A Rare Case of Hepatocellular Carcinoma in a Young Sudanese Female with Friedreich Ataxia
by Motwakil Imam Awadelkareim Imam*
Associate Professor of Internal Medicine, Faculty of Medicine, Shendi University, Consultant physician, Elmek Nimer University Hospital, Sudan
*Corresponding author: Motwakil Imam Awadelkareim Imam, Associate Professor of Internal Medicine, Faculty of Medicine, Shendi University, Consultant physician, Elmek Nimer University Hospital, Sudan
Motwakil Imam Awadalkareim-ORCID ID: 0000-0002-46799255
Received Date: 14 March 2025
Accepted Date: 18 March 2025
Published Date: 27 March 2025
Citation: Imam MIA (2025) A Rare Case of Hepatocellular Carcinoma in a Young Sudanese Female with Friedreich Ataxia. Arch Surg Clin Case Rep 8: 245. DOI: https://doi.org/10.29011/2689-0526.100245
Abstract
Friedreich Ataxia (FA) is a rare autosomal recessive neurodegenerative disorder characterized by progressive ataxia, cardiomyopathy, and diabetes mellitus [1-3]. Hepatocellular carcinoma (HCC) is an unusual complication in FA patients, with few cases reported in the literature [4-10]. We present a case of a young Sudanese female with FA who developed HCC, highlighting the rare association and discussing the potential pathophysiological mechanisms. The patient presented with abdominal pain, vomiting, and weight loss. Imaging studies revealed a liver mass consistent with HCC. After histopathological confirmation, the patient underwent treatment, but the prognosis remained poor. This case emphasizes the importance of considering liver complications in FA patients However, HCC occurring in patients with FA is exceedingly rare, with only a few cases documented. This case report presents a young Sudanese female with FA who developed HCC, providing insight into the possible connection between mitochondrial dysfunction in FA and hepatocarcinogenesis.
Keywords: Hepatocellular Carcinoma; Friedreich Ataxia; Frataxin; Sudan.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver tumor and the sixth most common cause of cancer worldwide. Cirrhosis is present in 75– 90% of individuals with HCC and is an important risk factor for the disease. The risk is between 1% and 5% in cirrhosis caused by hepatitis B and C. There is also an increased risk of cirrhosis due to hemochromatosis, alcohol, NASH, and α1-antitrypsin deficiency[11-15]. A genetic neurodegenerative condition known as Friedreich ataxia is brought on by a decrease in the expression of the mitochondrial frataxin protein. Making up half of all hereditary ataxias, this is the most prevalent type of ataxia. It can manifest in its classic form or association with a vitamin E deficiency syndrome determined by genetics; both forms are clinically identical. Friedreich's ataxia presents before 25 years of age with progressive staggering gait, frequent falling, and titubation. The lower extremities are more severely involved than the upper limbs [13-17].Dysarthria occasionally is the presenting symptom; rarely, progressive scoliosis, foot deformity, nystagmus, or cardiomyopathy is the initial sign. Patients with Friedreich's ataxia have undetectable or deficient levels of frataxin mRNA, compared with carriers and unrelated individuals; therefore, it seems that the illness is brought on by a loss of the protein frataxin expressed. A mitochondrial protein is called frataxin engaged in the balance of iron. Iron build-up in mitochondria is caused by the mutant frataxin gene's iron transporter no longer coding for it, which produces intramitochondrial iron that is oxidized. Too much oxidized iron causes irreversible cell damage and the oxidation of cellular components as a result[15-23].
Case Presentation
Patient Information: A 24-year-old Sudanese female, diagnosed with Friedreich ataxia at the age of 15, presented to our clinic in Elmek Nimer University Hospital with complaints of right upper quadrant abdominal pain, vomiting, weight loss, and generalized fatigue over the past five months. She had no history of liver disease alcohol use or significant exposure to hepatotoxic substances. No past medical history of diabetes mellitus and hypertension. Her family history was significant for consanguinity, with one sister and two brothers with Friedreich ataxia with no known history of malignancies. Her diagnosis of FA is based on clinical examination and a strong family history of Friedreich ataxia.
Clinical Examination: On physical examination, the patient had signs of progressive ataxia, dysarthria, and distal muscle weakness consistent with her FA diagnosis. There was moderate hepatomegaly on abdominal palpation but no signs of jaundice or ascites were noted.
Investigations: Routine laboratory investigations showed mildly elevated liver enzymes (AST, ALT) and elevated alpha-fetoprotein (AFP) levels (400 ng/ mL).
An abdominal ultrasound revealed a hypoechoic focal hepatic mass in the right lobe of the liver measuring 8 cm in diameter. Subsequent contrast-enhanced CT of the abdomen confirmed a heterogeneous liver mass with arterial enhancement and venous washout, suggesting hepatocellular carcinoma. No evidence of chronic liver disease or cirrhosis was observed Figure 1.
Histopathology: A liver biopsy was performed, and histopathological analysis confirmed the diagnosis of hepatocellular carcinoma. There was no evidence of underlying cirrhosis or chronic viral hepatitis.
Treatment: The patient was deemed unsuitable for surgical resection due to the size and location of the tumor. She was started on palliative care with transarterial chemoembolization (TACE) as a means of controlling tumor progression.
Unfortunately, the patient's clinical condition deteriorated, and she succumbed to her illness three months after diagnosis.
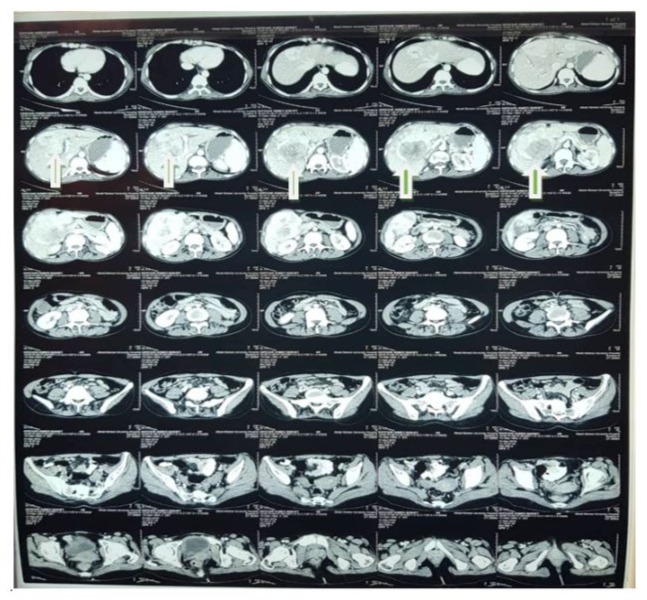
Figure 1: Contrast-enhanced CT of the abdomen show a heterogeneous liver mass with arterial enhancement and venous washout, suggestive of hepatocellular carcinoma. No evidence of chronic liver disease or cirrhosis was observed.
Discussion
Friedreich Ataxia (FA) is a mitochondrial disorder, and accumulating evidence suggests that mitochondrial dysfunction plays a role in carcinogenesis [5-9]. The frataxin deficiency seen in FA leads to impaired iron-sulfur cluster biosynthesis, resulting in mitochondrial iron overload and increased oxidative stress. These mechanisms may contribute to DNA damage and, ultimately, tumorigenesis, including the development of hepatocellular carcinoma. HCC typically arises in the setting of chronic liver disease, most commonly due to viral hepatitis or cirrhosis. However, our patient had no history of chronic liver disease, and imaging revealed no signs of cirrhosis. This implies that different mechanisms, perhaps connected to the oxidative stress and mitochondrial dysfunction seen in FA patients, may be involved in the development of HCC in FA patients [24-27].
While rare, this case underscores the importance of considering the possibility of liver malignancies in FA patients, especially in those presenting with nonspecific symptoms such as abdominal pain or weight loss. Given the poor prognosis associated with HCC, early detection and monitoring for liver abnormalities in FA patients could be critical.
To our knowledge, this is one of the few reported cases of HCC occurring in a patient with FA.
This case highlights the need for further research into the relationship between FA and hepatocarcinogenesis, particularly regarding the role of mitochondrial dysfunction in cancer development.
Conclusion
This case report describes a rare occurrence of hepatocellular carcinoma in a young Sudanese female with Friedreich Ataxia. While FA is not typically associated with liver malignancies, mitochondrial dysfunction, and oxidative stress may play a role in the development of HCC in these patients Clinicians should remain vigilant for the possibility of liver disease in FA patients, particularly when nonspecific symptoms such as abdominal pain or weight loss are present.
Further research is needed to explore the pathophysiological mechanisms linking FA with cancer and to determine appropriate screening strategies for this rare association.
Acknowledgments: We would like to thank the patient and her family for their cooperation and consent to publish this case. We also thank the medical staff involved in the patient's care and the pathology department for their contributions to the diagnostic process.
Note on The Authors' Contribution
While in the medical general ward, the patient's treatment plan was decided upon in consultation with a consultant MIA physician. He served as the patient's primary consultant physician. After giving it a thorough review, he gave the manuscript his approval to be submitted as is. The patient was seen by MHE, a resident medical registrar. In addition to conducting the literature review, drafting the manuscript, critically reviewing it, and approving its submission at the end, he was involved in the planning of several investigations for the patient. He was also involved in retrieving high-quality images for publication from the pathology and radiology archives. Both authors read and approved the final manuscript.
Conflict of Interest: Conflict of interest declared none.
Ethical Considerations: Informed consent from patient.
Funding: This research did not receive any specific grant from any funding agencies in the public, commercial, or not-for-profit sectors.
References
- Lill R. and M¨uhlenhoff, U. (2008) Maturation of iron–sulfur proteins in eukaryotes: mechanisms, connected processes, and diseases. Annu. Rev. Biochem. 77: 669–700.
- Rouault, T. A. and Tong, W. H. (2008) Iron–sulfur cluster biogenesis and human disease. Trends Genet.24, 398–407.
- Gerber, J, M¨uhlenhoff, U. and Lill, R. (2003) An interaction between frataxin and Isu1/Nfs1 that is crucial for Fe/S cluster synthesis on Isu1. EMBO Rep. 4: 906–911.
- M¨uhlenhoff, U, Richhardt, N, Ristow, M, Kispal, G. and Lill, R. (2002) the yeast frataxin homolog Yfh1p plays a specific role in the maturation of cellular Fe/S proteins. Hum. Mol. Genet. 11: 2025–2036.
- Stehling, O, Elsasser, H. P, Bruckel, B, M¨uhlenhoff, U. and Lill, R. (2004) Iron–sulfur protein maturation in human cells: evidence for a function of frataxin. Hum. Mol. Genet. 13: 3007–3015.
- Gakh, O, Park, S, Liu, G, Macomber, L, Imlay, J. A, et al (2006) Mitochondrial iron detoxification is a primary function of frataxin that limits oxidative damage and preserves cell longevity. Hum. Mol. Genet. 15: 467–479.
- Karlberg, T, Schagerlof, U, Gakh, O, Park, S, Ryde, U, et al (2006) The structures of frataxin oligomers reveal the mechanism for the delivery and detoxification of iron. Structure 14: 1535–1546.
- Cossee, M, Puccio, H, Gansmuller, A, Koutnikova, H, Dierich, A, et al (2000) Inactivation of the Friedreich ataxia mouse gene leads to early embryonic lethality without iron accumulation. Hum. Mol. Genet. 9, 1219–1226.
- Puccio H, Simon D, Cossee M, Criqui-Filipe P, Tiziano F, et al (2001) Mouse models for Friedreich ataxia exhibit cardiomyopathy, sensory nerve defect and Fe–S enzyme deficiency followed by intramitochondrial iron deposits. Nat. Genet. 27, 181–186.
- Ristow M, Mulder H, Pomplun D, Schulz T. J, M¨uller-Schmehl K, et al () Fex, pancreatic islets causes diabetes due to loss of β-cell mass. J. Clin. Invest. 112: 527–534.
- Thierbach, R, Schulz, T. J, Isken, F, Voigt, A, Mietzner, B, et al. (2005) Targeted disruption of hepatic frataxin expression causes impaired mitochondrial function, decreased life span, and tumor growth in mice. Hum. Mol. Genet. 14: 3857–3864.
- Al-Mahdawi, S, Pinto, R. M, Varshney, D, Lawrence, L, Lowrie, M. B, et al (2006) GAA repeat expansion mutation mouse models of Friedreich ataxia exhibit oxidative stress leading to progressive neuronal and cardiac pathology. Genomics 88: 580–590.
- De Pas, T, Martinelli, G, De Braud, F, Peccatori, F, Catania, C, et al (1999) Friedreich’s ataxia and intrathecal chemotherapy in a patient with lymphoblastic lymphoma. Ann. Oncol. 10: 1393.
- Ackroyd, R, Shorthouse, A. J. and Stephenson, T. J. (1996) Gastric carcinoma in siblings with Friedreich’s ataxia. Eur. J. Surg. Oncol. 22: 301–303.
- Barr, H, Page, R. and Taylor, W. (1986) Primary small bowel ganglioneuroblastoma and Friedreich’s ataxia. J. R. Soc. Med. 79: 612–613.
- Kidd, A, Coleman, R, Whiteford, M, Barron, L. H, Simpson, S. A. et al (2001) Breast cancer in two sisters with Friedreich’s ataxia. Eur. J. Surg. Oncol. 27: 512–514.
- Shoichet, S. A, B¨aumer, A. T, Stamenkovic, D, Sauer, H, Pfeiffer, A. F, et al (2002) Frataxin promotes antioxidant defense in a thiol-dependent manner resulting in diminished malignant transformation in vitro. Hum. Mol. Genet. 11: 815–821.
- Schulz, T. J, Thierbach, R, Voigt, A, Drewes, G, Mietzner, B. H, et al (2006) Induction of oxidative metabolism by mitochondrial frataxin inhibits cancer growth: Otto Warburg revisited. J. Biol. Chem. 281: 977–981.
- Ikeda, S, Biswas, T, Roy, R, Izumi, T, Boldogh, I, et al (1998) Purification and characterization of human NTH1, a homolog of Escherichia coli endonuclease III: direct identification of Lys-212 as the active nucleophilic residue. J. Biol. Chem. 273: 21585–21593.
- Cunningham, R. P, Asahara, H, Bank, J. F, Scholes, C. P, Salerno, J. C, et al (1989) Endonuclease III is an iron-sulfur protein. Biochemistry. 28:4450–4455.
- Sweasy, J. B, Lang, T. and DiMaio, D. (2006) Is base excision repair a tumor suppressor mechanism? Cell Cycle 5: 250–259.
- Boal, A. K, Yavin, E, Lukianova, O. A, O’Shea, V. L, David, S. S. et al. (2005) DNA-bound redox activity of DNA repair glycosylases containing [4Fe–4S] clusters. Biochemistry 44: 8397–8407.
- Rudolf J, Makrantoni V, Ingledew W. J, Stark M. J. and White M. F. (2006) The DNA repair helicases XPD and FancJ have essential iron–sulfur domains. Mol. Cell 23: 801–808.
- Dhe-Paganon S, Shigeta R, Chi Y. I, Ristow M. and Shoelson S. E. (2000) Crystal structure of human frataxin. J. Biol. Chem. 275: 30753-30756.
- Glatt H. and Meinl W. (2004) Use of genetically manipulated Salmonella typhimurium strains to evaluate the role of sulfotransferases and acetyltransferases in nitrofen mutagenicity. Carcinogenesis 25: 779-786.
- Glatt H, Piee A, Pauly K, Steinbrecher T, Schrode R, et al (1991) Fjord- and bay-region diol-epoxides investigated for stability, SOS induction in Escherichia coli, and mutagenicity in Salmonella typhimurium and mammalian cells. Cancer Res. 51: 1659–1667.
- Ristow M, Pfister M. F, Yee A. J, Schubert M, Michael L, et al (2000) Frataxin activates mitochondrial energy conversion and oxidative phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 97: 12239–12243.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.