A Case of Triad Co-Existence of Bullus Pemphigoid, Psoriasis Vulgaris and Esophageal Cancer
by John Peter Niyigena Mateme1,2*
1International Education College (IEC), Zhejiang Chinese Medical University, Hangzhou, China
2Dermatology and Venereology, Hangzhou First People’s Hospital, Hangzhou, China
*Corresponding Author: John Peter Niyigena Mateme, International Education College (IEC), Zhejiang Chinese Medical University, Hangzhou, China and Dermatology and Venereology, Hangzhou First People’s Hospital, Hangzhou, China
Received Date: 08 September 2025
Accepted Date: 11 September 2025
Published Date: 15 September 2025
Citation: Peter JNM. (2025). A Case of Triad Co-Existence of Bullus Pemphigoid, Psoriasis Vulgaris and Esophageal Cancer. Ann Case Report. 10: 2409. https://doi.org/10.29011/2574-7754.102409
Abstract
Bullous pemphigoid (BP) and psoriasis vulgaris are chronic and recurrent inflammatory autoimmune skin conditions. Their coexistence is a rare and clinically significant phenomenon that is observed mostly in men with Psoriasis typically preceding BP by an average of 15-20 years, while esophageal cancer can rarely coexist with bullous pemphigoid with an average cases of 4% however this coexistence is still not well understood. The pathophysiology that lead to BP in individuals with psoriasis remain unknown despite many controversial mechanisms suggested. Due to the potential for conflicting therapies of psoriasis that may aggravate treating this comorbidity remain challenging.
In this rare case we describe 65-year-old patient, where BP emerged after 8 years history of psoriasis vulgaris that has been has previously been treated with methotrexate and topical corticosteroids , clinically presented with hyperkeratotic plaques and tense blisters on both psoriatic plaques and unaffected skin sparing the mucous membranes that erupted a week before the visit to our clinic, the patient had also the history of dysphagia and upper gastrointestinal bleeding , histopathology showed hyperkeratosis and parakeratosis, sub epidermal blisters with eosinophils and direct immunofluorescence revealing linear IgG/C3 deposits at the BMZ, The patient received treatment with methotrexate (12.5 mg/week) and methylprednisolone (16 mg/day). Within a week of admission, the patient's lesions began to improve gradually. This serves as a reminder that the therapeutic response of this rare case where bullous pemphigoid is associated with psoriasis vulgaris may be affectively managed with a combination therapy of methotrexate and methylprednisolone.
Keywords: Psoriasis Vulgaris; Bullus Pemphigoid; Esophageal Cancer; Methotrexate; Methylpredisisolone.
Introduction
BP and psoriasis are distinct chronic inflammatory skin conditions that have been rarely reported to coexist at the same time where the first report of such case was reported by Bloom in 1929. Clinically this rare comorbidity is presented with tense blisters on both psoriatic plaques and unaffected skin, typically appears several decades after the initial onset of psoriasis, with an average latency period of 15 to 20 years, meanwhile our patient developed these lesions eight years after psoriasis first diagnosis. Regarding esophageal cancer, there is some reported association with pemphigus (another autoimmune blistering disease) but less direct evidence of coexistence with bullous pemphigoid or psoriasis vulgaris. Some studies note an increased risk of esophageal squamous cell carcinoma in patients with pemphigus, possibly due to chronic mucosal inflammation and autoimmunity, but similar direct links with BP or psoriasis are less clear. On histopathology the findings revealed sub epidermal blisters containing eosinophils for BP, hyperkeratosis for psoriasis vulgaris , and direct immunofluorescence that identifies linear deposits of IgG and C3 at the basement membrane zone .Thus this case report highlights a unique and rare instance of the concurrent presentation of these otherwise common skin conditions, emphasizing the challenges in diagnosis and treatment these two conditions due to the potential for conflicting therapies: such as UV phototherapy and biologics, including TNF-α inhibitors for psoriasis that are thought to worsen BP.
The reported treatment options consist of systemic corticosteroids and methotrexate as the first option, while IL-17 inhibitors, such as secukinumab, have shown dual efficacy in certain cases. Additionally, emerging treatments like JAK inhibitors, including upadacitinib, have shown promise in recent studies. However, the tapering of treatment poses a risk of disease relapse, necessitating careful and gradual adjustments in dosage.
Case Presentation
The patient is a 65-year-old Caucasian male with a documented history of relapsing chronic plaque psoriasis spanning eight years before, along with a 20-year history of hypertension reported to be stable with ongoing long-term oral valsartan, and benign prostatic hyperplasia. He has previously been treated with methotrexate at a dosage of 12.5 mg per week during exacerbation of the lesions. Recently, he presented with a sudden onset of flaccid vesicles and crusted erosions affecting the face, trunk, and both upper and lower extremities. These lesions were accompanied by burning pain, although the patient did not exhibit any fever.
During the initial consultation, a physical examination of the patient revealed multiple well-defined, irregularly shaped erythematous indurated plaques covered with thick silver white scales located on the scalp, post auricular, and some areas of the trunk and both extremities in consistency to Psoriasis with a Psoriasis Area and Severity Index (PASI) score of 30. Additionally, flaccid vesicles, bullae and crusted erosions were noted on the face, trunk, and extremities sparing the oral mucosa and genitals with negative Nikolsky sign also in compliance with BP both on psoriatic plaques and other unaffected skin. During the consultation, a 4-mm skin punch biopsy was performed on a fresh bulla and on a psoriatic plaque for routine histological analysis, and an additional specimen was collected from the perilesional skin for direct immunofluorescence (DIF).
The histopathological analysis using hematoxylin and eosin staining revealed a sub corneal cleft accompanied by acantholytic keratinocytes within the granular layer parakeratosis with neutrophilic infiltration, and superficial perivascular lymphocytic infiltrates, including eosinophils in the dermis. These findings were consistent with a diagnosis of psoriasis vulgaris and bullous pemphigoid. Direct immunofluorescence (DIF) indicated intercellular liear deposition of complement component C3, with immunoglobulin G (IgG) graded at +2, and IgA, IgM, Fp was found to be negative in the epidermis. The C-reactive protein (CRP) level was positive at 5+ level, and the Euroimmun enzyme-linked immunosorbent assay (ELISA) for desmoglein 1 yielded a positive result with a ratio of 7.616, whereas desmoglein 3 was negative with a ratio of 0.173. Additional diagnostic evaluations, including complete blood count, urinalysis, fasting blood sugar, lipid profile, and assessments of kidney and liver function, showed unremarkable results.
The patient was treated with methotrexate (12.5 mg/week) and oral methylprednisolone (16 mg QID), that was later tapered to15mg monthly and 30mg/day of methylprednisolone with the addition of glutathione injection 1.8 g daily to prevent hepatotoxicity. One week post-admission, the skin lesions showed improvement compared to the initial presentation. The patient was subsequently discharged with a plan for monthly follow-up. (Figure 1-3)
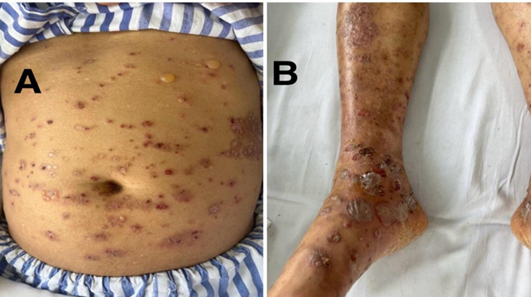
Figure 1A and 1B: well demarkated psoriatic plaques and bullae and crusted erosions lesions on the abdomen and lower limb before initiation of treatment
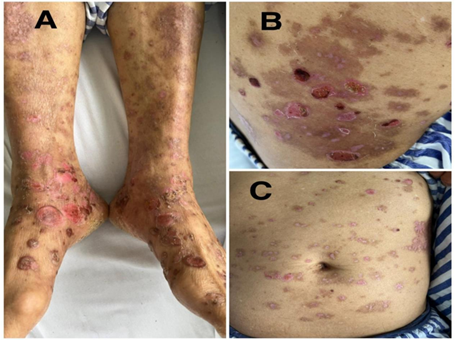
Figure 2A, B and C: improving psoriatic plaque and bullus lesions on lower limb, right lower back and abdomen after treatment with methotrexate and methylprednisolone
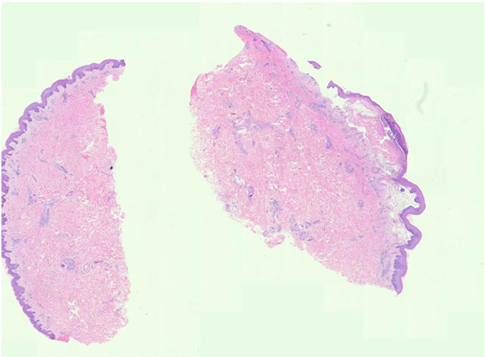
Figure 3: Histopathology showing: parakeratosis, acanthosis with neutrophilic infiltrationin the epidermis, and superficial perivascular lymphocytic infiltrates, including eosinophils in the dermis in consistance with Psoriasis (Left). while subepidermal blisters as a subcorneal cleft(split) accompanied by acantholytic keratinocytes within the granular layer parakeratosis containing eosinophils and lymphocytes infiltration, fibrin deposits and perivascular inflammation for BP(Right).
Discussion
The coexistence of BP and psoriasis vulgaris presented in this case is rare yet clinically important condition few cases of this comorbidity have been reported the first case BP and psoriasis was described by Bloom in 1929. This initial description marked the recognition of the association between these two conditions, with BP recognized as the most reported of all autoimmune blistering disorder associated with psoriasis. The chronic inflammation characteristic of psoriasis may instigate BP through a mechanism known as epitope spreading, wherein the exposure of antigens in the basement membrane zone (such as BP180/BP230) results in the production of autoantibodies wherein the inflammatory skin damage in psoriasis can unveil hidden skin proteins, triggering an autoimmune response that leads to BP. moreover to this another mechanism suggested to link these two conditions is the shared immunopathogenic mechanisms, particularly the disregulation of the Th17/IL-17 axis, further connect these two diseases. [1-7]
There have been reports of esophageal squamous cell cancer with pemphigus vulgaris coexisting. Nevertheless, it has never been documented that pemphigus's esophageal involvement that has been mistaken for esophageal cancer. When there is a history of PV and symptoms such dysphagia and odynophagia are present, esophageal involvement should be taken into consideration [8]. Most patients with esophageal lesions are middle-aged. When esophageal involvement is diagnosed, the majority of case reports in the literature classify individuals with PV as having either cutaneous or oral lesions [9, 10]. At the same time, 87% of patients in a research on pemphigus's esophageal involvement had oral mucosal involvement [11]. Isolated esophageal relapse, is an unforeseen situation that does not involve oral lesions.
The epitope spreading mechanism links psoriasis and BP through a process where primary autoimmune or inflammatory tissue damage in psoriasis exposes previously hidden (cryptic) antigens in the skin. This exposure triggers a secondary autoimmune response against these newly revealed epitopes, leading to the development of BP. Psoriasis causes chronic inflammation and damage to the skin, including the basement membrane zone, which can release sequestered antigens like BP180 and BP230, the main targets in BP [1, 5, 11]. This antigen exposure initiates epitope spreading, where the immune system broadens its attack from initial psoriasis-related antigens to new epitopes on skin proteins, inducing autoantibodies characteristic of BP [4, 5]. Studies show that in BP patients, IgG autoantibodies initially target the BP180 ectodomain, followed by intramolecular and intermolecular spreading to other epitopes and antigens, which parallels the immune activation seen in psoriasis-associated skin inflammation [5, 12].
Th17 cells and their key cytokine IL-17 play central roles in the pathogenesis of both psoriasis and BP, providing a pathogenic link between these diseases. In psoriasis, IL-17A and IL-17F produced mainly by Th17 cells drive keratinocyte hyper proliferation and inflammation. IL-23 from dendritic cells promotes Th17 cell maturation, which then secrete IL-17 to sustain skin inflammation and psoriatic lesion formation [1,2,5] while in BP, traditionally considered a Th1/Th2-mediated autoimmune blistering disease, recent evidence shows Th17 cells and IL-17 also contribute to disease exacerbation. IL-17 is elevated in BP skin and blister fluid, associated with eosinophilic infiltration and basement membrane damage. Dysregulated Th17/Treg balance promotes autoreactive T cells and autoantibody production in BP [6,9].
Thus, the Th17/IL-17 axis is a shared immunopathogenic pathway linking psoriasis and BP. IL-17-driven inflammation promotes keratinocyte activation in psoriasis and inflammatory cell infiltration and tissue damage in BP, explaining their coexistence and overlapping immune features [6].
Clinically, this patient presented with tense bulous blisters on both well-defined psoriatic plaques and unaffected skin, such lesions typically appear several decades after the initial onset of psoriasis, with an average latency period of 15 to 20 years, however in our case they presented only after 8 years of the first diagnosis of psoriasis. Histopathological findings releaved sub epidermal blisters containing eosinophils from the blister sample, while hyperkeratosis was found in psoriatic plaques, and direct immunofluorescence that identified linear deposits of IgG and C3 at the basement membrane, the patient had comorbidity of Benign Prostatic hyperplasia with no significant symptoms and pulmonary emphysema of both lungs with bullae in the right upper lung that was adequately treated with antibiotics. [13-20]
Management of such cases presents challenges due to the potential for conflicting treatments where therapies such as UV phototherapy and biologics, including TNF-α inhibitors for psoriasis, are thought to worsen bulous pemphigoid lesions. Nonetheless, some studies suggested treatment options consist of systemic corticosteroids and methotrexate, as first line treatment. Methotrexate offers a range of clinical advantages due to its anti-inflammatory, cytotoxic, and immunomodulatory effects. It functions as a dihydrofolate reductase inhibitor, believed to directly inhibit epidermal hyper proliferation while also exerting immunosuppressive actions. A case study by Tripathy et al. documented the use of methotrexate and mycophenolate mofetil in a patient with concurrent psoriasis and pemphigus foliaceous. The addition of oral corticosteroids, such as prednisolone, was necessary due to incomplete resolution of blisters, leading to continuous improvement of lesions and prevention of flare-ups upon cessation of prednisolone. Another case report by Zheng et al. described the successful use of methotrexate in combination with methylprednisolone for a patient with psoriasis and pemphigus vulgaris, resulting in a gradual resolution of lesions. In our case we used methotrexate (12.5 mg/week) and oral methylprednisolone (16 mg/day), with the addition of glutathione injection (atomolan) at 1.8 g daily to mitigate hepatotoxicity, this has shown a good result in our patient few days after initiation of the treatment. [20-30]
Meanwhile other options like IL-17 inhibitors, such as secukinumab, have shown dual efficacy in certain cases. Additionally, emerging treatments like JAK inhibitors, including upadacitinib, have shown promise in recent studies. However, the tapering of treatment poses a risk of disease relapse, necessitating careful and gradual adjustments in dosage.
The observed overlap highlights the necessity for a multidisciplinary approach to effectively manage immunosuppression and mitigate potential triggers. It is essential to conduct further investigations into the common mechanisms and develop targeted therapies to enhance outcomes in this intricate comorbidity. [30-37]
Conclusion
This rarity of this case highlights the importance of recognizing the coexistence of BP, psoriasis vulgaris and esophageal cancer, emphasizing the need for integrated management strategies that address both conditions effectively while minimizing treatment-related complications. Investigating predisposing and triggering factors, conducting re-biopsies, and performing additional evaluations as the disease progresses may provide deeper insights. Nevertheless, effectively managing this condition remains a considerable challenge. Therefore, establishing a therapeutic pathway is essential to elucidate the most effective treatment options and to address potential therapeutic challenges associated with these conditions.
Declaration of patient consent: The authors affirm that they have secured all necessary patient consent forms. In these forms, the patient(s) have granted permission for their images and other clinical data to be published in the journal. The patients are aware that their names and initials will remain confidential, and reasonable measures will be taken to protect their identities; however, complete anonymity cannot be assured.
Financial Support and Sponsorship: There was no financial support or sponsorship received.
Conflicts of Interest: The authors declare that there are no commercial or financial relationships that could be perceived as potential conflicts of interest related to this article.
References
- Maronese CA, Cassano N, Genovese G, Foti C, Vena GA, et al. (2022). The Intriguing Links Between Psoriasis and Bullous Pemphigoid. J Clin Med 12: 328.
- Di Lernia V, Peccerillo F, Ficarelli E. (2023). Therapeutic Management of a Case of Severe Psoriasis Coexistent With Bullous Pemphigoid in the Elderly. Psoriasis (Auckl) 13: 27–31.
- Yin Z, Li Y, Su T, Gopee S, Chen B. (2022). Coexistence of Psoriasis and Bullous Pemphigoid: Combination Treatment With Secukinumab and Methotrexate. Indian J Dermatol Venereol 88: 566–568.
- Rao R, Gupta A, Yunis F, Handettu S, Chandrashekar B. (2012). Coexistence of Psoriasis With Bullous Pemphigoid. Indian Dermatol Online J 3: 119–121.
- Drenovska K, Valeva E, Shahid M, Vassileva S. (2023). Case Report: Coexistence of Bullous Pemphigoid and Psoriasis: Therapeutic Challenge and IL-17A–Targeted Parallel Treatment Strategy. Front Med 10: 1148660.
- Koerber WA, Price NM, Watson W. (1978). Coexistent psoriasis and bullous pemphigoid: a report of six cases. Arch Dermatol. 114: 1643-6.
- Li H, Wang H, Qiao G, Liu Y, Zhang F, et al. (2023). Concurrent bullous pemphigoid and psoriasis vulgaris successfully treated with Janus kinase inhibitor tofacitinib: A case report and review of the literature. Int Immunopharmacol 122: 110591.
- Su F, Wang T, Qin Q, Xie Z. (2024). Upadacitinib for the management of bullous pemphigoid coexisting with psoriasis vulgaris: A case report and literature review. J Dermatolog Treat 35: 1.
- Timofte MA, Căruntu C, Bălăceanu-Gurău B, Mărgăritescu I, Giurcăneanu C, et al. (2025). Bullous pemphigoid overlapping psoriasis vulgaris: A rare case report and literature review. Clin Pract 15: 91.
- National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). (2025). Psoriasis: Symptoms, causes, & risk factors. NIAMS. Retrieved In Online
- NHS. (2024). Psoriasis – Symptoms. NHS. Retrieved In Online.
- American Academy of Dermatology (AAD). (2025). Psoriasis: Signs and symptoms. AAD. Retrieved In Online.
- Cleveland Clinic. (2023). Psoriasis: What it is, symptoms, causes, types & treatment. Cleveland Clinic. Retrieved In Online.
- Yale Medicine. (2025). Psoriasis. Yale Medicine. Retrieved In Online.
- Mayo Clinic. (2025). Psoriasis – Symptoms and causes. Mayo Clinic. Retrieved In Online.
- National Psoriasis Foundation (NPF). (2025). Psoriasis: Symptoms, causes, images & treatment. NPF.
- NHS. (2025). Psoriasis. NHS. Retrieved In Online.
- Nair PA, Badri T. (2023). Psoriasis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
- Jones C, Gupta M, Uddin S, Oakley A. (2023). Psoriasis. Autoimmune/Autoinflammatory.
- Mayo Clinic. (2025). Bullous pemphigoid – Symptoms and causes. Mayo Clinic.
- Bullous Pemphigoid – Medscape. Retrieved In Online.
- Bullous Pemphigoid: Know the Facts – WebMD. Retrieved In Online.
- Bullous pemphigoid – NHS. Retrieved In Online.
- Bullous pemphigoid: Signs and symptoms. Retrieved In Online.
- Bullous pemphigoid - Penn Medicine. Retrieved In Online.
- Bullous Pemphigoid: Causes, Symptoms & Treatment. Retrieved In Online.
- Bullous Pemphigoid - StatPearls - NCBI Bookshelf. Retrieved In Online.
- Bullous Pemphigoid: Causes, Symptoms, and Treatment – DermNet. Retrieved In Online.
- Bullous Pemphigoid - Dermatologic Disorders - Merck Manuals. Retrieved In Online.
- Coexistence of Psoriasis and Bullous Pemphigoid: Combination Treatment with Secukinumab and Methotrexate. International Journal of Dermatology and Venereology. 10: 0000000000000240.
- Di Lernia V, Peccerillo F, Ficarelli E. (2023). Therapeutic Management of a Case of Severe Psoriasis Coexistent with Bullous Pemphigoid in the Elderly. Psoriasis: Targets and Therapy. 2023: 27-31.
- Hunt A, Hunt S. (2024). Coexisting Psoriasis and Anti-P200 Pemphigoid: A Case Report. J of Skin. 8: 1581-1585.
- Drenocska K, Valeva E, Shahid M, Vassileva S. (2023). Case Report: Coexistence of bullous pemphigoid and psoriasis. Front Med. 10: 1148660.
- Koerber WA, Norman MP, William W. (1978). Coexistent Psoriasis and Bullous Pemphigoid: A Report of Six Cases. Arch Dermatol. 11: 1643:1646.
- Yin Z, Li Y, Su T, Gopee S, Chen B. (2024). Coexistent psoriasis and bullous pemphigoid treated with a combination of secukinumab and methotrexate. Int J Dermatol Venereol. PP: E046 - E052.
- Maria-Alexandra T, Constantin C, Bălăceanu-Gurău B, Irina M, Giurcăneanu C. (2025). Bullous Pemphigoid Overlapping Psoriasis Vulgaris: A Rare Case Report and Literature Review. Clin. Pract. 15: 91.
- Su F, Wang T, Qin Q, Xie Z. (2024). Upadacitinib for the management of bullous pemphigoid coexisting with psoriasis vulgaris: a case report and literature review. Journal of Dermatological Treatment. PP: 2302394.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.