rius M, Valdés‑Bango M, et al. (2021) Transvaginal ultrasound for diagnosis of deep endometriosis involving uterosacral ligaments, torus uterinus and posterior vaginal fornix: prospective study. Ultrasound Obstet Gynecol. déc 58: 926‑932. 25. Zhou Y, Su Y, Liu H, Wu H, Xu J, et al. (2021) Accuracy of transvaginal ultrasound for diagnosis of deep infiltrating endometriosis in the uterosacral ligaments: Systematic review and meta‑analysis. J Gynecol Obstet Hum Reprod. mars 50: 101953.
- 26. Maple S, Chalmers KJ, Bezak E, Henry K, Parange N (2023) Ultrasound Characteristics and Scanning Techniques of Uterosacral Ligaments for the Diagnosis of Endometriosis: A Systematic Review. J Ultrasound Med. juin 42: 1193‑1209.
- 27. Rousset P, Florin M, Bharwani N, Touboul C, Monroc M, et al. (2023) Deep pelvic infiltrating endometriosis: MRI consensus lexicon and compartment‑based approach from the ENDOVALIRM group. Diagn Interv Imaging. mars 104: 95‑112.
- 28. Gerges B, Li W, Leonardi M, Mol BW, Condous G (2021) Meta‑analysis and systematic review to determine the optimal imaging modality for the detection of uterosacral ligaments/torus uterinus, rectovaginal septum and vaginal deep endometriosis. Hum Reprod Open. 4 nov 2021: hoab041. 29. Leonardi M, Uzuner C, Mestdagh W, Lu C, Guerriero S, et al. (2022) Diagnostic accuracy of transvaginal ultrasound for detection of endometriosis using International Deep Endometriosis Analysis (IDEA) approach: prospective international pilot study. Ultrasound in Obstetrics & Gynecology. 60: 404‑413.
30. Kökçü A, Yanik F, Cetinkaya M, Alper T, Kandemir B, et al. (2002) Histopathological evaluation of the connective tissue of the vaginal fascia and the uterine ligaments in women with and without pelvic relaxation. Arch Gynecol Obstet. avr 266: 75‑78.
Abstract
Background: Uterosacral Ligament (USL) is the most frequent location of deep infiltrating endometriosis. Despite an abundant literature on USL anatomy in genital prolapse, few data are available on normal USL anatomy. This explains the absence of consensus on criteria to diagnose by imaging techniques this specific endometriosis location. Therefore, the aim of the present study was to assess the normal anatomy of USL.
Material and methods: Five fresh and five embalmed cadavers of adult female were dissected. The anatomic characteristics of USL at the insertion to the torus uterinum were assessed by measuring the distance between the inner edges of the USLs at the uterine insertion and the distance between the torus uterinum and the upper edge of the posterior vaginal cuff. For the 20 hemipelvis, the length and thickness of the USLs were evaluated every centimeter from the uterus to the sacral insertions. Comparisons of measures between the right and left USL and between fresh and embalmed cadavers were performed.
Results: Distance between the inner edges of the USLs at uterine insertion was 1.4±0.3cm. Distance between the torus uterinum and the upper edge of the vaginal cuff was 1.0±0.4cm. For the 20 hemipelvis, the mean length of the USL was 7±0.95cm. USL transverse thickness varied according to the distance from uterine insertion and was steady between 2 and 5cm from uterine insertion with a mean value of 0.5±0.2cm. The length of the right USL was longer (7.1 vs. 6.8cm) (p<0.01). No difference in the transverse thickness was found between right and left USL. No difference in USLs measures were found between fresh and embalmed cadavers.
Conclusion: Our results allow to determine the portion of USL where the measures are reproducible. Moreover, measures of normal USL were evaluated contributing to define cut-off of abnormal USL applicable to imaging techniques in women with suspicion of endometriosis.
Keywords: Anatomy; Cranio-caudal thickness; Endometriosis; Transverse thickness; Uterosacral ligament
Nabil Louafi1, Elie Zerbib1*, Yohann Dabi1, Clément Ferrier1, Adrien Crestani1, Kamila Kolanska1, Amélia Favier1, Isabelle Thomassin-Naggara2, Marc Bazot2, Cyril Touboul1, Emile Daraï1
1Department of Gynaecology, Obstetrics and Reproductive Medicine, Sorbonne University, Hôpital Tenon, 4 Rue de la Chine, 75020 Paris, France
2Département d’Imageries Radiologiques et Interventionnelles Spécialisées (IRIS), Hôpital Tenon, Assistance Publique-Hôpitaux de Paris, France; Sorbonne Université, INSERM U938 Équipe Biologie et Thérapeutiques du Cancer, France
Corresponding author: Elie Zerbib, Department of Gynaecology, Obstetrics and Reproductive Medicine, Sorbonne University, Hôpital Tenon, 4 Rue de la Chine, 75020 Paris, France
Citation: Louafi N, Zerbib E, Dabi Y, Ferrier C, Adrien Crestani A, et al. (2025) Anatomic Study of the Uterosacral Ligaments: Impact on Endometriosis Diagnosis J Surg 10: 11380 DOI: 10.29011/2575-9760.011380
Received Date: 07 July 2025; Accepted Date: 14 July 2025; Published Date: 16 July 2025
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.