A Case Report of Pulmonary Complications Following Prolonged Antibiotic Therapy for Recurrent Breast Prosthesis Infection
by Omar Tujjar1, Fabio Massimo Abenavoli2*
1Department of Intensive Care and Pain Medicine, Consultant in Anaesthesia, Director Zenobia Medical Limited - Anaesthetic Services, Dublin, Ireland
2Plastic and Maxillo-Facial Surgeon, Consultant AVOCA Clinic The Avoca Clinic, Kilmacanogue, Co Wicklow, A98 WK27. Dublin, Ireland
*Corresponding Author: Fabio Massimo Abenavoli, Plastic and Maxillo-Facial Surgeon, Consultant AVOCA Clinic The Avoca Clinic, Kilmacanogue, Co Wicklow, A98 WK27. Dublin, Ireland
Received Date: 08 December 2025
Accepted Date: 12 December 2025
Published Date: 15 December 2025
Citation: Tujjar O, Abenavoli FM. (2025). A Case Report of Pulmonary Complications Following Prolonged Antibiotic Therapy for Recurrent Breast Prosthesis Infection. Ann Case Report. 10: 2479. DOI:10.29011/2574-7754.102479
Abstract
Background: Periprosthetic infection after breast reconstruction is a recognised complication that may necessitate prolonged antibiotic therapy and repeated surgical intervention. Although adverse effects of prolonged antimicrobial treatment are well documented, pulmonary structural changes have not previously been described in this context.
Case Presentation: A 44-year-old woman with a BRCA1 mutation and a strong family history of breast cancer underwent prophylactic subcutaneous mastectomy with immediate prosthetic reconstruction in June 2016. Within weeks, she developed a periprosthetic infection caused by Pseudomonas aeruginosa, which was managed initially with clindamycin and ciprofloxacin. Despite temporary improvement, infection recurred, leading to surgical removal of the implant, lavage of the pocket with antibiotics, and immediate reimplantation. Persistent infection with Staphylococcus species was subsequently identified, resulting in multiple courses of antibiotics, predominantly fluoroquinolones, over a 22-month period. Eventually, both implants were removed and autologous flap reconstruction was performed. Following extended antibiotic exposure, the patient developed progressive exertional dyspnoea. Imaging revealed bullous pulmonary changes and reduced lung volumes consistent with chronic structural damage.
Conclusion: This case highlights a possible association between prolonged antibiotic therapy, particularly fluoroquinolone exposure, and structural pulmonary change in a patient without pre-existing respiratory disease. Although causality cannot be confirmed, the temporal relationship justifies further investigation into potential pulmonary toxicity arising from prolonged antimicrobial use.
Keywords: Breast Implant Infection; Antibiotics; Pulmonary Disease; Periprosthetic Infection; Cephalosporins.
Introduction
Breast prosthesis implantation, whether performed for reconstructive or aesthetic purposes, is associated with recognised complications such as capsular contracture, rupture, seroma formation, and infection. The incidence of periprosthetic infection is reported to range between 2% and 29% [1], and it may occur either early in the postoperative period or several weeks after surgery. The organisms most frequently implicated are Staphylococcus aureus and coagulase-negative staphylococci, although Pseudomonas aeruginosa and other Gram-negative bacteria are also identified in a proportion of cases [2-6].
Management strategies depend on the severity and clinical presentation of infection. In most circumstances, removal of the prosthesis with delayed reimplantation is recommended once infection has resolved completely. In carefully selected cases, immediate prosthesis exchange may be attempted following thorough pocket lavage and with appropriate antimicrobial coverage. Close collaboration with infectious disease specialists is essential to ensure optimal antibiotic selection and duration of therapy.
Adverse effects of prolonged or repeated antibiotic use are well documented and may involve multiple organ systems, including the gastrointestinal tract, liver, kidneys, and musculoskeletal and nervous systems. Reported systemic effects extend to allergic, autoimmune, and metabolic disturbances, and increasing evidence suggests that antibiotic-induced gut dysbiosis may mediate the link between antimicrobial exposure and the development of chronic disease.
However, pulmonary complications directly attributable to antibiotic therapy are not well recognised. Apart from a recently reported association between antibiotic exposure and increased risk of lung cancer [7], structural or inflammatory pulmonary injury secondary to antibiotic use has not been described. The present case report describes a patient who developed bullous pulmonary changes and loss of lung volume following prolonged antibiotic therapy for recurrent breast prosthesis infection.
Patient Information
A 44-year-old woman underwent prophylactic subcutaneous mastectomy with immediate breast prosthesis implantation in June 2016. The indication for surgery was a confirmed BRCA1 mutation and a strong family history of breast cancer.
Her past medical history was otherwise unremarkable, and she had no previous respiratory or systemic conditions. Preoperative clinical assessment and baseline investigations revealed no abnormalities. She was a lifelong non-smoker. Documented allergies included cephalosporins and fentanyl.
Clinical Findings
Within several weeks of surgery, the patient developed swelling of the left breast, which progressed to the formation of a small fistula near the areola. Clinical examination demonstrated local inflammation and fluid collection, and ultrasound imaging confirmed the presence of a seroma. Microbiological analysis of aspirated fluid cultured Pseudomonas aeruginosa.
Despite repeated antibiotic therapy with clindamycin and ciprofloxacin, the patient experienced recurrent swelling, local erythema, and persistent drainage. Sixteen weeks after the initial operation (October 2016), the prosthesis was explanted, the pocket was thoroughly irrigated with antibiotic solution, and a new implant was inserted. A surgical drain was left in situ for eight weeks because of continuous serous drainage. Microbiological culture of the drainage fluid on one occasion identified Staphylococcus species.
Between December 2016 and April 2018, the patient continued to experience intermittent episodes of inflammation of the left breast, characterised by redness, pain, swelling, and low-grade fever, each treated with antibiotics. In total, she received the following antimicrobial agents: levofloxacin (500–750 mg daily for 45 days), amoxicillin–clavulanate (for seven days), azithromycin (for three days), sulfamethoxazole–trimethoprim (800 mg for 25 days), ciprofloxacin (500 mg for 22 days), and clindamycin (300 mg for 25 days).
Overall, the patient received systemic antibiotic therapy for approximately 127 days between June 2016 and April 2018, in addition to 10 days of postoperative prophylaxis following the June and October procedures. She developed intolerance to the final course of levofloxacin, which caused lower limb oedema and pain.
Diagnostic Assessment
The initial periprosthetic infection was caused by Pseudomonas aeruginosa. In October 2016, surgical exploration of the infected breast was performed, during which the prosthesis was removed, the pocket was irrigated, and a new implant was inserted. After several weeks, infection recurred, and microbiological culture in December identified Staphylococcus species. The patient subsequently experienced multiple episodes of local inflammation of the left breast, characterised by redness, pain, swelling, and intermittent fever. Each episode responded only temporarily to antibiotic treatment, resulting in a chronic low-grade infection of the prosthetic site.
Following approximately 22 months of repeated antibiotic exposure, and about one year after the initial breast surgery, the patient developed progressive respiratory symptoms, including exertional dyspnoea and reduced exercise tolerance. Radiological evaluation by computed tomography and magnetic resonance imaging demonstrated multiple bullous changes and distal peripheral atelectasis, with overall reduction in pulmonary parenchymal volume. These findings were consistent with structural lung damage resembling chronic obstructive pulmonary disease (COPD).
Differential diagnoses considered included allergic lung disease, chronic infection, and pre-existing emphysematous change. However, preoperative evaluation and previous medical history revealed no evidence of pulmonary pathology, and the patient had not reported any respiratory symptoms prior to antibiotic exposure.
Therapeutic Intervention
Over a period of 22 months, the patient received multiple courses of systemic antibiotics, most of which were fluoroquinolones, accounting for approximately 67 days of total exposure. In addition to medical management, she underwent surgical lavage of the periprosthetic pocket using antibiotic-containing irrigation solutions, followed by immediate replacement of the prosthesis. A surgical drain was left in situ for approximately eight weeks due to the persistence of serous effusion.
Despite these measures, infection recurred repeatedly. The patient ultimately underwent bilateral explantation of the breast implants at another centre, followed by delayed autologous flap reconstruction. Postoperative healing was achieved without further infectious complications, although the cosmetic result was suboptimal owing to repeated inflammation and multiple surgical interventions.
Follow-up and Outcomes
The patient experienced eventual wound healing and stabilisation of her reconstructive course following autologous flap reconstruction. However, she developed significant pulmonary sequelae manifesting as bullous lung disease and reduced pulmonary volume as the CT scan images of the lung clearly show with pulmonary emphysema with some millimetre-sized blebs and pulmonary cyst (Figure 1, 2). These changes resulted in chronic breathlessness, reduced capacity for physical activity, and long-term impairment in quality of life. On the walking test and pulmonary function test the patient showed a reduction of the value of the range expected for her age, height and sex.

Figure 1: CT Scan of the lungs of the Patient: coronal view. There is a pulmonary emphysema with some millimeter-sized blebs and pulmonary cyst. This finding was absent in June 2016 before the first surgical intervention.
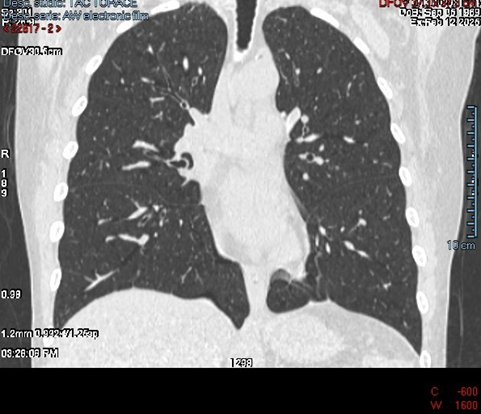
Figure 2: axial view showing pulmonary emphysema with some millimeter-sized blebs and pulmonary cyst.
Discussion
This case illustrates the complexity of managing recurrent breast prosthesis infection and highlights a possible, previously unrecognised association between prolonged antibiotic exposure and structural pulmonary change. The adverse effects of fluoroquinolones and other broad-spectrum antibiotics are well documented and include tendinopathy, neuropathy, hepatotoxicity, cardiac arrhythmias, and gastrointestinal dysbiosis [8-10]. However, to the best of our knowledge, structural pulmonary alterations such as bullous transformation and parenchymal volume loss have not been previously reported in association with antibiotic therapy.
The temporal relationship between extended antibiotic exposure and the subsequent development of pulmonary abnormalities in this patient is suggestive but not conclusive of causation. Nonetheless, several features strengthen the clinical suspicion of a drug-related effect. The patient had no history of respiratory disease, no autoimmune or systemic inflammatory disorder, and no environmental or occupational exposures that could explain the findings. Moreover, the onset of symptoms followed multiple courses of fluoroquinolones, a class of drugs known to cause connective tissue toxicity in tendons and aortic walls. It is conceivable that a similar mechanism involving oxidative stress or extracellular matrix degradation might contribute to parenchymal lung damage, though this hypothesis requires further investigation.
Emerging evidence supports that broad-spectrum antibiotics can alter host immune homeostasis and inflammatory responses in the lung. Experimental studies have demonstrated exaggerated inflammation, altered macrophage function, and enhanced tissue destruction following prolonged antibiotic exposure [11-13]. These mechanisms provide a biologically plausible link between systemic antibiotic use and pulmonary structural injury.
Further systematic research is warranted to determine whether this represents a rare idiosyncratic reaction or part of a wider, under-recognised phenomenon. Pharmacovigilance analyses, prospective registries, and mechanistic studies could help to clarify whether long-term antimicrobial therapy poses a risk of pulmonary toxicity similar to that observed in other tissues.
Conclusion
We describe a patient who developed bullous lung disease and pulmonary volume loss following prolonged antibiotic therapy for recurrent breast prosthesis infection. While a definitive causal link cannot be established, the temporal sequence, biological plausibility, and absence of alternative explanations suggest a possible association not previously reported in the literature. Clinicians should be vigilant for unexplained respiratory symptoms in patients receiving prolonged or repeated courses of antibiotics, particularly fluoroquinolones. Further investigation is needed to elucidate the underlying mechanisms and to determine whether this finding represents an isolated case or a broader manifestation of antibiotic-induced pulmonary injury.
Declarations
Consent to publish declaration signed by the Patient: I consent that the manuscript, a case report of pulmonary complications following prolonged antibiotic therapy for recurrent breast prosthesis infection connected to my clinical story could be published
Consent to publish declaration from the Authors: (The manuscript includes details, images, or videos relating to an individual person, please provide a Consent to Publish declaration in the manuscript.) The Authors consent that the images and all the information can be published.
Funding Declaration in the manuscript: There are no Funding to support the manuscript.
References
- Ozturk CN, Ozturk C, Sigurdson SL, Magner WJ, Sheedy B, et al. (2021). Broad-Spectrum Antibiotics for Breast Expander/Implant Infection: Treatment-Related Adverse Events and Outcomes. Ann Plast Surg. 87: 396-401.
- Vuong LN, Dorsey D, Obernuefemann C, Pinkner J, Walker JN, et al. (2022). Characterization of Host-Pathogen-Device Interactions in Pseudomonas aeruginosa Infection of Breast Implants. Plast Reconstr Surg. 150: 260e-271e
- Mesa F, Cataño S, Tuberquia O. (2021). Study of Infections in Breast Augmentation Surgery with Implants in 9,691 Patients over 5 Years. Plast Reconstr Surg Glob Open. 9: e3752.
- Seng P, Bayle S, Alliez A, Romain F, Casanova D, et al. (2015). The Microbial Epidemiology of Breast Implant Infections in a Regional Referral Centre for Plastic and Reconstructive Surgery in the South of France. Int J Infect Dis. 35: 62-6.
- Seng P, Bayle S, Alliez A, Romain F, Casanova D, Stein A. (2015). Microbiology of Implant-Based Breast Reconstruction Infections: A Systematic Review. Int J Infect Dis. 35: 62-6.
- Banuelos J, Abu-Ghname A, Asaad M, Vyas K, Sohail MR, et al. (2019). Microbiology of Implant-Based Breast Reconstruction Infections: A Systematic Review. Ann Plast Surg. 85: 1- 194-201.
- Kim M, Park SJ, Choi S, Jeong S, Chang J, et al. (2023). Association of Antibiotic Use with Risk of Lung Cancer: A Nationwide Cohort Study. J Infect Public Health. 16: 1123-1130.
- Jonville-Béra AP, Largeau B, Di Meglio F, Pariente A. (2025). The Safety Profile of Fluoroquinolones. Infect Dis Now. 55: 105064.
- Schwaiblmair M, Behr W, Haeckel T, Märkl B, Foerg W, et al. (2012). Drug Induced Interstitial Lung Disease. Open Respir Med J. 6: 63-74.
- Dubrall D, Wicherski J, Below M, Görtzen-Patin J, Schmid M, et al. (2025). Analyses of Adverse Drug Reactions to Fluoroquinolones in Spontaneous Reports Before and After the Referral and in Clinical Routine Cases. Drugs R D. 25: 35-55.
- Kim NH, Choi BY, Kim ES, Kim SJ, Hong JY, et al. (2023). Systemic Antibiotics Cause Deterioration of Emphysema Associated With Exaggerated Inflammation and Autophagy. Exp Mol Med. 55: 2260-2268.
- Dörner PJ, Anandakumar H, Röwekamp I, Vernengo FF, Pascual-Leone BM, et al. (2024). Clinically Used Broad-Spectrum Antibiotics Compromise Inflammatory Monocyte-Dependent Antibacterial Defense in the Lung. Nat Commun. 15: 2788.
- Shen Q, Yang S, Wang S. (2025). Pharmacovigilance of Five Commonly Used Antibiotics in Acute Exacerbations of COPD (AECOPD): Analysis of the FDA Adverse Event Reporting System Database. Pulm Pharmacol Ther. 90: 102383.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.