A Case of Cryptogenic Organizing Pneumonia after Immunotherapy and Radiotherapy for a Stage IV Non-Small Cell Lung Cancer
by Fardeau E1*, Roelandts M1, Moretti L1, Remmelink M2, Stathopoulos K3, Ruiz Patino M4, Berghmans T5, Van Houtte P1
1Department Radiation Oncology, Institut Jules Bordet, Université Libre Bruxelles, Belgium
2Laboratory Pathological Anatomy, Belgium
3Department Radiology, Institut Jules Bordet, Université Libre Bruxelles, Belgium
4Department Thoracic Surgery, Hopital Erasme, Cliniques Universitaires Bruxelles, Université Libre Bruxelles, Belgium
5Clinic Medical Oncology, Institut Jules Bordet, Université Libre Bruxelles, Belgium
*Corresponding author: Fardeau E, Department Radiation Oncology, Institut Jules Bordet, Université Libre Bruxelles, Belgium
Received Date: 16 October 2024
Accepted Date: 21 October 2024
Published Date: 23 October 2024
Citation: Fardeau E, Roelandts M, Moretti L, Remmelink M, Stathopoulos K, et al (2024) A Case of Cryptogenic Organizing Pneumonia after Immunotherapy and Radiotherapy for a Stage IV Non-Small Cell Lung Cancer. Ann Case Report. 9: 2021. https://doi.org/10.29011/2574-7754.102021
Abstract
Lung toxicity is a major challenge especially when combining immunotherapy and radiotherapy.We present the case of a patient developing a cryptogenic organizing pneumonia (COP) after a multimodality treatment for a stage IV non-small cell lung cancer (NSCLC).
Keywords: Lung cancer; Immunotherapy; Radiotherapy; Cryptogenic organizing pneumonia.
Introduction
Lung cancer is still the leading cause of mortality by cancer worldwide with 2.1 million new cases detected each year. Since 2015, programmed death 1 inhibitors (PDL) are playing a major role in the management of lung cancer especially for stage IV non-small cell lung cancer [1,2]. Among them, pembroluzimab is the frontline setting with or without a platinum-based chemotherapy according to the PD-L1 tumor expression. Nevertheless, one major concern is the possible immune related adverse events, which can be very severe and even lethal. Among them, the rate of respectively any grade or grade 3 and 4 pneumonitis are about 3.6% and 1.1% [3]. Lung toxicity is also a major issue after thoracic radiotherapy and a major concern when combined with anti-PD-1/PD-L1 agents. Therefore, we present here an unusual complication seen after a multimodality treatment.
Clinical Case
A 73 years old man, 40 pack-year former smoker complained of cough and dyspnea leading to the diagnosis of a mass in the left lower pulmonary lobe. Endobronchial biopsies showed a non-squamous NSCLC moderately differentiated. Molecular test for EGFR mutation was negative but discovered an exon 20 mutation of the HER2 gene. In immunohistochemistry, the PD-L1 tumor expression was 15% and no overexpression of ALK and ROS1 was found. The PET-scan showed the primary lung tumor, a bilateral adrenal uptake without other distant lesions or pathological nodes. The brain MRI was normal.
With the intent of an oligo metastatic approach, induction chemotherapy started with cisplatin and pemetrexed. After 3 cycles, there was a stability of all lesions. The multidisciplinary board decided pursuing with a radio-chemo radiotherapy for the primitive lung lesion followed by a stereotactic body radiotherapy (SBRT) on both adrenals. The chest chemo radiotherapy delivered a dose of 66 Gy in 33 fractions over 47 days. The dosimetry values for the lungs were a mean dose of 15 Gy and a V20 of 24%.The patient tolerated well this treatment. A PET performed at the end of the chemo radiotherapy found a 5 mm hyper metabolic nodule in the right lower lobe and a progressive disease in the left adrenal. Patient started pembrolizumab (200 mg/q3w). The control brain-MRI showed a unique left frontal lesion, treated by radiosurgery (Gamma-Knife).
Two months later, the patient developed an episode of dry cough, causing a pneumothorax. The chest CT did not find the lung nodule and did not reveal any interstitial or alveolar opacity nor disease progression. The pneumothorax was resolved with a chest tube but the cough did not stop despite a symptomatic treatment. After two weeks, the cough continued and the blood test found a CRP increased at 250 mg/L. A new CT found heterogeneous condensations all over the lungs. A combined therapy of Amoxicilline-Clavulanate and corticosteroids resolved this episode. A new PET-CT showed an infiltration related to the radiotherapy in the left lung, a clearance of the right lung nodule and of the left adrenal. Partial response was obtained and pembroluzimab was pursued. Six months later, he developed new right cerebellar metastases and was treated again by radiosurgery without stopping pembrolizumab.
A follow up PET-CT, performed one year after the end of the chest radiotherapy, showed a new hyper metabolic opacity in the posterior lower right lobe highly suspicious for a new neoplastic lesion and in a different location as the one observed earlier (Figure 1). This lesion appeared in an area receiving low dose radiotherapy from the prior course, between 2 and 15 Gy. Its volume and extension towards the mediastinum did not allow a new radiation course. A new brain MR did not reveal any progression or new lesion. A transthoracic biopsy was not performed due to the location of the lesion.
Finally, in the absence of any other side of tumor progression, the patient underwent a lobectomy with a mediastinal lymph node dissection and the pathology revealed a COP and no tumor (Figure 2).Following this results, pembrolizumab was interrupted after 15 cycles. The patient had no more any symptoms and did not receive any corticotherapy. One year later, a new progression occurred with lung, liver and adrenals metastases justifying starting pembrolizumab. Six months later, a PET-CT showed a partial response without any recurrence of the COP but an intense uptake in different mediastinal lymph nodes but with a negative EBUS.Three months later, a new PET-CT showed a complete metabolic response of the initial progressing lesions and an increase in FDG uptake at the level of mediastinal lymph nodes without morphological modification.
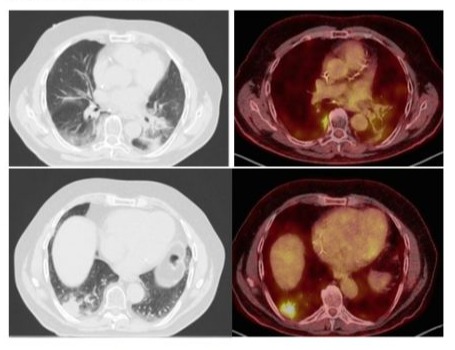
Figure 1: Axial chest HRCT and PET/FDG scan images of localised organising pneumonia manifesting as a single sub pleural mass-like opacity with abnormal 18F ‐fluorodeoxyglucose uptake in the lower third of right lung (lateral and posterior basal segments) associated with satellite infra-centimetric nodular opacities. This radiological aspect is similar to sub pleural metastatic lesion. On the left side, the radiation induced fibrosis.
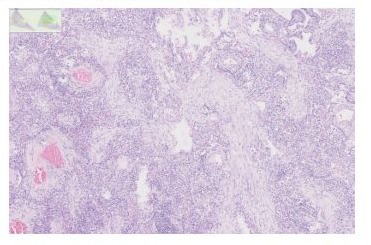
Figure 2: This high power view shows the typical branching appearance of the granulation tissue plugs and the mild interstitial inflammatory infiltrate.
Discussion
The present case highlighted the problems evaluating a patient after multiple treatments including radiation, anti-PD-1/PD-L1 agents, surgery and chemotherapy. The interpretation is often difficult to differentiate a tumor progression from a possible toxicity related to the treatments due to the lack of specific symptoms nor radiologic appearance, which range from COP to ground glass opacities or interstitial patterns [4]. Pneumonitis is a common and potentially severe complication of thoracic radiation therapy and immune checkpoint inhibitors. Often, in the clinical trials, all types of lung injuries are registered as “Pneumonitis”, but we know that we can find at least two clinical features. On one hand, the radiation pneumonitis, which results of direct radiation induced damage and on the other hand, the COP, which results of a T-Cell mediated immune response to an aggression of the alveolar epithelium.
COP is a nonspecific histopathological lesion and can be associated with various clinical context and radiological patterns. The heterogeneity of the features on CT and the absence of pathognomonic signs make it difficult to differentiate localised COP from other lung pathologic entities and in particular from primary and metastatic lung tumours [5]. Focal COP is a rare form that is associated with discrete unifocal lesions on radiological images. It can easily be misdiagnosed as lung cancer because of mass‐like pattern and positive PET‐CT scan. High resolution computed tomography (HRCT) is the imaging method of choice for evaluating COP. The most common finding in patients with a COP is consolidation and ground glass opacities-GGO, which are usually bilateral and peripheral but are nonspecific. These opacities often migrate spontaneously [6]. Their size is variable, ranging from a few centimetres to a whole lobe. A solitary focal opacity is an uncommon presentation of COP and known as a focal COP. The diagnosis is usually made after a biopsy of a nodule or mass removed because of a suspicion of bronchogenic carcinoma. It is also described that COP can overlap with other types of interstitial pneumonia, particularly idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia. This pattern is characterised by a relative lack of consolidation and GGO, with a predominance of reticular opacities with architectural distortion. Furthermore, the reversed halo sign is initially considered as COP-specific finding, but it is also described in several other diseases.
On the other hand, COP can also present as centrilobular nodules of 3-5 mm and small infra-centimetric nodular opacities that are typically ill defined. The differential diagnosis with metastasis is crucial, especially in patients with cancer history, given that there is an association between COP and cancer [6].
In addition, another important aspect in the HRCT evaluation of COP is the distribution of opacities. Subpleural/peribronchovascular distribution and a perilobular pattern can be found in approximately 60% of cases [7,8]. Bilateral lung involvement predominated and the middle third of the lung is the most commonly affected area followed by the lower third. Last, PET‐CT scan does not give us any evidence to distinguish focal COP from lung carcinoma [9].
Existence of COP after radiotherapy where described after breast irradiation since 2000 [9] but surprisingly rarely in lung cancer. Recently, a retrospective study found a pattern of immune pneumonitis which appears under ICI and after radiotherapy in the low-dose or fall-off dose region in the treatment of NSCLC but the timing of the immune related pneumonitis was not different for patients receiving or not chest radiotherapy [11].
Several case reports describe COP after ICI and radiotherapy. The timing between the drug administration and radiotherapy varied from one month to several months or even years. In the three cases reported by Sakaguchi, COP developed after a first treatment, 13 courses or after one year of treatment with nivolumab [12]. The chest radiotherapy was completed one month or 27 months earlier. In the series of Watanabe et al, 31 patients out of 231 treated with anti-PD-1 therapy developed interstitial lung disease including 16 cases of COP, the number of cycles ranged from 1 to 27 with a median of 9 [13]. All patients had received nivolumab. Patients with a COP developed later the toxicity compared to ground glass opacities and had a better survival.
In the present case, we had the pathological proof of a COP. Its management is very specific and a misdiagnosis can lead to an overly aggressive treatment like in this case or for example, a use of multiple courses of meaningless antibiotics drugs. The COP treatment consists in oral corticosteroids and is based on CTCAE symptoms-grade [14,15].
The link between radiotherapy and ICI in the genesis of COP in not established. We know that the CD8+ T-cells are related with the development of COP lesions [16]. Because of this finding, we can try to connect the different elements of our case: on one hand, a nonlytic radiation dose can enhance the tumor cells’ susceptibility to CD8+ T-cell–mediated immune attack or serve as additional targets for immunotherapy [17]. On other hand, we know that the PD-1/PD-L1 pathway involve the CD8+ T-cells [18].
Moreover, the issue of the ICI discontinuation after a side effect and after a potential corticosteroid treatment seems to be widely open which highlights the need of a good assessment of any side effect [19].
Clearly, as ICIs is more and more widely used in the management of stage III and IV NSCLC, clinicians should be aware of the differential diagnosis between radiation-induced pneumonitis and other pathologies.
References
- Gray J.E, Villegas A, Daniel D, Vicente D, Murakami S, et al (2020) Three-Year Overall Survival with Durvalumab after Chemo radiotherapy in Stage III NSCLC-Update from PACIFIC.J Thorac Oncol. 15:288-293.
- Reck M, Rodríguez-Abreu D, Robinson A.G, Hui R, Csőszi T, et al; (2016) KEYNOTE-024 Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer.N Engl J Med. 375:1823-1833.
- Khunger M, Rakshit S, Pasupuleti V, Hernandez AV, Mazzone P, et al (2017) Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: A systematic review and meta-analysis of trials. Chest 152:271–281.
- Suresh K, Naidoo J, LinC.T. , Danoff S. (2018) Immune checkpoint immunotherapy for Non-Small Cell Lung Cancer: benefits and pulmonary toxicities Chest. 154:1416-1423.
- Cordier JF. (2006) Cryptogenic organising pneumonia. Eur Respir J. 28:422–446.
- Drakopanagiotakis F, Paschalaki K, Abu-Hijleh M, Aswad B, Karagianidis N, et al (2011) Cryptogenic and secondary organizing pneumonia: clinical presentation, radiographic findings, treatment response, and prognosis. Chest. 139:893–900.
- Chang J, Han J, Kim DW, et al. (2002) Bronchiolitis obliterans organizing pneumonia: clinicopathologic review of a series of 45 Korean patients including rapidly progressive form. J Korean Med Sci. 17:179-186.
- Drakopanagiotakis F, Polychronopoulos V, Judson MA. (2008) Organizing pneumonia. Am J Med Sci. 335:34–39.
- Baha A, Yildirim F, Kokturk N, Akdemir UO, Demircan S, et al (2016) 18 F-FDG uptake in focal organising pneumonia mimicking bronchial carcinoma. Clin Respir J. 10:740-745.
- Sato H, Ebi J, Tamaki T, Yukawa A, Nakajima M, et al (2018) Incidence of organizing pneumonia after whole-breast radiotherapy for breast cancer, and risk factor analysis. J Radiat Res. 59:298-302.
- Voong K.R, Hazell S.Z, Fu W, Lin C.T, Ding K, et al. (2019) Relationship between prior radiotherapy and checkpoint-inhibitor pneumonitis in patients with advanced non-small cell lung cancer. Clin.Lung Ca. 20:e470-9.
- Sakaguchi M, Maebayashi T, Aizawa T, Ishibashi N, Okada M. (2019) Organizing pneumonia after thoracic radiotherapy followed by anti-PD-1 antibody treatment for patients with lung cancer: Three case reports. Thoracic cancer. 10:1503-07.
- Watanabe S, Ota T, Hayashi M, Ishikawa H, Otsubo A, et al (2020) Prognostic significance of the radiologic features of pneumonitis induced by anti-PD-1 therapyCancer Medicine. 9:3070-77
- Haanen J.B.A.G, Carbonnel F, Robert C, Kerr K.M, Peters S, et al (2017) Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 28:iv119-iv142.
- Otani K, Seo Y, Ogawa K. (2017) Radiation-Induced organizing pneumonia: A characteristic disease that requires symptom-oriented management. Int. J. Mol .Sci. 18:281.
- Majeski E.I, Harley R.A, Bellum S.C, London S.D, London L. (2003) Differential role for T cells in the development of fibrotic lesions associated with reovirus 1/L-induced bronchiolitis obliterans organizing pneumonia versus Acute Respiratory Distress Syndrome. Am. J. Respir. Cell Mol. Biol. 28:208-17.
- Garnett C.T, Palena C, Chakraborty M, Tsang K.Y, Schlom J, et al (2004) Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 64:7985-9
- Ribas A, Wolchok J.D. (2018) Cancer immunotherapy using checkpoint blockade. Science. 359:1350-1355.
- Simonaggio A, Michot .JM. Voisin A.L, Le Pavec J, Collins M, et al (2019) Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol. 5:1310-7.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.