Analgesic Effect of a New Topical Gel Formulation Based on Cannabis Sativa Oil Enriched in Cannabidiol, Escin, Bromelain, Boswellia Extract, Glucosamine Sulphate, Methylsulfonylmethane and Methylsalycylate in Patients with Osteoarthritis
by Massimo Biondi1, Annalisa Curcio2*, Assunta Amicone1, Fabiana Nano2, Michele Pironti2
1Orthopedics Department, ASL Napoli 2 Nord, Italy.
2Medical Department, Aqma Italia S.p.A, Italy
*Corresponding author: Annalisa Curcio, Medical Department, Aqma Italia S.p.A., Naples, Italy.
Received Date: 08 August, 2024
Accepted Date: 15 August, 2024
Published Date: 20 August, 2024
Citation: Biondi M, Curcio A, Amicone A, Nano F, Pironti M (2024) Analgesic Effect of a New Topical Gel Formulation Based on Cannabis Sativa Oil Enriched in Cannabidiol, Escin, Bromelain, Boswellia Extract, Glucosamine Sulphate, Methylsulfonylmethane and Methylsalicylate in Patients with Osteoarthritis. J Orthop Res Ther 9: 1356. https://doi.org/10.29011/2575-8241.001356
Abstract
Background: Osteoarthritis (OA) is the most frequent form of arthritis worldwide, with an increasing trend of frequency for the higher incidence in the population of risk factors, like age and obesity. New local remedies for pain and inflammation management are useful for reducing adverse events related to oral anti-inflammatory drugs administration. The aim of this study is to evaluate the analgesic effect of a new topical gel formulation based on cannabis sativa oil enriched in CBD, escin, bromelain, Boswellia extract, glucosamine sulphate, methylsulfonylmethane (MSM) and methylsalicylate (Cibides lipogel®), in patients with OA. Methods: 53 outpatients with osteoarthritis diagnosis, were allocated to treatment with the topical formulation applied three times a day for 14 days and evaluated at baseline, after 7 and 14 days of treatment, by assigning a score in a 100-mm Visual Analogue Scale (VAS) and by filling a questionnaire for quality-of-life assessment. Results: A statistically significant VAS score reduction versus baseline, for each timepoint evaluated, was registered, with a reduction of 56.3% of VAS score at T2 versus T0. Furtherly, in most patients the score related to every question of the questionnaire for quality-of-life is decreased, showing an improvement of quality of life. Conclusions: This study confirmed that this new topical formulation is effective for pain management in patients with OA and can represent a potential alternative to systemic and local NSAIDs use, without side effects.
Keywords: Osteoarthritis; Cannabis sativa oil; Cannabidiol; Escin; Bromelain; Boswellia extract
Introduction
Osteoarthritis (OA) is the most frequent form of arthritis, affecting about 527 million people worldwide, according to latest reports on 2019 [1]. OA global prevalence increased by 113% from 1990 to 2019 and this trend can mainly be due to the increase in the mean age of the population and in the frequency of obesity, two factors involved in the major incidence risk of this disease [1].
OA is a chronic and degenerative joint disease, characterized by progressive loss of joint function, caused by a complex pathophysiological mechanism, including cartilage degradation, loss of intact subchondral bone, synovial hyperplasia and inflammation, and instability of the tendons and ligaments [2,3]. OA clinical symptoms include pain, stiffness, swelling, loss of normal joint function, that can lead to motor disability, like issues in walking or running. OA-related disability represents a very high health and socioeconomic burden, which showed an increase of 114.5% in years lived with disability due to OA from 1990 to 2019. OA incidence is higher in women and in older people, with a peak in 60-64 years population. The most affected by OA joint is the knee, followed by the hand and hip [1,2].
Management of OA consists of several approaches depending on the joint involved, the severity of disease, and the patient type (i.e., patients with co-morbidity). Overall, interventions can be educational, behavioral, and physical, including lifestyle and training exercises, along with pharmaceutical therapies, like topical, oral, and intra-articular medications, or, in case of worsening of OA, treatment with surgical procedures [3-5].
Currently, pharmacological OA treatment is limited to reduce symptoms, but not to remove OA causes and/or progression disease, and the main classes of drugs commonly used are nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, opioids, and chondroprotective agents [3-5].
Since long-term use of oral NSAIDs can lead to several adverse events, (mainly gastrointestinal, renal, and cardiovascular) national and international guidelines recommend the use of topical analgesic treatments, when possible and effective [3-5]. In particular, the 2019 Osteoarthritis Research Society International (OARSI) guidelines and the 2019 American College of Rheumatology/Arthritis Foundation Guideline stated that topical NSAIDs are strongly recommended for patients with knee OA and conditionally recommended for patients with hand OA [3,4]. The Italian Society for Rheumatology has implemented guidelines stating that topical pharmacological treatments are preferred over systemic treatments, especially for mild to moderate pain and when only a few joints are affected [5].
Several studies confirmed that topical and oral NSAIDs showed similar efficacy in relieving pain and improving OA, with a slightly higher safety profile for topical agents [6-9]. Furtherly, increasing interest is arising about the topical use of naturally derived compounds for pain-relieving and anti-inflammatory action in different patient settings [10,11].
Recently, a study conducted on patients with knee osteoarthritis showed efficacy of hemp seed oil and terpenes contained in several herbal compounds administered as food supplement, in improving joint pain and knee function [12]. Also, topical application of hemp oil extract improved inflammation and pain in several study with in vivo models and humans. Cannabis sativa oil extract contains cannabinoids or phytocannabinoids derivative, including delta (9)-tetrahydrocannabinol (Δ9-THC), the psychoactive compound, responsible for the drug abuse of this plant, and cannabidiol (CBD), which is one of more studied active constituents of the plant, with important analgesic and anti-inflammatory effects and without psychotropic activity [13,14].
Several studies explored CBD topical activity, including a study evaluating CBD efficacy in reducing pain and disability in patients with thumb basal joint arthritis [15], and a study conducted on patients with peripheral neuropathy, showing improvement in pain and other disturbing sensations after transdermal application of CBD oil in this patient’s type [16]. CBD topical activity in reduction of pain and inflammation was studied also in a rat model of arthritis, confirming its therapeutic potential for relief of arthritis symptoms [17].
Other active ingredients of natural origin have been studied for their effect following topical applications, for example, glucosamine sulphate, in combination with chondroitin sulphate and camphor, showed efficacy in pain reduction in patients with knee osteoarthritis [18]. Escin, a triterpene glucoside isolated from the seeds of horse chestnut (Aesculus hippocastanum), also showed efficacy in pain reduction in patient with acute impact injuries [19]. Another study, where escin was studied in combination with Boswellia serrata extract and other herbal ingredients, reported improvements in clinical symptoms of patients with localized neck/shoulder pain [20]. Topical use of a gel preparation with methyl salicylate was evaluated in patients with rheumatoid arthritis, showing local analgesic and anti-inflammatory effect [21].
A new topical composition based on cannabis sativa oil enriched in CBD, escin, bromelain, Boswellia extract, glucosamine sulphate, methylsulfonylmethane (MSM) and methylsalicylate (Cibides lipogel®) was developed in order to exploit its beneficial effect as analgesic and anti-inflammatory agent in several conditions affecting joints, muscles, tendons and/or ligaments. This formulation was studied adding a lot of active ingredients, for obtaining a synergism of action compared to the action of single compounds and, thus, improving analgesic and antiinflammatory efficacy. This formulation was already studied in vitro with a permeability study, which allowed to choose the best type of vehicle between four several formulations, and the better final formulation of the product [22]. Another study conducted oh this topical product showed its efficacy as analgesic agent in patients with localized pain related to acute minor musculoskeletal conditions [23].
The aim of this study is to evaluate the use of the new topical formulation based on cannabis sativa oil enriched in CBD, escin, bromelain, Boswellia extract, glucosamine sulphate, methylsulfonylmethane (MSM) and methylsalicylate (Cibides lipogel®) for pain reduction and improvement of quality of life in patients with osteoarthritis.
Materials and Methods
This is an observational study, included in the normal clinical practice of the investigators, conducted on a total of 53 outpatients of both sexes (12 males, 41 females), mean age 56.6 (±13.0) years, with osteoarthritis diagnosis, that were allocated to treatment with the gel formulation based on cannabis sativa oil enriched in CBD, escin, bromelain, Boswellia extract, glucosamine sulphate, methylsulfonylmethane (MSM) and methylsalicylate (Cibides lipogel®, AQMA Italia S.p.A.) three times a day for 14 days. Patient were instructed about the way of use of the product, that was applied on the painful part and massaged gently until absorption. Subjects were informed of the study procedures and provided written informed consent. Local ethic boards approved the protocol. The study was conducted in accordance with the Declaration of Helsinki guidelines regarding ethical principles for medical research involving human subjects.
Patients were evaluated at 3 timings: T0 (baseline), T1 (after 7 days of treatment), and T2 (after 14 days of treatment). All patients filled in an assessment questionnaire at each timepoint, assigning a score from 0 to 100 to perceived pain, using a 100-mm Visual Analogue Scale (VAS). The questionnaire included a part of quality-of-life assessment, consisting of the assignation of a score from zero to two to following questions: 1. Do you carry out all your daily activities with some difficulty? 2. Do you carry out all your daily activities feeling stiffness in your movements? 3. Do you carry out all your daily activities feeling pain when moving? 4. Do you carry out all your daily activities feeling intense effort? The score was assigned as follows: 0 = no; 1 = slightly; 2 = yes. Safety was evaluated collecting information about adverse events eventually reported. The last two questions of the evaluation form regarded the benefit obtained from the gel application after 14 days of treatment registered with following parameters: a) no benefit; b) real benefit; and c) great benefit; and patients judgment of the treatment as excellent, good, fair, satisfactory, or unsatisfactory.
The primary objective was to assess the analgesic effect of the gel application on localized pain related to osteoarthritis, by using the reduction of VAS score from T0 to T2. The secondary outcome was to obtain information on the improvement of quality of life after treatment.
Statistical analysis was performed using Paired T-test with Microsoft excel analysis program for Windows 11 Pro, by comparing all VAS scores registered in the study collected at T0, T1, and T2. The differences were considered significant when P<0.05.
Results
This observational study was conducted to evaluate the use of the new topical composition based on cannabis sativa oil enriched in CBD, escin, bromelain, Boswellia extract, glucosamine sulphate, methylsulfonylmethane (MSM) and methylsalicylate (Cibides lipogel®) for pain reduction and improvement of quality of life in 53 consecutive outpatients with osteoarthritis. Most of the enrolled patients had knee OA (83%, N=44), the remaining patients had hand OA (13.2%, N=7) or hip OA (3.8%, N=2). The analgesic effect of topical application 3 times a day of the new composition in study was evaluated analysing VAS scores reduction from T0 (baseline) to T2 (after 14 days of treatment). Mean VAS scores collected at each time are reported in Table 1.
|
Timepoint1 |
T0 |
T1 |
T2 |
P value |
|
Mean VAS score |
71.51 |
29.15 |
15.28 |
<0.001 |
|
Standard deviation (± SD) |
9.39 |
9.04 |
6.41 |
- |
|
1T0: baseline; T1: 7 days after first application; T2: 14 days after first application. Means ± SD (N=53). P<0.001 for all timepoints versus baseline. |
||||
Table 1: Mean VAS scores over treatment period.
Results showed a statistically significant VAS score reduction versus baseline for each timepoint evaluated, with a mean reduction from 71.51 registered at T0 to 15.28 registered at T2 (P<0.001), corresponding to a 56.3% of reduction (Figure 1).
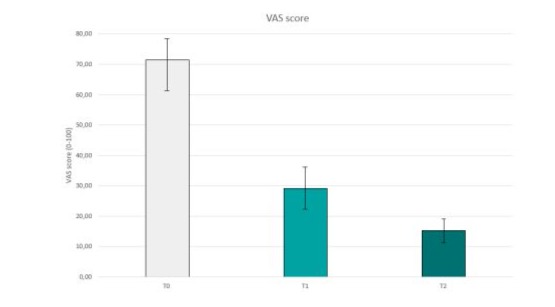
Figure 1: Reduction of mean VAS scores from T0 to T2, after treatment with gel formulation based on cannabis sativa oil enriched in CBD, escin, bromelain, Boswellia extract, glucosamine sulphate, methylsulfonylmethane (MSM) and methylsalicylate (Cibides lipogel®) three times a day for 14 days. N=53; P<0.001.
Regarding the secondary outcome of improvement of quality of life after treatment with the gel formulation in study for 14 days, the results showed that most patients reduced the score related to every question evaluated, corresponding to an improvement of quality of life (Table 2).
The percentage of patients assigning 2-point score to each question at time T2 after 14 days of gel treatment is zero, meaning that after treatment all patients did not show overt pain and/or intense effort during movement. The majority of patients assigned 1-point score to each question at time T2, indicating a slight level of pain, stiffness and difficulty during movement. A little percentage of patient (ranging from 17% to 4%) assigned 0-point score to each question, indicating none pain, nor effort during movement. Figure 2 shows the improvements in scores of quality of life-related questions.
|
Q11 |
Q11 |
Q22 |
Q22 |
Q33 |
Q33 |
Q44 |
Q44 |
|
|
(T0) |
(T2) |
(T0) |
(T2) |
(T0) |
(T2) |
(T0) |
(T2) |
|
|
% patients with score =2 |
62% |
55% |
79% |
87% |
||||
|
% patients with score =1 |
38% |
83% |
45% |
92% |
21% |
91% |
13% |
96% |
|
% patients with score =0 |
17% |
8% |
9% |
4% |
||||
|
1Q1= question 1: Do you carry out all your daily activities with some difficulty? 2Q2= quention 2: Do you carry out all your daily activities feeling stiffness in your movements? 3Q3= question 3: Do you carry out all your daily activities feeling pain when moving? 4Q4= quention 4: Do you carry out all your daily activities feeling intense effort? The score was assigned as follows: 0 = no; 1 = slightly; 2 = yes. |
||||||||
Table 2: Percentage of patients assigning a score to 4 questions for quality-of-life assessment at T0: baseline and T2: after 14 days of treatment with gel formulation based on cannabis sativa oil enriched in CBD, escin, bromelain, Boswellia extract, glucosamine sulphate, methylsulfonylmethane (MSM) and methylsalicylate (Cibides lipogel®).
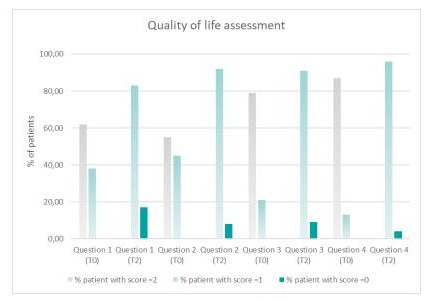
Figure 2: Improvements in scores assigned to quality of liferelated questions by patients at T0: baseline and T2: after 14 days of treatment with gel formulation based on cannabis sativa oil enriched in CBD, escin, bromelain, Boswellia extract, glucosamine sulphate, methylsulfonylmethane (MSM) and methylsalicylate (Cibides lipogel®).
Overall, the gel application was well tolerated, and no adverse events were reported. Furtherly, all patients reported to having benefit from the treatment, in particular, 49% of patients (N=26) reported a great benefit and 51% (N=27) of patients reported a real benefit from treatment. Last question of the evaluation form, regarding patients’ judgment of the treatment, showed that 49% of patient (N=26) evaluated treatment as excellent, 40% of patients (N=21) evaluated treatment as good, and the remaining 11% (N=6) of patient evaluated the treatment as fair.
Discussion
OA represents a growing global issue, related to the increasing incidence of risk factors, like obesity and the higher population mean age. Currently, the main interventions for OA include moderate exercise, weight loss and the treatment with analgesic drugs and NSAIDs, both topically and for systemic use [1,4].
The use of topical remedies for OA can allow to reduce the need of oral anti-inflammatory and analgesic drugs that can be frequently responsible of adverse events, so emerging interest is arising to topical treatments with analgesic and anti-inflammatory action.
For this reason, this study aimed to evaluate a new topical formulation based on cannabis sativa oil enriched in CBD, escin, bromelain, Boswellia extract, glucosamine sulphate, methylsulfonylmethane (MSM) and methylsalicylate (Cibides lipogel®) for pain reduction and improvement of quality of life in patients with OA.
The results of this study showed that topical treatment with the gel formulation was effective in pain reduction since VAS scores decreased in statistically significant way, in 53 patients with OA after topical application 3 times a day for 14 days. This new formulation also allowed to improved quality of life of the participants to the study as confirmed by the increase in percentage of patients with lower score assigned to the questions administered through quality-of-life questionnaire.
This new topical composition based on cannabis sativa oil enriched in CBD, escin, bromelain, Boswellia extract, glucosamine sulphate, methylsulfonylmethane (MSM) and methylsalicylate (Cibides lipogel®) was already showed its efficacy in pain reduction in 60 patients with localized pain related to acute minor musculoskeletal conditions, such as tendinitis of the upper or lower limbs, low back pain, knee and ankle sprains/contusions, sport-related softtissue injury (sprains, strains and contusions) of upper or lower limbs, cervicalgia (neck pain), myalgias, arthrosis, carpal tunnel syndrome [23]. Results here reported, despite the limitations deriving from the small case series and the lack of a control group, since the study was designed as observational, showed efficacy of this formulation also in patients with OA.
In conclusion, this study confirmed that this new topical formulation based on cannabis sativa oil enriched in CBD, escin, bromelain, Boswellia extract, glucosamine sulphate, methylsulfonylmethane (MSM) and methylsalicylate (Cibides lipogel®) is effective for pain management and improvement of quality of life in patients with OA and can represent a potential alternative to systemic and local NSAIDs use, without side effects.
Funding
This research received financial support by Aqma Italia S.p.A (Milan, Italy) for covering the costs of the article processing charge. The study was a non-profit spontaneous investigatorinitiated trial and the above company did not interfere with the study design, conduct, or interpretation.
Ethical Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by local Ethic Committee of Aqma Italia S.p.A. in Naples (protocol n. 20220002610 on 26 October 2022).
Conflicts of Interest: The authors declare no conflicts of interest.
References
- Long H, Liu Q, Yin H, Wang K, Diao N, et al. (2022) Prevalence Trends of Site-Specific Osteoarthritis From 1990 to 2019: Findings from the Global Burden of Disease Study 2019. Arthritis Rheumatol 74: 11721183.
- Yao Q, Wu X, Tao C, Gong W, Chen M, et al. (2023) Osteoarthritis: pathogenic signaling pathways and therapeutic targets. Signal Transduct Target Ther 8(1): 56.
- Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, et al. (2020) 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res (Hoboken) 72(2): 149-162.
- Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, et al. (2019) OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage 27(11): 1578-1589.
- Ariani A, Manara M, Fioravanti A, Iannone F, Salaffi F, et al. (2019) The Italian Society for Rheumatology clinical practice guidelines for the diagnosis and management of knee, hip and hand osteoarthritis. Reumatismo 71(S1): 5-21.
- Wang Y, Fan M, Wang H, You Y, Wei C, et al. (2022) Relative safety and efficacy of topical and oral NSAIDs in the treatment of osteoarthritis: A systematic review and meta-analysis. Medicine (Baltimore) 101(36): e30354.
- Rannou F, Pelletier JP, Martel-Pelletier J (2016) Efficacy and safety of topical NSAIDs in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin Arthritis Rheum 45(4 Suppl): S18-21.
- Ling T, Li JJ, Xu RJ, Wang B, Ge WH (2020) Topical diclofenac solution for osteoarthritis of the knee: an updated meta-analysis of randomized controlled trials. Biomed Res Int 2020: 1758071.
- Wolff DG, Christophersen C, Brown SM, Mulcahey MK (2021) Topical nonsteroidal anti-inflammatory drugs in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Phys Sportsmed 49(4): 381-391.
- Oltean H, Robbins C, van Tulder MW, Berman BM, Bombardier C, et al. (2014) Herbal medicine for low-back pain. Cochrane Database Syst Rev 12: CD004504.
- Derry S, Wiffen PJ, Kalso EA, Bell RF, Aldington D, et al. (2017) Topical analgesics for acute and chronic pain in adults - an overview of Cochrane Reviews. Cochrane Database Syst Rev 5: CD008609.
- Farì G, Megna M, Scacco S, Ranieri M, Raele MV, et al. (2023) Hemp Seed Oil in Association with β-Caryophyllene, Myrcene and GingernExtract as a Nutraceutical Integration in Knee Osteoarthritis: A Double-Blind Prospective Case-Control Study. Medicina (Kaunas) 59(2): 191.
- Bruni N, Della Pepa C, Oliaro-Bosso S, Pessione E, Gastaldi D, et al. (2018) Cannabinoid Delivery Systems for Pain and Inflammation Treatment. Molecules 23: 2478.
- Tubaro A, Giangaspero A, Sosa S, Negri R, Grassi G, et al. (2010) Comparative topical anti-inflammatory activity of cannabinoids and cannabivarins. Fitoterapia 81(7): 816-819.
- Heineman JT, Forster GL, Stephens KL, Cottler PS, Timko MP, et al. (2022) A Randomized Controlled Trial of Topical Cannabidiol for the Treatment of Thumb Basal Joint Arthritis. J Hand Surg Am 47(7): 611620.
- Xu DH, Cullen BD, Tang M, Fang Y (2020) The Effectiveness of Topical Cannabidiol Oil in Symptomatic Relief of Peripheral Neuropathy of the Lower Extremities. Curr Pharm Biotechnol 21(5): 390-402.
- Hammell DC, Zhang LP, Ma F, Abshire SM, McIlwrath SL, et al. (2016) Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain 20: 936-948.
- Cohen M, Wolfe R, Mai T, Lewis D (2003) A randomized, double blind, placebo-controlled trial of a topical cream containing glucosamine sulfate, chondroitin sulfate, and camphor for osteoarthritis of the knee. J Rheumatol 30(30): 523-528.
- Wetzel D, Menke W, Dieter R, Smasal V, Giannetti B, et al. (2002) Escin/diethylammonium salicylate/heparin combination gels for the topical treatment of acute impact injuries: a randomised, double blind, placebo controlled, multicentre study. Br J Sports Med 36(3): 183-188.
- Hu S, Belcaro G, Cesarone MR, Feragalli B, Cotellese R, et al. (2021) A sport cream (Harpago-Boswellia-ginger-escin) for localized neck/ shoulder pain. Minerva Med 112(2): 255-260.
- Jurca T, Józsa L, Suciu R, Pallag A, Marian E, et al. (2020) Formulation of Topical Dosage Forms Containing Synthetic and Natural AntiInflammatory Agents for the Treatment of Rheumatoid Arthritis. Molecules 26(1): 24.
- Curcio A, Nano F, Pironti M, Marchitto N, Pannozzi A, et al. (2021) In vitro Study Evaluating the Influence of Vehicle in the Permeability Process of a Topical Composition Containing Cannabis Sativa Oil, Escin, Bromelain, Glucosamine Sulphate, Methylsulfonylmethane, Methylsalicylate and Boswellia Extract, Designed for Local Treatment of Musculoskeletal Painful and Inflammatory Conditions. J Clin Chem Lab Med 4: 188.
- Favara M, Curcio A, Nano F, Rossi M, Vettore M, et al. (2022) Evaluation of the analgesic effect of a new topical composition based on Cannabis sativa oil enriched in cannabidiol, escin, bromelain, Boswellia extract, glucosamine sulphate, methylsulfonylmethane and methylsalicylate. Orthop Muscular Syst 11: 351.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.