A Potent Calcium Dietary Consumption Improves Bone Mineral Density in Asian Older Adults
by Donghoon Yoon1, Alex Lee2, Irawan Yusuf3-5, Terawan Putranto6, Lyana Setiawan7, Endang Hoyaranda8, Server Bozdogan9, J.W Saputro9, Jae Youl Cho10, Sang Soo Lee11, Jaehong Lee2, Seong Hwan Roh2, Akira Kubo12, Paul K Lee2*, Jong Y. Park13*
1University of Arkansas for Medical Science, Little Rock, AR, USA
2Calcium and Bone Health Institute (CBHI), Coquitlam, BC, Canada
3Siloam Hospitals, Tangerang, Indonesia
4Mochtar Riady Institute for Nanotechnology, Indonesia
5Faculty of Medicine, Hasanuddin University, Indonesia
6Central Army Hospital, Jakarta, Indonesia
7Dharmais National Cancer Hospital, Jakarta, Indonesia
8Prodia Group, Jakarta, Indonesia
9Advanced Calcium Research Institute, Mersin, Turkiye
10Sungkyunkwan University, Gyeonggi-do, S. Korea
11Hallym University, Kangwon-do, S. Korea
12Tokai University, Tokyo, Japan
13Moffitt Cancer Center, Tampa, FL, USA
* Corresponding author: Jong Y. Park, Moffitt Cancer Center, Tampa, FL, USA, and Paul K Lee, Calcium and Bone Health Institute (CBHI), Coquitlam, BC, Canada
Received Date: 24 September 2025
Accepted Date: 01 October 2025
Published Date: 06 October 2025
Citation: Yoon D, Lee A, Yusuf I, Putranto T, Setiawan L, et al. (2025) A Potent Calcium Dietary Consumption Improves Bone Mineral Density in Asian Older Adults. J Family Med Prim Care Open Acc 9: 290. https://doi.org/10.29011/2688-7460.100290
Abstract
Osteoporosis is a long-term bone disease that causes a gradual loss of bone density and increases the risk of bone fractures. The Asia Pacific region shows a higher prevalence of osteoporosis and associated fractures. Increasing calcium uptake is a well-known method to improve bone health. We took a potent calcium carbonate (Sigma Anti-bonding Molecule Calcium Carbonate, SAC). This clinical investigation evaluates the impact of a potent calcium carbonate supplement on Bone Mineral Density (BMD) in an Asian cohort. A total of 82 participants were enrolled/stratified into three groups based on T- score: normal (n=18), osteopenia (n=31), and osteoporosis (n=33). T-scores, BMD, and speed of sound (SOS) were measured at baseline and following ~eight months of SAC intervention. All measurements were conducted at the distal radius, a clinically relevant site for assessing peripheral bone density.
After intervention, T-scores improved significantly (from -2.0 to -0.85, p<0.0001) along with increased BMD (from 0.95 to 1.10 g/ cm², p<0.0001) across all ages and genders. SAC treatment also significantly increased SOS values from 3,946 to 4,079 (p < 0.0001). 65% of osteopenia participants (20/31) improved to normal after treatment, while 64% of osteoporosis participants (21/33) moved to osteopenia. Remarkably, 12% of baseline osteoporosis participants (4/33) transitioned into the normal bone density range. We found that a dietary supplement of potent calcium, SAC, leads to significant improvements in bone health in Asian individuals, a population with higher susceptibility to osteoporosis. These results suggest that SAC may be a useful option to attenuate osteoporosis.
Keywords: Osteoporosis; Osteopenia; Bone mineral density; Sigma Anti-bonding Molecule Calcium Carbonate (SAC)
Introduction
Osteoporosis is a chronic skeletal disorder defined by reduced bone mass and microarchitectural deterioration, which increases bone fragility and fracture risk [1]. Fractures due to osteoporosis are a major source of morbidity and mortality in older adults, imposing a substantial socioeconomic burden worldwide [2]. As the most common metabolic bone disease, osteoporosis is progressive and associated with pain, disability, and loss of independence [3,4]. Large population-based studies have shown excess mortality in individuals with osteoporosis compared with the general population, even when treated, partly due to fracturerelated complications [5].
The prevalence of osteoporosis is rising with global population aging. It affects ~10% of the general population and up to 30% of post-menopausal women [6]. In Asia and other high-income regions, including Japan, South Korea, Taiwan, Singapore, and Australia, the burden of osteoporotic fractures is particularly significant [7]. Racial and ethnic differences are evident; for example, Asian adults often have lower bone mineral density (BMD) than White counterparts, largely due to differences in skeletal size and body composition [8,9].
Pharmacological therapies, particularly bisphosphonates, are widely prescribed for patients at high fracture risk. Meta-analyses confirm that bisphosphonates reduce overall fracture incidence (odds ratio 0.62, p<0.001). Among several medication options, bisphosphonates are frequently prescribed as first-line therapy for patients with high fracture risk [10,11]. However, these agents do not fully halt disease progression, and residual fracture risk remains high. Adjunctive calcium and vitamin D supplementation are often recommended, although the optimal regimen remains uncertain [12]. Consequently, more effective and targeted treatment options for osteoporosis are urgently needed. Additionally, research results showed that even if osteoporosis is treated, the mortality rate and fractures still increase [5,13].
Calcium carbonate is a common supplement derived from natural sources such as oyster shell. Sigma Anti-Bonding Molecule Calcium Carbonate (SAC) is a modified form with weakened sigma antibonding, yielding higher bioavailability than conventional calcium carbonate [14]. In animal studies, SAC improved bone density by modulating osteocalcin, collagen, and mineral deposition without adverse effects [15]. Despite promising preclinical evidence, its efficacy in humans has not been evaluated. This study aimed to assess the effect of SAC supplementation on bone mineral density in an Asian population.
Materials and Methods
Study Design and Participants
This retrospective study was conducted at a medical clinic in Vancouver, Canada. Participants were recruited through newspapers, social media, and word-of-mouth advertisements. Participants received 7.6 mg SAC daily, administered as 5 ml of solution diluted in 500 ml of water per 70 kg body weight, taken twice daily on an empty stomach or between meals. Individuals were excluded if they had: 1) received osteoporosis treatment within the past year; 2) significant hepatic impairment (cirrhosis, active hepatitis); 3) impaired renal function or history of kidney/urinary stones; 4) inability to perform light exercise or maintain regular meals; 5) secondary osteoporosis requiring other treatments. After providing written informed consent, all participants underwent baseline bone density assessment using ultrasound and were prescribed SAC. The protocol was approved by the Institutional Review Board (Protocol no. MCC23681).
Bone Density Measurement
Bone density was assessed with a portable ultrasound device (BeamMed™ MiniOmni™). Daily calibration was performed using a standard block. Measurements were taken from the distal radius with the participant seated and forearm resting on the device [16].
Data Processing and Transformation
Ultrasound-derived speed of sound (SOS) values were converted to BMD and T-scores using Asian population reference standards from the World Health Organization (WHO) and regional epidemiological studies. Data conversion was validated to ensure consistency and minimize errors. The standard deviation (SD) of BMD in individuals compared to young adults is referred to as the T score. Based on this score, individuals were classified as normal (T-score > −1.0), osteopenia (−2.5 < T-score < −1.0), or osteoporotic (T-score ≤ −2.5) [4]. A T-score of 0 was defined as a BMD value of 1.2 g/cm2.
Sample Size Calculation
The sample size was calculated using G*Power 3.1 (HeinrichHeine-Universität Düsseldorf, Germany) to ensure adequate statistical power (0.80) at an alpha level of 0.05. For the chi-square test, an effect size of 0.6 (medium to large, based on prior literature) and 2 degrees of freedom were assumed, totalling 82 participants.
Statistical analysis
The efficacy of SAC was evaluated based on changes in bone mineral density (BMD), T-score, and speed of sound (SOS) of the distal radius after approximately 8 months of treatment.
Baseline Characteristics
Baseline characteristics of the study participants were compared across three clinical groups (normal, osteopenia, and osteoporosis). Numerical variables were analyzed using one-way analysis of variance (ANOVA) for comparisons across the three groups. Categorical variables were analyzed using the chi-square test or Fisher’s exact test when expected cell counts were less than 5.
Within-Group Changes: Changes in BMD, T-score, and SOS from baseline to post-treatment within the same group were analyzed using a paired t-test.
Between-Group Comparisons: Between-group differences in BMD changes after treatment were analyzed using analysis of covariance (ANCOVA), with baseline BMD as a covariate. For T-score and SOS, similar ANCOVA models were applied, with baseline T-score and SOS as respective covariates. To address multiple comparisons across BMD, T-score, and SOS, a Bonferroni correction was applied, adjusting the significance level to p < 0.0167 (0.05/3).
All statistical analyses were performed using IBM SPSS Statistics version 26 (IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics
A total of 82 participants participated in this study. Table 1 presents the baseline characteristics of the participants, which showed no significant differences in the measured parameters except average age. The average age was approximately 54 for normal, 62 for osteopenia, and 64 for the osteoporosis group (p<0.0001).
|
Variables |
Normal (n=18) |
Osteopenia (n=31) |
Osteoporosis (n=33) |
p value |
|
|
Age ± SD |
54.0 ±9.7 |
62.3 ± 9.7 |
63.6 ± 7.1 |
<0.0001 |
|
|
Gender |
Male |
8 |
8 |
5 |
0.07 |
|
Female |
10 |
23 |
28 |
||
|
Weight ± SD (kg) |
60.5 ± 11.8 |
58.5 ± 8.4 |
58.2 ± 5.6 |
0.65 |
|
|
Height ± SD (cm) |
163.0 ± 6.0 |
161.3 ±6.8 |
157.9 ± 4.9 |
0.10 |
|
|
BMI ± SD |
22.6 ± 3.2 |
22.4 ± 2.3 |
23.0 ± 1.9 |
0.86 |
|
|
Intervention ± SD (days) |
279 ± 223 |
215 ± 249 |
273 ± 272 |
0.59 |
|
Table 1: Characteristics of participants.
Changes of T-score, SOS, and BMD by clinical group, gender, and age group
T-scores, SOS, and BMDs were measured at baseline and ~8 months of SAC. As expected, the mean differences from baseline were statistically significant among clinical groups (p<0.0001, Figure 1). After treatment, SAC treatment significantly increased T score (-2.01 vs. -0.85, p<0.0001, Table 2), SOS (3,946 vs. 4,079, p<0.0001, Table 2), and BMD (0.95 vs. 1.10 g/cm2, p<0.0001, Table 3) regardless of gender and age groups. These improvements were more pronounced among participants with osteoporosis and in male subjects (p<0.0001) (Figure 1D). The efficacy of SAC was more evident in the men's group overall (Figure 1D, Table 2).
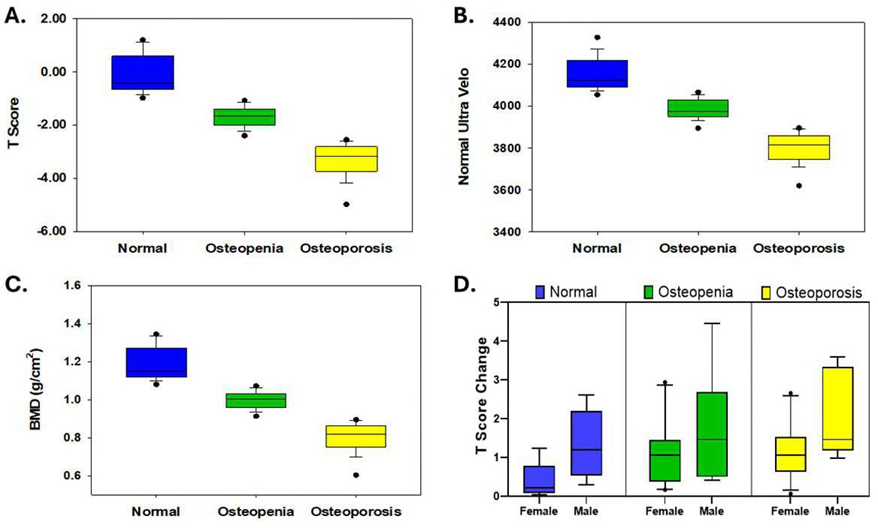
Figure 1: Baselines of T-Score, SOS, BMD, T Score Changes with 10th, 25th, median, 75th, 90th values in clinical groups. A: Median of T-scores are -0.11 (normal) vs -1.68 (osteopenia) vs. -3.34 (osteoporosis, p<0.0001). B: Median of SOS Scores are 4,153 vs 3,983 vs 3,798 (p<0.0001), and C: Median of BMD are 1.19 vs 1.00 vs 0.80 (p<0.0001). D. T score change after SAC treatment. Male and osteoporosis groups showed higher efficacy than female and other clinical groups (p<0.0001).
|
Gender |
values |
number |
p value |
|
|
Age ± SD |
Female |
58.0 ± 10.2 |
61 |
0.858 |
|
Male |
58.5 ± 11.0 |
21 |
||
|
SOS ± SD (%) |
Female |
2.91 ± 0.02 |
61 |
0.004 |
|
Male |
4.75 ± 0.03 |
21 |
||
|
T-Score ± SD (%) |
Female |
51.8 ±45.3 |
61 |
0.001 |
|
Male |
262.0 ± 494.2 |
21 |
||
|
BMD ± SD (%) |
Female |
13.6±10.2 |
61 |
0.035 |
|
Male |
20.0±15.9 |
21 |
||
|
Menopause |
||||
|
Age ± SD |
Yes |
61.3 ± 6.23 |
48 |
<0.0001 |
|
No |
43.5 ± 8.89 |
13 |
||
|
SOS ± SD (%) |
Yes |
3.16 ± 2.04 |
48 |
0.075 |
|
No |
2.00 ± 2.04 |
13 |
||
|
T-Score ± SD (%) |
Yes |
48.6 ±40.7 |
48 |
0.288 |
|
No |
63.8 ± 59.7 |
13 |
||
|
BMD ± SD (%) |
Yes |
15.1±10.2 |
48 |
0.028 |
|
No |
8.11±8.34 |
13 |
||
Table 2: Changes of T-score, SOS, and BMD by gender or menopausal status.
|
Gender |
T-score |
|||||||
|
Baseline ± SD |
After ± SD |
N |
p value |
|||||
|
All |
-2.0±1.38 |
-0.85±1.51 |
82 |
<0.0001 |
||||
|
Normal |
All |
-0.11±0.69 |
0.70±1.09 |
18 |
<0.0001 |
|||
|
Normal |
Female |
-0.22±0.76 |
0.21±0.63 |
10 |
0.01 |
|||
|
Normal |
Male |
0.03±0.62 |
1.33±1.26 |
8 |
0.004 |
|||
|
Osteopenia |
All |
-1.68±0.37 |
-0.46±1.11 |
31 |
<0.0001 |
|||
|
Osteopenia |
Female |
-1.69±0.40 |
-0.63±0.89 |
23 |
<0.0001 |
|||
|
Osteopenia |
Male |
-1.64±0.37 |
0.04 ±1.56 |
8 |
0.013 |
|||
|
Osteoporosis |
All |
-3.34±0.66 |
-2.06±0.98 |
33 |
<0.0001 |
|||
|
Osteoporosis |
Female |
-3.37±0.69 |
-2.23±0.91 |
28 |
<0.0001 |
|||
|
Osteoporosis |
Male |
-3.20±0.48 |
-1.10±0.78 |
5 |
0.015 |
|||
|
SOS |
||||||||
|
All |
3946±153 |
4079±164 |
82 |
<0.0001 |
||||
|
Normal |
All |
4153±79 |
4246±113 |
18 |
<0.0001 |
|||
|
Normal |
Female |
4163±87 |
4212±71 |
10 |
0.012 |
|||
|
Normal |
Male |
4140±71 |
4289±144 |
8 |
0.004 |
|||
|
Osteopenia |
All |
3983±48 |
4123±122 |
31 |
<0.0001 |
|||
|
Osteopenia |
Female |
3995±44 |
4116±100 |
23 |
<0.0001 |
|||
|
Osteopenia |
Male |
3949±42 |
4141±180 |
8 |
0.13 |
|||
|
Osteoporosis |
All |
3798±76 |
3946±105 |
33 |
<0.0001 |
|||
|
Osteoporosis |
Female |
3804±79 |
3934±104 |
28 |
<0.0001 |
|||
|
Osteoporosis |
Male |
3769±56 |
4011±89 |
5 |
0.015 |
|||
|
BMD |
||||||||
|
All |
0.95 ±1.65 |
1.10 ± 0.18 |
82 |
<0.0001 |
||||
|
Normal |
All |
1.19±0.08 |
1.28±0.13 |
18 |
<0.0001 |
|||
|
Normal |
Female |
1.17±0.09 |
1.22±0.08 |
10 |
0.01 |
|||
|
Normal |
Male |
1.20±0.07 |
1.36±0.15 |
8 |
0.004 |
|||
|
Osteopenia |
All |
1.00±0.05 |
1.14±0.13 |
31 |
<0.0001 |
|||
|
Osteopenia |
Female |
1.00±0.05 |
1.12±0.11 |
23 |
<0.0001 |
|||
|
Osteopenia |
Male |
1.00±0.04 |
1.20±0.19 |
8 |
0.013 |
|||
|
Osteoporosis |
All |
0.80±0.08 |
0.95±0.12 |
33 |
<0.0001 |
|||
|
Osteoporosis |
Female |
0.80±0.08 |
0.93±0.11 |
28 |
<0.0001 |
|||
|
Osteoporosis |
Male |
0.82±0.06 |
1.07±0.09 |
5 |
0.015 |
|||
Table 3: T-score, SOS, BMD changes by gender and clinical groups.
The status of menopause is critical to bone health. As expected, participants with menopause showed significantly lower values of SOS (3,888 vs. 4,110, p<0.0001), T-score (-2.64 vs. -0.69, p<0,0001), and BMD (0.88 vs. 1.12, p<0.0001). The percentage increase in BMD after treatment was significantly higher in the menopausal group compared to the non-menopausal female group (15.1% vs. 8.1%, p=0.028). This association was not observed in changes of SOS (p=0.075) and T-score (p=0.288, Table 2).
Aging is a well-established risk factor for osteoporosis and plays a critical role in bone health deterioration. Significant inverse relations between age and bone health were observed on T-score (Pearson correlation with age, -0.41, p<0.0001), SOS (Pearson correlation with age, -0.43, p<0.0001), and BMD (Pearson correlation with age, -0.41, p<0.0001) (Figure 2). Efficacy of SAC was observed in all age groups. Therefore, there is no significant difference in efficacy among age groups (SOS change p=0.114, T-Score change p=0.324, BMD change p=0.124) (Figure 3).
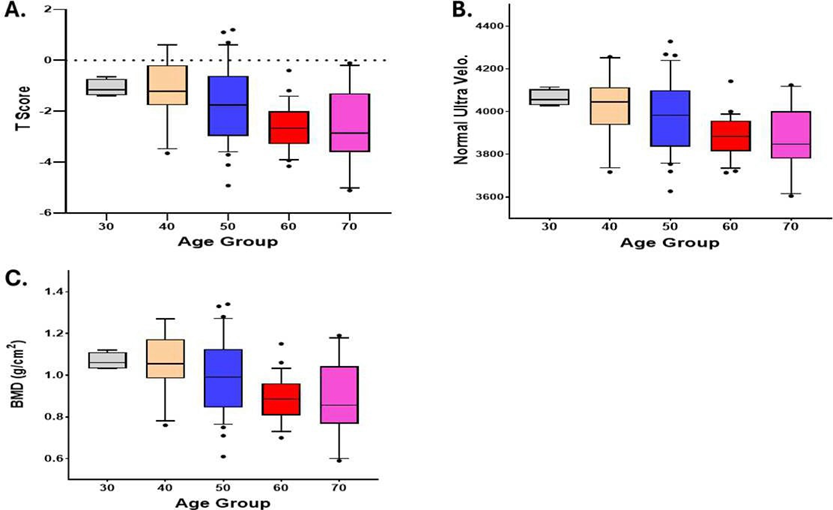
Figure 2: Inverse relationship between age and bone health. A: T-score (p<0.0001), B: SOS (p<0.0001), C: BMD (p<0.0001)
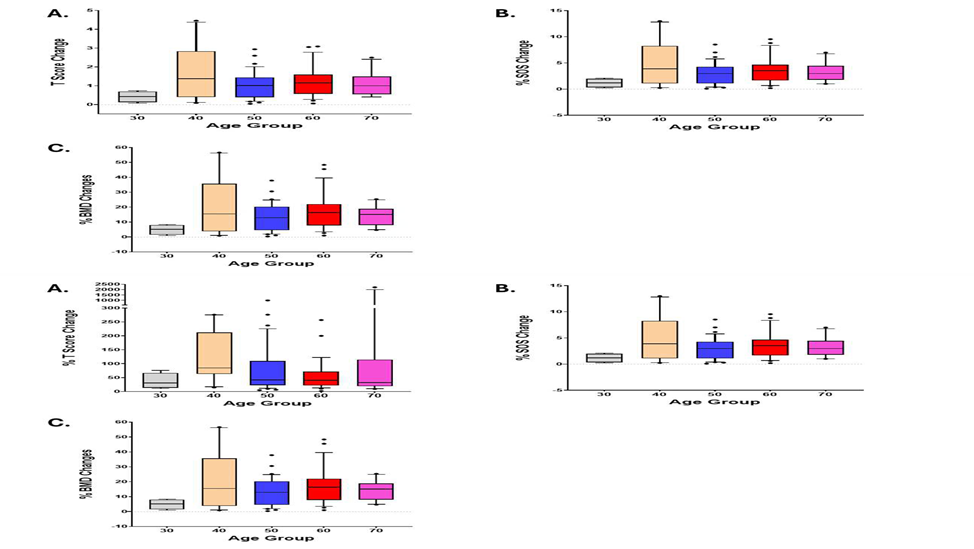
Figure 3: Efficacy of SAC by age groups. Efficacy of SAC was observed in all age groups. There is no significant difference in efficacy among age groups. A: T-score change p=0.324, B: SOS change p=0.114, and C: BMD change p=0.124.
Among 82 participants, 65% of the osteopenia participants (20/31) improved to the normal range after 8 months of treatment, while 64% of participants with osteoporosis (21/33) were reclassified into the osteopenia group. Moreover, 12% of osteoporosis participants (4/33) improved to the normal range (Figure 4).
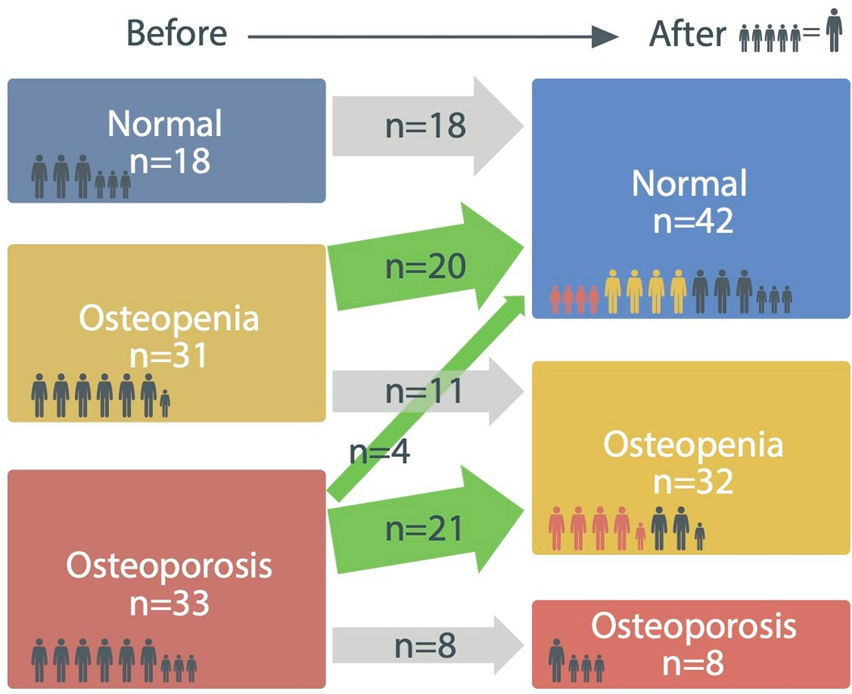
Figure 4: Distribution of clinical group before and after treatment based on T-score. 65% of participants with osteopenia (20/31) were improved to the normal group after treatment, while 64% of participants with osteoporosis (21/33) became the osteopenia group. Notably, 12% of osteoporosis participants (4/33) improved to the normal range.
Discussion
In this study, we observed that 8 months of daily SAC supplementation significantly improved BMD, T-score, and SOS in both men and women across all age groups and all clinical groups. To the best of our knowledge, this is the first study to investigate the effects of SAC in humans.
Calcium plays a crucial role in many physiological processes. In mammals, serum calcium is tightly maintained in a narrow range (1-1.3 mM) [17]. Normally, dietary calcium is absorbed via the lumen with the aid of Vitamin D, but it is immediately bound to albumin, becoming physiologically inactive.
The SAC is a calcium carbonate formulation characterized by weak sigma anti-bonding interaction with water molecules, forming Ca(H₂O)₆. Due to its loosely bound molecules, it enables absorption into the bloodstream without vitamin D, staying bioavailable ionized calcium. Such elevation of bioavailable calcium in the body resulted in an increase in extracellular calcium. In the bone environment, these extracellular Ca2+ signals interact with BMP and promote osteoblast differentiation, resulting in bone formation from the bone marrow-mesenchymal stem cells and increasing BMD, depicted in Figure 5 [18]. Furthermore, it is likely to reduce parathyroid hormone secretion, mitigating calcium loss and suppressing osteoporosis progression. As shown in the animal model [15]. We hypothesized that oral consumption of SAC increases serum bioactive calcium without or with minimal interruption of total calcium homeostasis and improves patients’ osteoporosis status.
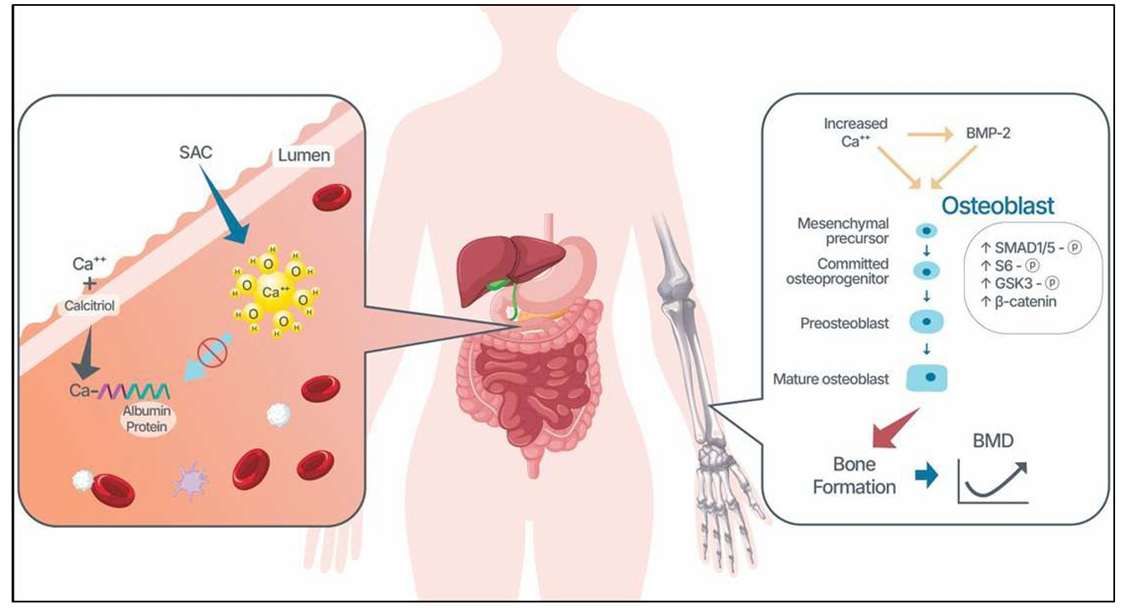
Figure 5: Proposed mechanism of how SAC improves BMD. Due to a weak intermolecular bonding of SAC with water molecules, forming Ca(H₂O)6, the SAC enables absorption into the bloodstream without vitamin D, staying bioavailable as ionized calcium. It elevates extracellular calcium in the bone microenvironment. This extracellular calcium and BMP-2 cooperatively promote the osteoblast differentiation/activity, increasing bone formation.
SAC differs from conventional calcium carbonate in its weak sigma anti-bonding, which enhances solubility and absorption, allowing bioactive calcium to remain in circulation without reliance on vitamin D [14]. Increased extracellular calcium may stimulate osteoblast differentiation via BMP signalling and suppress parathyroid hormone, thereby reducing bone resorption [15, 18].
Based on a 16% increase in BMD by SAC treatment, a significantly reduced fracture risk was anticipated in participants (Table 3) as previous studies demonstrated an inverse relationship between BMD change and fracture risk [19]. A meta-analysis study reported that a calcium supplement is effective in increasing BMD and reducing fractures of the bone [19, 20].
Menopause is one of the established risk factors for osteoporosis. It leads to low bone density and progresses into a decrease in BMD [21]. In fact, osteoporosis affects 30% of post-menopausal women [6]. In our study, the percentage of BMD after treatment of the menopausal group showed higher improvement compared to the non-menopausal female group (15.15% vs. 8.11%, p=0.028). Estrogen deficiency due to menopause can, in part, explain osteoporosis. Low estrogen levels lead to the outflow of calcium from the bones. This process lowers parathyroid hormone level and calcium absorption, contributing further to calcium loss from the bone [22, 23].
Our findings should be interpreted cautiously. First, the small sample size and retrospective design limit generalizability. Second, ultrasound, though cost-effective and widely available, is less established than DXA for guiding osteoporosis management. Third, most participants were East Asian, so findings may not extrapolate to other populations.
Despite these limitations, SAC demonstrated consistent efficacy and safety, making it a promising candidate for osteoporosis prevention and treatment. Larger, prospective randomized controlled trials with DXA-based endpoints and fracture outcomes are warranted.
Asian populations are more susceptible to osteoporosis than individuals of other racial backgrounds. Based on BMD, 32 % of 65-79 years old, and 18% of 50-64 years old Asian females have osteoporosis [24]. In addition, low calcium intake, a small body frame, and low physical activity contribute to a higher risk.
A larger, well-designed study with higher statistical power and a longer follow-up period is needed to further validate our findings. We are aware of the limitations of this study. Although ultrasound measurement costs less and has been proposed as a potential alternative to DXA measurements of bone mineral density (BMD), there is still uncertainty in the incorporation of ultrasound in the clinical management of osteoporosis. However, ultrasound is more widely used in clinics. Significant progress has been made, and new studies are promising. The second limitation is a small sample size, which limits generalizability. Finally, most participants are of East Asian descent. Therefore, these findings may not be extrapolated to other populations. Despite these limitations, SAC demonstrated consistent efficacy and safety, making it a promising candidate for osteoporosis prevention and treatment. Larger, prospective randomized controlled trials with DXA-based endpoints and fracture outcomes are warranted.
Acknowledgements: We give special thanks to all participants.
Author Contribution
Formal Analysis: Donghoon Yoon, Paul K Lee, Jong Y. Park
Visualization: Donghoon Yoon, Jong Y. Park
Original Draft: Donghoon Yoon, Paul K Lee, Alex Lee, Jong Y. Park
Investigation: Donghoon Yoon, Paul K Lee, Alex Lee, Jae Youl Cho, Sang Soo Lee, Jaehong Lee, S.H. Roh, Akira Kubo, Jong Y. Park
Software: Donghoon Yoon, Paul K Lee, Alex Lee, Jong Y. Park
Validation: Donghoon Yoon, Paul K Lee, Alex Lee, Jong Y. Park
Writing—Review and Editing: Donghoon Yoon, Paul K Lee, Alex Lee, Irawan Yusuf, Terawan Putranto, Lyana Setiawan, Endang Hoyaranda, Server Bozdogan, J.W Saputro. Jae Youl Cho, Sang Soo Lee, Jaehong Lee, S.H. Roh, Akira Kubo, Jong Y. Park
Conceptualization: Donghoon Yoon, Paul K Lee, Alex Lee, Jae Youl Cho, Sang Soo Lee, Jaehong Lee, S.H. Roh, Jong Y. Park
Data Curation: Donghoon Yoon, Paul K Lee, Alex Lee, Irawan Yusuf, Terawan Putranto, Lyana Setiawan, Endang Hoyaranda, Server Bozdogan, J.W Saputro, Sang Soo Lee, Jaehong Lee, S.H. Roh, Akira Kubo, Jong Y. Park
Funding Acquisition: Paul K Lee, Alex Lee
Project Administration: Donghoon Yoon, Paul K Lee, Alex Lee
Resources: Donghoon Yoon, Paul K Lee, Alex Lee
Supervision: Donghoon Yoon, Paul K Lee, Alex Lee
All authors are to declare non-financial interests in relation to the submitted work.
References
- Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, et al. (2014) Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int 25: 2359-2381.
- Özmen S, Kurt S, Timur HT, Yavuz O, Kula H, et al. (2024) Prevalence and Risk Factors of Osteoporosis: A Cross-Sectional Study in a Tertiary Center. Medicina (Kaunas) 60: 2109.
- Aibar-Almazán A, Voltes-Martínez A, Castellote-Caballero Y, AfanadorRestrepo DF, Carmen Carcelén-Fraile MD, et al. (2022) Current Status of the Diagnosis and Management of Osteoporosis. Int J Mol Sci 23: 9465.
- Song J, Zhao J, Liu T, Li Y, Dang X, et al. (2023) Prevalence and Risk Factors of Osteoporosis in a Chinese Population: A Cross-Sectional Study in Xi’an, Shaanxi Province, China. Med Sci Monit 29: e942346.
- Abrahamsen B, Osmond C, Cooper C (2015) Life Expectancy in Patients Treated for Osteoporosis: Observational Cohort Study Using National Danish Prescription Data. J Bone Miner Res 30: 1553-1559.
- Bijelic R, Milicevic S, Balaban J (2017) Risk Factors for Osteoporosis in Post-menopausal Women. Med Arch 71: 25-28.
- Chandran M, Brind’Amour K, Fujiwara S, Ha YC, Tang H, et al. (2023) Prevalence of osteoporosis and incidence of related fractures in developed economies in the Asia Pacific region: a systematic review. Osteoporos Int 34: 1037-1053.
- Al Anouti F, Taha Z, Shamim S, Khalaf K, Al Kaabi L, et al. (2019) An insight into the paradigms of osteoporosis: From genetics to biomechanics. Bone Rep 11: 100216.
- Jain RK, Vokes T (2017) Dual-energy X-ray Absorptiometry. J Clin Densitom 20: 291-303.
- Byun JH, Jang S, Lee S, Park S, Yoon HK, et al. (2017) The Efficacy of Bisphosphonates for Prevention of Osteoporotic Fracture: An Update Meta-analysis. J Bone Metab 24: 37-49.
- Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, et al. (2019) Pharmacological Management of Osteoporosis in Post-menopausal Women: An Endocrine Society* Clinical Practice Guideline. J Clin Endocrinol Metab 104: 1595-1622.
- Rosen HN (2025) Calcium and vitamin D supplementation in osteoporosis.
- Black DM, Eastell R, Adams AL (2020) Atypical Femur Fracture Risk versus Fragility Fracture Prevention with Bisphosphonates. Reply. N Engl J Med 383: 2189-2190.
- Lee PK (2018) Systemic literature review of the effects of Sigma AntiBonding Molecule Calcium Carbonate (SAC). Canada: CBHI.
- Choi SY, Park D, Yang G, Lee SH, Bae DK, et al. (2011) Effects of Sigma Anti-bonding Molecule Calcium Carbonate on bone turnover and calcium balance in ovariectomized rats. Lab Anim Res 27: 301307.
- Hans D, Métrailler A, Rodriguez EG, Lamy O, Shevroja E (2022) Quantitative Ultrasound (QUS) in the Management of Osteoporosis and Assessment of Fracture Risk: An Update. Adv Exp Med Biol 1364: 7-34.
- Brown EM (1991) Extracellular Ca2+ sensing, regulation of parathyroid cell function, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol Rev 71: 371-411.
- Aquino-Martínez R, Artigas N, Gámez B, Rosa JL, Ventura F (2017) Extracellular calcium promotes bone formation from bone marrow mesenchymal stem cells by amplifying the effects of BMP-2 on SMAD signalling. PLoS One 12: e0178158.
- Eastell R, Vittinghoff E, Lui LY, McCulloch CE, Pavo I, et al. (2022) Validation of the Surrogate Threshold Effect for Change in Bone Mineral Density as a Surrogate Endpoint for Fracture Outcomes: The FNIH-ASBMR SABRE Project. J Bone Miner Res 37: 29-35.
- Fraser LA, Vogt KN, Adachi JD, Thabane L (2011) Fracture risk associated with continuation versus discontinuation of bisphosphonates after 5 years of therapy in patients with primary osteoporosis: a systematic review and meta-analysis. Ther Clin Risk Manag 7: 157-166.
- Marshall D, Johnell O, Wedel H (1996) Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 312: 1254-1259.
- Kaplan B, Hirsch M (2004) Current approach to fracture prevention in post-menopausal osteoporosis. Clin Exp Obstet Gynecol 31: 251-255.
- Nordin BE, Need AG, Steurer T, Morris HA, Chatterton BE, Horowitz M, et al. (1998) Nutrition, osteoporosis, and aging. Ann N Y Acad Sci 854: 336-351.
- Lo JC, Yang W, Park-Sigal JJ, Ott SM (2023) Osteoporosis and Fracture Risk among Older US Asian Adults. Curr Osteoporos Rep 21: 592-608.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.