Acute Respiratory Distress Syndrome Related to Methotrexate: A Rare and Potentially Fatal Adverse Effect
by Ibtissam Nabih1*, K. Khaleq1,2, S. Haddady1, A. Bouhouri1, A. Belhouss3
1Anesthesiology-Intensive Care Medicine Department, Ibn Rochd, University Hospital Center, Faculty of Medicine and Pharmacy, Hassan II University, Casablanca, Morocco
2Laboratory (LC-BENS), Faculty of Medicine and Pharmacy, Hassan II University, Casablanca, Morocco
3Forensic Medicine Department, Ibn Rochd, University Hospital Center, Faculty of Medicine and Pharmacy, Hassan II University, Casablanca, Morocco
*Corresponding author: Ibtissam Nabih, Anesthesiology-Intensive Care Department, Ibn Rochd, university hospital center, Casablanca, Morocco
Received Date: 23 June 2025
Accepted Date: 10 December, 2025
Published Date: 12 December, 2025
Citation: Nabih I, Khaleq K, Haddady S, Bouhouri A, Belhouss A (2025) Acute Respiratory Distress Syndrome Related to Methotrexate: A Rare and Potentially Fatal Adverse Effect. Int J Nurs Health Care Res 8: 1685. https://doi.org/10.29011/2688-9501.101685
Abstract
Methotrexate (MTX) is an antifolate widely used in oncology and rheumatology for its antiproliferative and immunosuppressive properties. While commonly associated with dermatological, gastrointestinal, and hematological side effects, MTX-induced interstitial pneumonitis remains a rare but severe complication that can progress to acute respiratory distress syndrome (ARDS). we report a case of severe ARDS likely induced by MTX, highlighting the importance of early recognition and appropriate management of this serious complication.
We report the case of a 22-year-old male with a family history of Behçet’s disease and suspected lupus with multisystemic involvement, including hematologic, articular, and renal manifestations. The patient was initially treated with corticosteroids and subsequently prescribed MTX at 20 mg/day. On Day 5 of treatment, he developed severe respiratory distress (SaO₂: 5%), hemodynamic instability (tachycardia at 160 bpm, non-recordable blood pressure), and altered consciousness (Glasgow Coma Scale: 3/15). Imaging revealed a bilateral diffuse alveolo-interstitial syndrome, and a chest CT scan showed extensive ground-glass opacities and alveolar consolidations in the lower lobes. Laboratory findings demonstrated pancytopenia, acute renal failure, and hepatic cytolysis. Despite intensive care management, the patient deteriorated and died on Day 13. Post-mortem biopsies confirmed alveolar edema and hemorrhage, centrilobular hepatic necrosis, and renal tubular necrosis.
This case underscores the importance of early recognition of MTX-induced pulmonary toxicity, particularly in patients with pre-existing comorbidities. Immediate drug discontinuation, corticosteroid therapy, and respiratory support are crucial in severe cases. Further research is needed to develop predictive biomarkers and personalized treatment strategies.
Key words: Methotrexate; Acute respiratory distress syndrome; Pneumonitis; Toxicity; Immunosuppression
Introduction
Methotrexate (MTX) is a folic acid antagonist widely used in oncology and rheumatology [1]. By inhibiting dihydrofolate reductase (DHFR), an essential enzyme in folic acid metabolism, it blocks the synthesis of tetrahydrofolic acid (THF), thereby disrupting the production of thymidines and purines necessary for DNA synthesis [1]. These mechanisms explain its efficacy in the treatment of certain cancers, severe rheumatoid arthritis (RA), and severe psoriasis.
However, MTX is associated with a variety of adverse effects, the most common being dermatological (photosensitivity, skin rashes, alopecia), gastrointestinal (nausea, vomiting, diarrhea), and hematological (leukopenia, thrombocytopenia) [2]. Among its rare but serious complications is interstitial pneumonia, first described in 1969 [3]. Its incidence is difficult to estimate but may range between 1% and 7% in patients receiving low doses of MTX, particularly in the context of RA (4). Although rare and unpredictable, this pneumonitis can progress to severe forms, including acute respiratory distress syndrome (ARDS), which can sometimes be fatal [4].
In this article, we report a case of severe ARDS likely induced by MTX, highlighting the importance of early recognition and appropriate management of this serious complication.
Case Presentation
This is a 22-year-old patient with a family history of Behçet’s disease, being followed up for strong suspicion of lupus with multisystem involvement, including hematologic (anemia, thrombocytopenia, lymphopenia), articular (subacute polyarthritis affecting large joints), and renal (positive proteinuria) involvement.
Immunological analyses showed negative anti-DNA, antinuclear, and anti-CCP antibodies, a positive rheumatoid factor, and a positive Coombs test.
The patient was treated with corticosteroid therapy at 60 mg/day. After a consultation with a general practitioner, methotrexate treatment at 20 mg/day was initiated, with a cumulative dose of 90 mg.
On Day 5 of treatment, he developed severe respiratory distress (SaO₂: 5%) with hemodynamic instability (non-recordable blood pressure, tachycardia at 160 bpm) and altered consciousness (Glasgow Coma Scale: 3/15). He was admitted to the intensive care unit, where he was intubated, ventilated, and stabilized with norepinephrine at 3 mg/h.
On clinical examination, erythematous-squamous lesions were noted on the face, with oral aphthosis. Pulmonary auscultation revealed decreased vesicular breath sounds in both lung fields with bilateral coarse crackles. The rest of the systemic examination was unremarkable.
The results of biological analyses are presented in the table below (Table 1).
|
Laboratory tests |
D1 |
D6 |
D8 |
D10 |
D11 |
D12 |
D13 |
|
Hemoglobin |
11,8 |
11,8 |
9,8 |
9,9 |
9,8 |
9,5 |
8,3 |
|
MCV |
87 |
92,7 |
91,7 |
89,6 |
85 |
87 |
82 |
|
MCHC |
32 |
31,1 |
31,5 |
31,6 |
31 |
31.5 |
31 |
|
WBCs |
4980 |
7000 |
2400 |
600 |
1500 |
1036 |
270 |
|
Neutrophils |
4921 |
2160 |
447 |
||||
|
Eosinophils |
15 |
14 |
28 |
1 |
|||
|
Basophils |
3 |
7 |
4 |
4 |
|||
|
Lymphocytes |
130 |
2002 |
187 |
133 |
|||
|
Monocytes |
23 |
56 |
19 |
13 |
|||
|
Platelets |
201000 |
590000 |
172000 |
19000 |
19000 |
17000 |
15000 |
|
PT |
70% |
62% |
48% |
44% |
31% |
31% |
|
|
aPTT |
28 |
57 |
26 |
29 |
43 |
64 |
|
|
CRP |
24 |
62 |
346,5 |
100,25 |
88 |
79 |
58 |
|
AST |
334 |
314 |
319 |
425 |
|||
|
ALT |
84 |
84 |
64 |
36 |
|||
|
Urea |
0,38 |
0,35 |
0,5 |
0,88 |
1,08 |
1,29 |
1,71 |
|
Creatinine |
5,41 |
9,6 |
8,75 |
7,67 |
8,6 |
17 |
32,3 |
Table 1: Biological profile before and after treatment with methotrexate.
Arterial gasometry showed (Table 2)
|
Gazométrie |
D6 |
D7 |
D13 |
|
PH |
7,15 |
7,28 |
7,57 |
|
PaO2 |
25 |
56 |
35 |
|
PaCO2 |
99,8 |
80,1 |
37 |
|
HCO3 |
14,6 |
33,9 |
34,5 |
|
PaO2/FiO2 |
25 |
56 |
35 |
Table 2: Arterial blood gas results after methotrexate treatment.
The chest X-ray revealed a diffuse bilateral alveolo-interstitial syndrome, filling both lung fields (Figure 2), in contrast to the initial chest X-ray (before methotrexate treatment), which was normal (Figure 1).
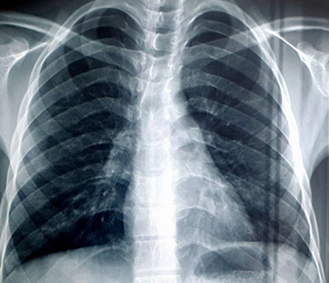
Figure 1: Chest X-ray Before Treatment.
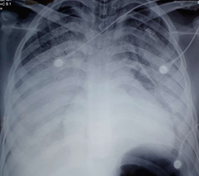
Figure 2: Chest X-ray on Day 5 of Treatment.
A chest CT scan was performed, revealing a diffuse bilateral ground-glass appearance, predominantly in the lower lobes, with confluent alveolar consolidation foci in the bilateral lower lobes, associated with adjacent peri-bronchovascular thickening (Figure 3).
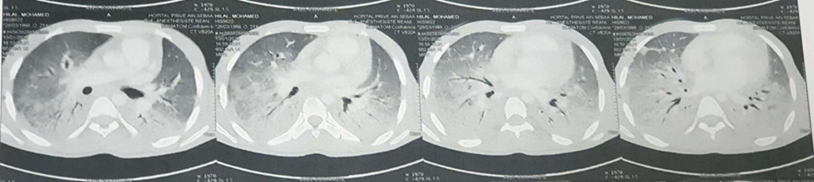
Figure 3: Chest CT Scan on Day 7 of Treatment.
The patient received rehydration with alkalinization and broad-spectrum antibiotic therapy. The evolution was marked by the persistence of severe ARDS, with the appearance of biological abnormalities consisting of pancytopenia with anemia at 8.3 g/dL, leukopenia at 270/mm³, and thrombocytopenia at 15,000/mm³. Laboratory findings also revealed acute renal failure with urea at 1.71 mmol/L and creatinine at 32.3 µmol/L, as well as hepatic cytolysis with AST/ALT at 425/84. The patient died on Day 13.
Post-mortem biopsies showed
- Liver biopsies: Centrilobular parenchymal necrosis.
- Renal biopsies: Tubular necrosis.
- Lung biopsies: Pulmonary edema and hemorrhage.
Discussion
Methotrexate (MTX) is an antifolate drug with antiproliferative and anti-inflammatory effects, classified among antimetabolites, which are cytotoxic agents with toxic effects against neoplastic cells by blocking cellular metabolism, in addition to its immunosuppressive effect [5]. Thus, methotrexate is widely used in the treatment of neoplastic diseases, as well as autoimmune and inflammatory disorders such as rheumatoid arthritis and psoriasis [2]. However, it can lead to rare but severe adverse effects [6]. Here, we report the case of a 22-year-old male patient who developed acute respiratory distress syndrome (ARDS) following methotrexate treatment. This case illustrates a rare but severe complication associated with this drug.
Methotrexate-induced interstitial pneumonia, although rare, is well described in the literature, with an estimated incidence of 7.6%, most frequently occurring within the first year of treatment [7]. However, the early onset of ARDS on Day 5 of treatment, as observed in our case, is exceptional.
The administration of MTX may induce either a hypersensitivity reaction, immunosuppression leading to viral or other recurrent infections [8], or direct pulmonary toxicity, resulting in pulmonary lesions characterized by hyperemia, edema, and infiltrates, as well as the release of endothelin-1, cytokines, and reactive oxygen species (ROS) [9]. In our case, post-mortem histopathological findings (alveolar edema and hemorrhage) suggest a severe inflammatory process.
Risk factors for MTX-induced pulmonary toxicity include advanced age, use of disease-modifying antirheumatic drugs (DMARDs), hypoalbuminemia, diabetes, and pre-existing pleuropulmonary involvement related to rheumatoid arthritis [4]. Moreover, recent studies highlight the role of genetic factors and interindividual variability in methotrexate elimination, contributing to an increased risk of drug-induced toxicity [10,11].
Pulmonary involvement due to MTX usually occurs within the first year of treatment, with reported extremes ranging from 4 months to 11 years after initiation [7,12].
Methotrexate-induced pneumonia is a diagnosis of exclusion [13], primarily due to the non-specific nature of symptoms, such as dry cough and dyspnea, with or without fever [7]. Additional investigations are necessary to rule out differential diagnoses. Chest X-ray typically reveals a bilateral diffuse interstitial syndrome [4], while thoracic CT scan shows characteristic ground-glass opacities with or without consolidation foci [13]. Bronchoalveolar lavage (BAL) analysis, although helpful, does not provide specific findings but may demonstrate increased CD4+ cells and an elevated CD4/CD8 ratio [14]. In severe cases, pulmonary biopsy, whether transbronchial or surgical, may be considered, especially when discontinuation of methotrexate does not lead to rapid clinical improvement [4]. Histological findings are generally non-specific, including signs of acute pneumonitis, alveolar type II cell hyperplasia or dysplasia, and interstitial infiltration [4].
The diagnostic criteria proposed by Searles and McKendry [15] and Carson et al. [16] are frequently used to identify methotrexate-induced pneumonitis. However, these criteria have not been clinically validated, making their practical application challenging. Moreover, differentiating infectious etiologies or interstitial lung diseases associated with rheumatoid arthritis remains difficult [7].
In terms of treatment, the first-line management consists of the immediate discontinuation of MTX. In many cases, this leads to spontaneous improvement of symptoms [17]. Systemic corticosteroids are often used, particularly in moderate to severe cases [13]. In advanced cases, oxygen therapy or respiratory support may be required to manage hypoxemia [18].
Several preventive treatments have recently been explored in studies, including serratiopeptidase and fisetin [19], febuxostat [20], cannabidiol [21], and pumpkin seed oil [22]. However, none of these treatments have been definitively validated, and further research is required to confirm their efficacy and safety.
A literature review estimated that 13% of severe MTX-induced pneumonitis cases could result in death if not diagnosed and treated promptly [23]. However, overall mortality remains low due to improved diagnostic and early treatment strategies.
This case highlights the need for thorough evaluation before initiating methotrexate therapy, especially in patients with complex comorbidities. Particular attention should be given to assessing pulmonary toxicity risk factors. Additionally, the implementation of a systematic follow-up protocol, including regular clinical assessments and paraclinical investigations, could allow earlier detection of complications, thereby reducing their severity.
Interindividual variability in response to methotrexate remains a promising area of research. Genetic studies may help identify predictive biomarkers of toxicity, thereby enabling personalized treatment approaches. Moreover, further exploration of the underlying pathophysiological mechanisms of methotrexate-induced pulmonary toxicity, such as inflammatory and immunological interactions, could lead to new targeted therapeutic approaches. These advancements would not only enhance the safety of methotrexate use but also expand its therapeutic applications while minimizing risks.
Conclusion
This case highlights a rare but severe complication of methotrexate, necessitating heightened vigilance from treatment initiation, particularly in patients with comorbidities. Methotrexate-induced pneumonitis, although uncommon, must be promptly identified through rigorous clinical, radiological, and biological assessment. Early drug discontinuation remains essential for improving prognosis, and systemic corticosteroids may be required in severe cases. Finally, further research is needed to better understand the underlying mechanisms and to develop predictive tools for personalized management.
Declarations
Contributors
All authors contributed to planning, literature review, and conduct of the review article. All authors have reviewed and agreed on the final manuscript.
Patient consent for publication
Informed consent was obtained from the patient’s legal representative, as the patient is deceased. The consent form is available upon request.
Ethics approval and consent to participate Not applicable. The patient is deceased, and informed consent was obtained from the legal representative.
Funding
No external funding was received from any public, commercial, or not-for-profit sector.
References
- Friedman B, Cronstein B (2019) Methotrexate mechanism in treatment of rheumatoid arthritis. Joint Bone Spine. 86: 301-307.
- Ahmed ZSO, Hussein S, Ghandour RA, Azouz AA, El-Sakhawy MA (2021) Evaluation of the effect of methotrexate on the hippocampus, cerebellum, liver, and kidneys of adult male albino rat: Histopathological, immunohistochemical and biochemical studies. Acta Histochem. 123: 151682.
- Manni JJ, Van den Broek P (1977) Pulmonary complications of methotrexate therapy. Clin Otolaryngol Allied Sci. 2:131-137.
- Jakubovic BD, Donovan A, Webster PM, Shear NH (2013) Methotrexate-induced pulmonary toxicity. Can Respir J. 20:153-155.
- Torres RP, Santos FP, Branco JC (2022) Methotrexate: Implications of pharmacogenetics in the treatment of patients with Rheumatoid Arthritis. ARP Rheumatol. 1: 225-229.
- Solomon DH, Glynn RJ, Karlson EW, Lu F, Corrigan C, et al. (2020) Adverse Effects of Low-Dose Methotrexate: A Randomized Trial. Ann Intern Med. 172: 369-380.
- Conway R, Low C, Coughlan RJ, O'Donnell MJ, Carey JJ (2014) Methotrexate and lung disease in rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheumatol. 66:803-12.
- Kim YJ, Song M, Ryu JC (2009) Mechanisms underlying methotrexate-induced pulmonary toxicity. Expert Opin Drug Saf. 8: 451-458.
- Kurt A, Tumkaya L, Turut H, Cure MC, Cure E, et al. (2015) Protective Effects of Infliximab on Lung Injury Induced by Methotrexate. Arch Bronconeumol. 51: 551-557.
- Mahmoud LB, Mdhaffar M, Frikha R, Ghozzi H, Hakim A, et al. (2018) Use of MTHFR C677T polymorphism and plasma pharmacokinetics to predict methotrexate toxicity in patients with acute lymphoblastic leukemia. Adv Clin Exp Med. 27: 1061-1068.
- Taylor ZL, Mizuno T, Punt NC, Baskaran B, Navarro Sainz A, et al. (2020) MTXPK.org: A Clinical Decision Support Tool Evaluating High-Dose Methotrexate Pharmacokinetics to Inform Post-Infusion Care and Use of Glucarpidase. Clin Pharmacol Ther.108:635-643.
- McKenna KE, Burrows D (2000) Pulmonary toxicity in a patient with psoriasis receiving methotrexate therapy. Clin Exp Dermatol. 25: 24-27.
- Lateef O, Shakoor N, Balk RA. (2005) Methotrexate pulmonary toxicity. Expert Opin Drug Saf. 4: 723-730.
- Schnabel A, Richter C, Bauerfeind S, Gross WL (1997) Bronchoalveolar lavage cell profile in methotrexate induced pneumonitis. Thorax. 52: 377-379.
- Searles G, McKendry RJ (1987) Methotrexate pneumonitis in rheumatoid arthritis: potential risk factors. Four case reports and a review of the literature. J Rheumatol. 14:1164-1171.
- Carson CW, Cannon GW, Egger MJ, Ward JR, Clegg DO (1987) Pulmonary disease during the treatment of rheumatoid arthritis with low dose pulse methotrexate. Semin Arthritis Rheum. 16:186-95.
- D'Elia T (2014) Methotrexate-induced pneumonitis: heterogeneity of bronchoalveolar lavage and differences between cancer and rheumatoid arthritis. Inflamm Allergy Drug Targets. 13: 25-33.
- Dai MS, Ho CL, Chen YC, Kao WY, Chao TY (2000) Acute respiratory distress syndrome following intrathecal methotrexate administration: a case report and review of literature. Ann Hematol. 79:696-699.
- Shahbaz M, Kamran SH, Anwar R (2021) Amelioration of Bleomycin and Methotrexate-Induced Pulmonary Toxicity by Serratiopeptidase and Fisetin. Nutr Cancer. 73:2774-2784.
- Zaki SM, Hussein GHA, Khalil HMA, Abd Algaleel WA (2021) Febuxostat ameliorates methotrexate-induced lung damage. Folia Morphol (Warsz). 80: 392-402.
- Ozmen O, Milletsever A, Tasan S, Selcuk E, Savran M (2024) The effects of cannabidiol against Methotrexate-induced lung damage. Basic Clin Pharmacol Toxicol. 134: 695-703.
- Abosrea AM, Aboul Ezz HS, Mahmoud SM, Mousa MR, Ahmed NA (2023) The potential role of pumpkin seeds oil on methotrexate-induced lung toxicity. Sci Rep. 13:7321.
- Imokawa S, Colby TV, Leslie KO, Helmers RA (2000) Methotrexate pneumonitis: review of the literature and histopathological findings in nine patients. Eur Respir J. 15: 373-381.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.