A Patient with Type II Von Willebrand Disease on a P2Y12 Antagonist Undergoing Coronary Artery Bypass Grafting: A postoperative Hemostatic Challenge
by Asami Naka1, Christine Schlömmer1, Ivonne Kröckel1, Arno Schiferer1, Arabella Fischer1, Peter Wohlrab1, Peter Quehenberger2, Bernd Jilma3, Barbara Steinlechner1*
1Department of Anaesthesiology, Intensive Care and Special Pain Medicine, Division of Cardiothoracic Surgery, Medical University of Vienna, Austria
2Clinical Institute of Laboratory Medicine, Medical University of Vienna, Austria
3Department of Clinical Pharmacology, Medical University of Vienna, Austria
*Corresponding author: Barbara Steinlechner, Department of Anaesthesiology, Intensive Care and Special Pain Medicine, Division of Cardiothoracic Surgery, Medical University of Vienna, Austria
Received Date: 09 November 2024
Accepted Date: 13 November 2024
Published Date: 15 November 2024
Citation: Naka A, Schlömmer C, Kroeckel I, Schiferer A, Fischer A, et al (2024) A Patient with Type II Von Willebrand Disease on a P2Y12 Antagonist Undergoing Coronary Artery Bypass Grafting: A postoperative Hemostatic Challenge. Ann Case Report. 9: 2064. https://doi.org/10.29011/2574-7754.102064
Abstract
Von Willebrand disease (VWD) is the most common inherited bleeding disorder. It is caused by a deficiency of von Willebrand factor (VWF), a key player in platelet adhesion and aggregation. Hemostatic coverage and prophylaxis with von Willebrand factor (VWF) in VWD patients with cardiovascular disease undergoing cardiac surgery requiring antiplatelet therapy are poorly understood.
Here, we report the findings of a VWD type II patient with three-vessel disease undergoing coronary artery bypass grafting (CABG) who required continuous antiplatelet therapy with clopidogrel until surgery. It was not possible to discontinue clopidogrel prior to surgery due to left main artery disease with 70% stenosis and intolerance to aspirin. CABG was performed with cardiopulmonary bypass support. Despite intra- and post-operative prophylaxis with VWF/factor VIII (FVIII) concentrate to minimise bleeding, severe bleeding continued for 48 hours after surgery. Regular monitoring of VWF and FVIII activity and platelet function, using the Multiplate analyser, was performed. Due to recurrent bronchial bleeding episodes, the patient also received other blood products such as erythrocyte and platelet transfusions, desmopressin, fibrinogen and prothrombin complex concentrates based on the point-of-care test results. The patient did not experience any thromboembolic events. After successful weaning from mechanical ventilation on day 34, the patient was transferred to the intermediate care unit on day 89 in good neurological and cardiorespiratory condition.
We recommend that clinicians perform platelet function testing when preoperative discontinuation of antiplatelet therapy, such as clopidogrel, is not possible.
Keywords: Von Willebrand Disease; Cardiovascular Disease; Coronary Artery Bypass Graft; Antiplatelet Therapy.
Introduction
Von Willebrand disease (VWD) is the most common inherited bleeding disorder, with an estimated prevalence in the general population ranging from 0.82% in Northern Italy [1] to 1.3% in the USA [2], and affecting men and women equally [3]. Patients with VWD have a quantitative or qualitative defect in von Willebrand factor (VWF).
Von Willebrand factor (VWF) is a multimeric glycoprotein with a half-life of 9-15 hours that mediates primary and secondary haemostasis. It is essential for adequate haemostasis by bridging the gap between platelets and the vascular lesion and by binding and stabilising Factor VIII.
Following vascular injury, VWF accumulates at the site of injury. In response to high endovascular shear stress, VWF changes conformation, leading to platelet adhesion and activation, which in turn leads to the binding of clotting factors and further release of VWF from endothelial cells and platelets. VWF exists in various sizes ranging from 500 - 10,000 kDa; the larger the multimer, the greater its affinity for collagen and platelets [4].
Especially during and after cardiac surgery with cardiopulmonary bypass (CPB), multifactorial haemostatic disturbances can occur [4], e.g. due to haemodilution by the priming solution of the bypass circuit [5], surgical blood loss, complement activation and thrombo-inflammation after contact of blood and cannula/tubes of the CPB [6], residual anticoagulation due to residual heparin after antagonisation with protamine or platelet dysfunction after the application of protamine itself [7].
Diagnosing VWD is challenging. There are three types of VWD: Types I and III show a quantitative deficiency (partial VWF deficiency with < 20 IU/dL in type I and total VWF deficiency with < 3 IU/dL in type III), whereas type II is characterised by qualitative defects. Type II is further subdivided into IIA, IIB, IIN and IIM according to the multimer size defect, the mechanism of multimer loss and the response to treatment with desmopressin [8].
The activity of high molecular weight VWF multimers can be measured by assessing collagen binding capacity (VWF:CB), platelet binding capacity (ristocetin cofactor activity; VWF:RCo), the ratio of VWF:RCo to VWF antigen (VWF:RCo/VWF:Ag) and VWF activity (VWF:Act). Ristocetin-induced platelet aggregation (RIPA) and RIPA mixing studies also aid in the diagnosis of type II VWD subtypes [9-13]. Current treatment options for individuals with VWD include VWF/FVIII replacement therapies, desmopressin, antifibrinolytics and hormone therapy [14]. There are no evidence-based guidelines for the integration of bleeding and ischaemic risk in VWD patients with cardiovascular disease. Antiplatelet management and laboratory monitoring in VWD patients undergoing coronary artery bypass grafting (CABG) remains unclear. This is the first report of a patient with type II VWD undergoing CABG who received VWF/FVIII replacement therapy while on antiplatelet therapy.
Clinical Case
Our male patient first visited the coagulation Center of the General Hospital in Vienna in 1995 because of recurrent epistaxis, prolonged bleeding after dental restoration and factor VIII deficiency. He was diagnosed with type I VWD (no laboratory values available). Since then, he has undergone several surgical procedures (laparoscopic cholecystectomy, hip replacement, inguinal hernia repair, dental extractions) and has been treated prophylactically with desmopressin and tranexamic acid without major bleeding complications.
In May 2021, the patient (then 75 years old, BMI 27.7, blood group A+) presented with severe triple-vessel disease with angina pectoris and dyspnoea, arterial hypertension, hypercholesterolaemia and chronic obstructive pulmonary disease GOLD stage I-II, for which he was referred for elective CABG. The initial diagnosis of VWD was revised to type II based on the following findings: VWF:RCo 17%, VWF:Ag 93%, VWF:RCo/VWF:Ag 0.18, VWF:Act 31%, absence of intermediate and large VWF multimers (Figure 1), and an unknown heterozygous mutation in exon 28.
One week prior to surgery, intracardiac catheterisation during which he received plasma-derived 7,200 IU VWF / 3,000 IU FVIII (i.e. 88 IU VWF/kg and 36 IU/kg FVIII), showed significant stenosis of the left main coronary artery (50-70%), severe stenosis of the proximal left anterior descending coronary artery (7090%), severe stenosis of the proximal circumflex ramus of the left coronary artery (70-90%), as well as significant stenosis at the ostium of the right coronary artery (50-70%) and severe stenosis in the middle (70-90%) and distal part of the right coronary artery (9099%). Because of the multifocal stenosis, percutaneous coronary intervention was not performed and the patient was referred to the Heart Team for CABG. To prevent graft occlusion in this high-risk patient, antiplatelet therapy was started with clopidogrel, a P2Y12 platelet receptor antagonist, due to a known allergy to aspirin. Clopidogrel 75 mg orally once daily was continued until the day of surgery.
CABG was performed under cardiopulmonary bypass. The priming solution of the bypass circuit consisted of 1,300 mL Ringer’s lactate, 200 mL human albumin 20% and 100 mL mannitol 20%. 460 mL of cardioplegic solution was used. Cardiopulmonary bypass (CPB) time was 92 min, aortic cross clamp time 72 min, lowest body temperature during CPB 34.7 °C. Intra-operative transesophageal echocardiography (TEE) showed good biventricular function with mitral regurgitation without evidence of severe aortic valve stenosis or hypertrophic obstructive cardiomyopathy that could cause acquired VWD [15]. For the perioperative transfusion algorithm, a target haemoglobin of > 8 g/dl was set, and coagulation factor replacement after CPB was performed according to ClotPro®. The required anticoagulation therapy with heparin (initial bolus 32,800 IU = 400 IU/kg; additional bolus of 10,000 IU was given for a target activated clotting time (ACT) of > 600 sec; total 42,500 IU) during CPB was antagonised by 260 mg protamine at the end of surgery. Partial thromboplastin time was monitored before (37.8 sec) and after CPB (39.9 sec); ACT was also measured regularly (at baseline: 119 sec; during CPB: > 600 sec; at end: 141 sec), indicating safe, therapeutic levels of anticoagulation. 800 mg tranexamic acid was administered intravenously at the start of surgery and in the priming solution of the CPB circuit.
As recommended by the coagulation Center, the patient’s VWF activity, FVIII activity, VWF:Ag and VWF:RCo levels were monitored throughout the operation and afterwards, and the infusion rate was adjusted to maintain the predetermined therapeutic level of VWF:RCo (Table 1). The initial replacement treatment consisted of 4,800 IU VWF/ 2,000 IU FVIII concentrate after the start of surgery prior to CPB. Following point-of-care testing with the ClotPro® viscoelastic whole blood coagulation analyser (Enicor GmbH, Munich, Germany), 2 g of fibrinogen and 100 mL of platelet concentrate were administered. A total of 2,000 mL of Ringer’s lactate and 643 mL of cell saver blood were infused during the entire operation. The amount of intraoperative blood loss is not documented. Haemoglobin, which was 15.4 g/dl at the start of the operation, was reduced to 9.5 g/dl at the end of the operation. The total length of stay in the operating theater was six hours.
The patient was admitted to the intensive care unit (ICU) in a stable cardiorespiratory condition. A dose of 2,400 IU VWF/1,000 IU FVIII was administered four hours after admission to the ICU (nine hours after the first intraoperative administration) and again two hours later because of severe bleeding from the chest tubes (total of 1,150 mL, haemoglobin from the mediastinal drain: 8.3 g/ dl, haematocrit: 25.7%). In addition, other blood products (1,500 mL erythrocyte transfusion, 300 mL platelet transfusion, 30 µg desmopressin, 1 g fibrinogen and 1,000 IU prothrombin complex concentrate) were required several hours after surgery. Of note, desmopressin was used as empirical therapy, in accordance with the 2021 ASH ISTH NHF WFH guidelines [9], although there was no real need for VWF as the patient was already substituted.
Point-of-care testing using viscoelastometry was performed regularly to assess bleeding risk. In view of the severe coronary stenosis, particularly of the left anterior descending coronary artery, we avoided excessive transfusions of clotting factors or platelets in agreement with the surgeon. A daily dose of 4,800 IU VWF/ 2,000 IU FVIII concentrate was administered until day 3 and then tapered to 2,400 IU VWF/ 1,000 IU FVIII until day 41 (Figure 2). Although VWF activity was successfully maintained at 70% (trough), significant bleeding (> 2,000 mL/day chest tube fluid loss on days 1 and 2, > 800 mL/day until day 7) and abnormal coagulation values persisted for more than one week after surgery (Figure 2). Therefore, an additional 2,400 mL of erythrocyte transfusion and 1,200 mL of fresh frozen plasma were administered until the third postoperative day. The amount of VWF/FVIII concentrate was adjusted according to the severity of bleeding episodes (i.e. amount of blood in the chest tubes and bronchial bleeding) and daily FVIII and VWF activity levels. The reintroduction of antithrombotic therapy with 75 mg clopidogrel on day 7 was guided by the results of the Multiplate analysis. However, antithrombotic therapy was discontinued on day 17 for several weeks due to recurrent episodes of bronchial bleeding.
Respiratory weaning was delayed due to intermittent pulmonary and oral bleeding and recurrent pneumonia despite antibiotic therapy. After initial successful extubation on day 2, the patient required reintubation on day 12 due to aspiration pneumonia associated with postoperative delirium. In addition, bronchoscopy and chest computed tomography revealed bilateral bronchial bleeding on day 20 despite prophylactic low molecular weight heparin (LMWH) and repeated attempts to restart clopidogrel to prevent graft occlusion. Clinically, the patient was hypoxemic and hypercapnic with a low tidal volume, which could not be improved by prone positioning or intrabronchial administration of tranexamic acid, desmopressin or epinephrine. LMWH and clopidogrel therapy was therefore withheld.
Tracheostomy was initially postponed due to the increased risk of bleeding and prolonged bronchial bleeding. Eventually, a surgical tracheostomy was performed by the otolaryngology department with increased VWF/FVIII replacement (600 IU VWF/250 IU FVIII; i.e., 7 IU/kg VWF and 3 IU/kg FVIII) on day 34. The patient was then successfully weaned from mechanical ventilation under physiotherapy. After consultation with the coagulation Center, prophylactic anticoagulation therapy with LMWH was resumed on day 42. Clopidogrel was not reintroduced until day 89. Intermittent VWF replacement was required up to three months after surgery due to recurrent blood in the sputum.
No thrombotic events or other serious adverse events were observed. Finally, the patient was discharged to the ICU on day 89 in good neurological and cardiorespiratory condition. However, the patient was readmitted to another ICU two months later for aspiration pneumonia and sepsis, and died six months after surgery in comfort terminal care.
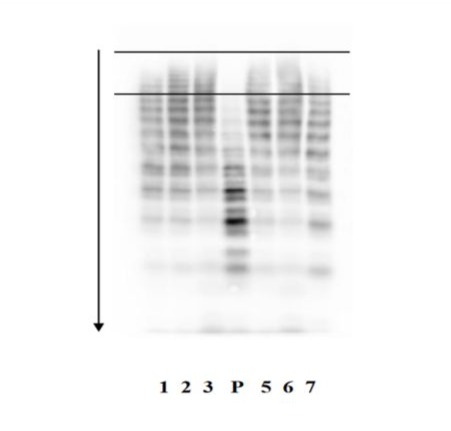
Figure 1: Westernblot analysis of von Willebrand factor multimers in plasma. Lane P - Plasma of our patient. Lane 1, 2, 3, 5, 6, 7 - Plasma of patients with normal VWF pattern. The two bars delineate the area where the bands of the heavy von Willebrand factor multimers are located. Owing to their high molecular weight they attach very early to the agarose gel during electrophoresis.
|
Before CPB |
After CPB |
||||
|
Before first substitution |
After first substitution (4800 IU VWF/ 2000 IU FVIII) |
Before second substitution |
After second substitution (2400 IU VWF/ 1000 IU FVIII) |
Reference range |
|
|
Activated partial prothrombin time (sec) |
37.8 |
34.8 |
38.4 |
39.9 |
27.0 – 41.0 |
|
VWF:Ag (%) |
121 |
374 |
119 |
250 |
60 – 180 |
|
VWF activity (%) |
25 |
161 |
22 |
136 |
48 – 170 |
|
VWF:RCo (%) |
NA |
149 |
15 |
119 |
60 – 180 |
|
FVIII activity (%) |
101 |
159 |
106 |
137 |
60 – 230 |
Table 1: Intraoperative laboratory parameters. Monitoring of PTT, FVIII activity, and VWF parameters in response to plasma-derived VWF/FVIII replacement therapy immediately before and after cardiopulmonary bypass. CPB=cardiopulmonary bypass; VWF:Ag=VWF antigen; VWF:RCo=VWF-ristocetin cofactor activity. NA=not assessed.
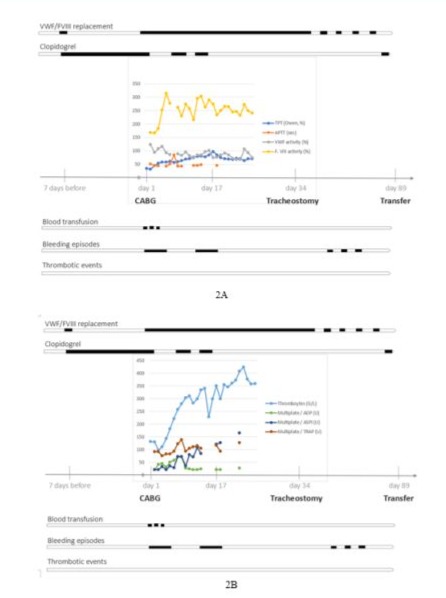
Figures 2A and 2B: Postoperative laboratory parameters and clinical course: Measurement of VWF antigen, VWF activity, VWF: RCo, FVIII activity and Multiplate could not be performed (n.p.) at ICU admission (laboratory closed in the evening). ICU = intensive care unit. POD = postoperative day. 20 mg subcutaneous low molecular weight heparin (LMWH) was given on POD 3 and POD 6, as well as between POD 8 to 14 regularly once daily. 75 mg clopidogrel was given from POD 7 to 16 once daily, except on POD 13. Anticoagulation was paused after POD 17 for several weeks because of recurring bronchial bleeding episodes.
Discussion
In this case report, we present a rare clinical case of a patient with VWD and severe three-vessel disease who underwent CABG with ongoing antiplatelet therapy with clopidogrel up to the time of surgery, experienced a severe postoperative bleeding episode and was treated with VWF/F VIII concentrates.
The patient fulfilled the criteria for the diagnosis of type II VWD (low platelet binding capacity (VWF:RCo: 17%), ratio of VWF:RCo to VWF:Ag <0.7 (VWF:RCo/VWF:Ag: 0.18) (Table 1) [14, 16]. However, we do not know the exact type II subtype as no preoperative RIPA test result was available and the RIPA test could not be performed postoperatively due to continuous VWF replacement. The absence of high molecular weight multimers in the preoperative VWF assessment led us to suspect that the patient had type IIA or IIB according to Sadler et al [8] (or IIA/IIB/IIC/IID according to Michiels et al [13]). Type IIB usually also presents with thrombocytopenia, probably due to sequestration of the VWF-platelet complex in the circulation, exacerbated by surgery or stress reaction [11], and we found a normal platelet count in the patient before surgery. Notably, many patients with type II VWD do not respond to desmopressin and require other forms of treatment [9]. The 2021 international guidelines for the management of VWD now suggest prophylaxis with VWF concentrate to prevent bleeding while on antiplatelet or anticoagulant therapy [9].
In collaboration with the Coagulation Center of the Vienna General Hospital, we were advised to ensure post-operative VWF activity with levels >70%. The patient received daily plasma-derived VWF/FVIII concentrate until POD 41, although we were puzzled by the patient’s history of previous surgical procedures managed with prophylactic desmopressin and tranexamic acid. Acquired type II triggered by stenosis was excluded by TEE.
One question is whether oral anticoagulation with the P2Y12 platelet receptor inhibitor clopidogrel should be given before or after surgery, despite the lack of intermediate- and highmolecular-weight multimers, which are associated with a high intra- and postoperative bleeding risk. Current evidence on the benefit of perioperative antithrombotic prophylaxis on bleeding complications is limited. In individuals with normal hemostasis, guidelines recommend indefinite aspirin plus clopidogrel started early postoperatively and continued for 12 months to prevent graft failure and improve clinical outcomes [17]. Clopidogrel 75 mg daily is a reasonable alternative for patients who are intolerant or allergic to aspirin. However, CABG should only be performed at 5 or more days after discontinuation of clopidogrel to allow for recovery of platelet function [17]. In this case, clopidogrel was not discontinued prior to surgery because the patient had a severe stenosis of the left main artery and the risk of pre- or intraoperative myocardial infarction without antithrombotic therapy was too high.
In individuals with VWD and cardiovascular disease, providing the necessary antiplatelet or anticoagulant therapy is probably preferable to no treatment, but bleeding risk should be reassessed throughout treatment [9]. However, the quality of the evidence is questionable because of the small number of patients and the lack of a direct comparison between antiplatelet or anticoagulant therapy and no therapy. A personalized treatment plan is therefore desirable. According to the European Society of Cardiology guidelines, the first-line treatment for non-ST-elevation acute coronary syndrome is aspirin (class I recommendation, level of evidence A). In addition, a P2Y12 receptor inhibitor is recommended in the absence of contraindications or excessive bleeding risk (Class I, Level A). Clopidogrel should only be used if prasugrel or ticagrelor are intolerable or contraindicated (Class I, Level C) [18]. Furthermore, the ESC statement on antiplatelet therapy in chronic coronary syndrome is ambiguous, adding “the optimal long-term antithrombotic therapy in patients at high risk of ischemic events is uncertain” [19]. More specifically, in patients with non-ST-elevation acute coronary syndrome undergoing CABG, the European Society of Cardiology states that “the risk of ischemic events, possibly related to suboptimal antiplatelet therapy while awaiting surgery, is less than 0.1%, while perioperative bleeding complications associated with antiplatelet therapy are higher than 10%” [18]. Considering that the cited studies were not even performed in patients with a focus on additional coagulation disorders [20,21], the necessity of preoperative antiplatelet treatment in patients with a higher bleeding risk must be questioned. Our VWD patient, who presented without acute myocardial infarction or hemodynamic instability, may have not needed preoperative anticoagulant therapy.
However, a large retrospective observational study of >330,000 CABG procedures found that although the incidence of perioperative venous thromboembolism in patients undergoing CABG is low (~1%), it is associated with increased in-hospital morbidity and mortality [22].
Regarding the whole case, there are some points that could be improved for the future. In the current case, the patient attended the coagulation Center preoperatively, but was not presented in time to the anesthetist (first contact was 24 hours before surgery). So, the first assessment of platelet function was made after admission to the ICU, when the patient was already clinically bleeding. Preoperative assessment of platelet function, possibly even before initiation of antiplatelet therapy, would have been desirable, as preoperative Multiplate test results correlate with postoperative bleeding in patients taking P2Y12 receptor antagonists [23]. Therefore, we encourage clinicians to regularly perform preoperative platelet function testing, especially when preoperative discontinuation of P2Y12 inhibitors such as clopidogrel or ticagrelor is not possible, to better tailor preoperative anticoagulation and postoperative substitution to patients’ needs. Another consideration is the use of different antiplatelet agents, such as glycoprotein IIb/ IIIa inhibitors, which have been shown to be highly effective in patients with unstable ACS and have a shorter plasma half-life of several hours compared to other antiplatelet agents with a plasma half-life of several days [24]. However, the high efficacy was only achieved when the drug was administered intravenously [25], making it unattractive for outpatients. Reported adverse effects included thrombocytopenia and the occurrence of major bleeding events, particularly in combination with heparin. The reported risk of bleeding in patients treated with glycoprotein IIb/IIIa inhibitors undergoing emergency CABG is equivocal. Patients treated with abciximab may have an increased risk of bleeding, whereas tirofiban and eptifibatide are thought to cause fewer bleeding events [26,27]. Randomized controlled trials investigating the peri- and postoperative bleeding risk in patients treated with glycoprotein IIb/IIIa inhibitors undergoing cardiac surgery are still lacking.
Informed consent: Written informed consent was not obtained from the patient, as this case report contains no patient-identifiable data.
Conflicts of interest: The authors declare that they have no competing interests related to the publication of this article.
References
- Rodeghiero, F, Castaman G, and Dini E (1987) Epidemiological investigation of the prevalence of von Willebrand’s disease. Blood, 69: 454-459.
- Werner E.J, B.E.H, Tucker E.L, Giroux D.S, Shults J, et al (1993) Prevalence of von Willebrand disease in children: A multiethnic study. The Journal of Pediatrics, 123: 893-8.
- Abe, K, Dupervill B, O Brien SH, Oakley M, Kulkarni R, et al, (2020) Higher rates of bleeding and use of treatment products among young boys compared to girls with von Willebrand disease. Am J Hematol, 95: 10-17.
- Stockschlaeder, M, R. Schneppenheim, and Budde U (2014) Update on von Willebrand factor multimers: focus on high-molecular-weight multimers and their role in hemostasis. Blood Coagul Fibrinolysis, 25: 206-16.
- Olmos Rodríguez, M, Hernandez JAB, Barcenas MTA, Cerezo AR, De Artinano MAVO, et al, (2015) Effect of priming solution and ultrafiltration on post-operative bleeding and blood transfusion in cardiac surgery. Randomized controlled trial. Rev Esp Anestesiol Reanim, 62: 81-9.
- Ekdahl, K.N, Fromell K, Mannes M, Grinnemo KH, Lang MH, et al, (2023) Therapeutic regulation of complement activation in extracorporeal circuits and intravascular treatments with special reference to the alternative pathway amplification loop. Immunol Rev, 313: 91-103.
- Tornudd, M, Ramstrom S, Kvitting JP, Alfredsson J, Nyberg L, et al, (2023) Platelet Function is Preserved After Moderate Cardiopulmonary Bypass Times But Transiently Impaired After Protamine. J Cardiothorac Vasc Anesth, 37: 1110-1120.
- Sadler, J.E, Budde U, Eikenboom CJ, Favaloro EJ, Hill FGH, et al (2006) Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J Thromb Haemost, 4: 2103-14.
- Connell, N.T, Flood VH, Brignardello-Petersen R, Abdul-Kadir R, Arapshian A, et al (2021) ASH ISTH NHF WFH 2021 guidelines on the management of von Willebrand disease. Blood Adv. 5: 301-325.
- Frontroth, J.P. and E.J. Favaloro, (2017) Ristocetin-Induced Platelet Aggregation (RIPA) and RIPA Mixing Studies. Methods Mol Biol, 1646: 473-494.
- Nichols, W.L, Hultin MB, lames AH, Manco-Johnson MJ, Montgomery RR, et al (2008) von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia, 14: 171-232.
- Teppone-Martin, O.L, M. Zhao, and Norris TE (2013) von Willebrand disease and cardiopulmonary bypass: a case report. Aana j, 81: p. 60-4.
- Michiels, J.J, Berneman Z, Gadisseur A, Van der Planken M, Schroyens W, et al, (2006) Classification and characterization of hereditary types 2A, 2B, 2C, 2D, 2E, 2M, 2N, and 2U (unclassifiable) von Willebrand disease. Clin Appl Thromb Hemost, 12: 397-420.
- Leebeek, F.W. and J.C. Eikenboom, (2016) Von Willebrand’s Disease. N Engl J Med, 375: 2067-2080.
- Berger, J, Schwartz J, Ramachandran S, D Leff J (2019) Review of von Willebrand Disease and Acquired von Willebrand Syndrome for Patients Undergoing Cardiac Surgery. J Cardiothorac Vasc Anesth, 33: 3446-3457.
- James, P.D, Connell NT, Ameer B, Paola JD, Eikenboom J, et al (2021) ASH ISTH NHF WFH 2021 guidelines on the diagnosis of von Willebrand disease. Blood Adv, 5: 280-300.
- Hillis, L.D, Smith PK, Anderson JL, Bittl JA, Bridges CR, et al (2012) Special Articles: 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Anesth Analg, 114: 11-45.
- Collet, J.P, Thiele H, Barbato E, Barthelemy O, Bauersachs J, et al (2021) 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J, 42: 1289-1367.
- Knuuti, J, Wijns W, Saraste A, Capodanno D, Barbato E, et al (2020) 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J, 41: 407-477.
- Fukui, T, Tabata M, Morita S, Takanashi S (2013) Early and longterm outcomes of coronary artery bypass grafting in patients with acute coronary syndrome versus stable angina pectoris. J Thorac Cardiovasc Surg, 145: 1577-83, 1583 e1.
- Malm, C.J, Hansson EC, Akesson J, Andersson M, Hesse C, et al (2016) Preoperative platelet function predicts perioperative bleeding complications in ticagrelor-treated cardiac surgery patients: a prospective observational study. Br J Anaesth, 117: 309-15.
- Panhwar, M.S, Ginwalla M, Kalra A, Gupta T, Kolte D, et al (2019) Association of Acute Venous Thromboembolism With In-Hospital Outcomes of Coronary Artery Bypass Graft Surgery. J Am Heart Assoc, 8: e013246.
- Ranucci, M, Colella D, Baryshnikova E, Di Dedda U (2014) Effect of preoperative P2Y12 and thrombin platelet receptor inhibition on bleeding after cardiac surgery. Br J Anaesth, 113: 970-6.
- Huber, K, Tirofiban (2000) (Aggrastat®) - ein Überblick über die derzeitige Publikations- und Studiensituation. Journal für Kardiologie - Austrian Journal of Cardiology, 7: 84-87.
- Darius H (2001) Parenterale und orale Glykoprotein IIb/IIIaAntagonisten bei instabiler Angina pectoris - gibt es noch eine Chance für oral wirksame Substanzen? Journal für Kardiologie - Austrian Journal of Cardiology, 8: 497-502.
- Pang, J.T, Fort S, Siega D, Cohen EA (2002) Emergency coronary artery bypass surgery in the era of glycoprotein IIb/IIIa receptor antagonist use. J Card Surg, 17: 425-31.
- Straub, A, Azevado R, Beierlein W, Wendel HP, Dietz K, et al (2005) Glycoprotein IIb/IIIa inhibition reduces prothrombotic events under conditions of deep hypothermic circulatory arrest. Thromb Haemost, 94: 115-22.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.