A Bovine Dermal Collagen Matrix (BDCM) Advances Readiness to Autografting: A Case Series
by Chinaemelum Akpunonu1, Michael Young1, Laura Pezzopane1, Nidhi Aravapalli1, Rachel Penny2, Ariel Rodgers1, Nicole Bernal1, John Loftus1*
1The Ohio State University Wexner Medical Center, Columbus, OH, USA
2AVITA Medical, Valencia, CA, USA
*Corresponding author: John Loftus, The Ohio State University Wexner Medical Center, Columbus, OH, USA
Received Date: 26 May 2025
Accepted Date: 30 May 2025
Published Date: 02 June 2025
Citation: Akpunonu C, Young M, Pezzopane L, Aravapalli N, Penny R, et al. (2025) A Bovine Dermal Collagen Matrix (BDCM) Advances Readiness to Autografting: A Case Series. J Surg 10: 11337 https://doi.org/10.29011/2575-9760.011337
Abstract
Dermal matrices have become fundamental in managing deep surgical and traumatic wounds, while promoting scar reduction and improved functional outcomes. However, conventional dermal matrices often require 2-4 weeks to achieve a sufficiently vascularized wound bed for autografting, which delays definitive wound closure and potentially increases the risk of infection, pain, and hospital length of stay. A young bovine dermal collagen matrix (BDCM) comprised of crosslinked, highly-purified, structurally intact, collagen type I and type III has demonstrated autograft readiness preclinically at 7 days. Our center was the first in the world to utilize the BDCM and to evaluate if the preclinical timeline translated into clinical utilization. A retrospective chart review was conducted from January to March 2025 to evaluate the early clinical use of the BDCM in two patients with full-thickness wounds. Data collected included surgical timelines, wound bed readiness, autograft outcomes, and complications. In case 1, a 67-year-old female with multiple comorbidities and a full-thickness hand wound achieved a well-vascularized wound bed by day 5 and underwent successful autografting on day 7 post-BDCM application with 100% graft take and reepithelialization. In case 2, a 48-year-old male with a full-thickness wound had a well-vascularized wound bed by day 10 and autografting on day 13 post-BDCM application with 80% graft take and functional recovery. Both cases demonstrated fast BDCM integration and a well-vascularized wound bed within 5-10 days. Our initial experience indicates the BDCM is a safe and effective dermal matrix option to advance speed to wound bed vascularization and autograft readiness.
Keywords: Autograft Readiness; Bovine Dermal Collagen Matrix; Burns; Dermal Matrix, Dermal Regenerative Template; Wounds
Introduction
Dermal matrices have expanded in use since their FDA approval in the late 1990s. They have been used in deep wounds with both surgical and traumatic etiologies, and their utilization has been associated with a reduction in scarring and contracture, and an increase in functional and cosmetic outcomes [1].
Dermal matrices act as a scaffold, allowing cell migration and adhesion to occur. Angiogenesis allows delivery of nutrients and immune cells to promote tissue regeneration [2-4]. This rapid revascularization is critical for successful dermal matrix integration and eventual support of viable tissue. One of the main uses of dermal matrices is to vascularize a wound bed for definitive closure such as split-thickness skin grafting and Autologous Skin Cell Suspension (ASCS). The typical timeline after dermal matrix application to wound bed preparation with well-vascularized tissue ranges from 2-4 weeks [3,5]. This timeline results in delayed surgical procedures for definitive wound closure, increasing the risk of infection, pain, and scarring. It may also increase hospital length of stay for those who are treated as inpatients [6,7]. Therefore, there exists an unmet need for a dermal matrix that reduces the time from placement to autograft readiness.
Pre-clinically, a new young bovine dermal collagen matrix (BDCM, CohealyxTM, Regenity Biosciences, Paramus, NJ) has shown autograft readiness at 7 days with formation of a robust and well-vascularized wound bed [8]. The BDCM is comprised of crosslinked, highly-purified, structurally intact, collagen type I and type III [8]. The importance of collagen is that it supports all phases of wound healing [9]. Both the age and source of collagen in dermal matrices can impact tissue regeneration and inflammation. The BDCM has been engineered with an optimal pore size to promote cellular migration and vascular tissue formation [10]. Besides its structure, the way the material is processed impacts a dermal matrices’ strength, porosity, resorption rate, and pore shape [8]. Pre-clinical outcomes were attributed to the biological structure and material processing in manufacturing the BDCM.
Our group was the first in the world to utilize this BDCM, and to our knowledge, to also publish on its clinical use. The authors were interested to see if the preclinical timeline translated in clinical utilization. The results below demonstrate the BDCM’s early use as a two-stage procedure to prepare the wound bed and minimize the time to autografting.
Methods
A retrospective chart review was conducted at a single university medical center from January to March 2025 for patients treated with BDCM. In addition to written informed surgical consent, separate photo consents were obtained from each patient for publication of their clinical cases and accompanying images.
Case 1
A 67-year-old Caucasian female patient presented to our burn unit after sustaining a full-thickness contact burn injury to the palmar surface of the left hand and anterior forearm from a syncopal episode. Her total body surface area (TBSA) burn was 1.5%. She had a history of uncontrolled diabetes mellitus, diabetic foot ulcers, and prior amputations. She had initially undergone surgical excision in the operative setting and application of a temporary, bilayer, Biosynthetic Wound Matrix (BWM, PermeaDerm®, Stedical Scientific, Inc., Carlsbad, CA) for wound temporization. After adherence of the BWM, the BWM was removed, and she received both a 1:1 meshed Split-Thickness Skin Graft (mSTSG) and Autologous Skin Cell Suspension (ASCS). The patient was discharged from the hospital after post-operative day 7. At her first outpatient clinic visit, it was determined she had 99% graft take and 100% reepithelialization. The left hand was placed in moisturizer, and she was instructed to follow-up in 1 month. However, the patient delayed follow-up for 2 months. When she returned to the clinic, she had a small area of graft loss on the palmar surface of the hand and had been self-treating with diabetic foot cream at home. The wound was 32 centimeters squared with some fibrinous debris and hypergranulation tissue (Figure 1).
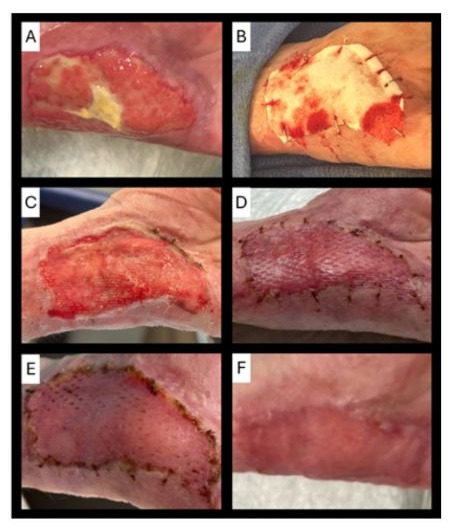
Figure 1: Case 1: A 67-year-old female with a full-thickness injury of the left hand. The left hand wound prior to excision (A); intra-operative left hand with BDCM pre-hydration (B); left hand well-vascularized wound bed on day 5 post-BDCM application (C); left hand on post-operative day 3 from 1:1 mSTSG (D); left hand on post-operative day 10 from 1:1 mSTSG (E); left hand on post-operative day 24 from 1:1 mSTSG (F).
She was admitted on the day of surgery and underwent excision in the operative setting, resulting in a full-thickness surgical wound. Once hemostasis was achieved, the BDCM was placed along with non-adherent petrolatum-based gauze, black foam sponge, and negative pressure wound therapy (NPWT) at 100 mmHg for 5 days. An ace wrap and wrist splint were utilized to prevent pulling on the NPWT cord or dressing. On day 5, her NPWT was removed, and she was deemed ready for autografting as her wound bed was well-vascularized. Due to operative setting availability, she was scheduled for autografting 2 days after graft readiness was observed. On day 7 post-BDCM application, she had debridement and a 1:1 mSTSG. She was discharged home on post-operative day 1. On post-operative day 3, she had 100% take and was completely healed in clinic. She was rechecked in clinic on post-operative day 10 and good durability of the healed skin graft was noted. She was rechecked in clinic on post-operative day 24 and was in moisturizer and a tubular elastic bandage. She was instructed to follow-up for assessment of long-term functional and aesthetic outcomes.
Case 2
A 48-year-old Caucasian male patient presented to our burn unit after sustaining a full thickness contact burn injury to the right hand, right upper extremity, and anterior trunk from having a syncopal episode while cooking on the stovetop (figure 2). He sustained a burn injury to 11.25% TBSA. His comorbidities include major depressive disorder, bipolar disorder, generalized anxiety disorder, and insomnia. He underwent a full trauma workup to rule out etiologies for his syncopal episode and ensure he sustained no head trauma. He had daily topical dressings changes with antimicrobial cream and non-adherent petrolatum-based gauze. He underwent excisional debridement in the operative setting on post-burn day 2, resulting in a full-thickness surgical wound. Once hemostasis was achieved, the BDCM was applied to his right hand and wrist to cover an area of 440 centimeters squared and more proximally a temporary, bilayer, BWM was applied to the right upper extremity and trunk for wound temporization. The BDCM was hydrated on the wound bed with saline flushes and secured using intermittent staples and 4-0 resorbable sutures. The BDCM was then covered with a small pore non-adherent primary dressing, antimicrobial ointment with non-adherent petrolatum-based gauze, gauze rolls, ace wraps, and cohesive bandage. Range of motion was started on post-operative day 1 to the right hand and wrist. The antimicrobial ointment and non-adherent petrolatum-based gauze was replaced on post-operative day 3. He was taken back to the operative setting on post-operative day 5. The BDCM had integrated and there were a few areas that underwent light hydrosurgical debridement. A conservative course was taken and he was placed in a non-adherent dressing, black foam sponge, and negative pressure wound therapy for 5 days. His NPWT was kept in place until post-application day 10 when it was determined he was ready for autografting and had a well-vascularized wound bed. Due to operative setting availability, he was scheduled for autografting 3 days after graft readiness was observed. He had 1:1 mSTSG with ASCS to the right hand and wrist on post-application day 13. After autografting, the patient had better than anticipated range of motion, minimal edema, and 80% graft take was observed. Silver nitrate was applied to close a few remaining areas on the metacarpal phalangeal joints. Additional outpatient functional assessment completed on postoperative day 31.
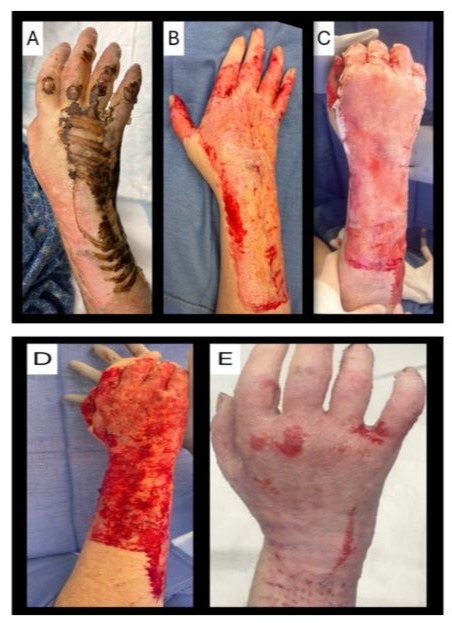
Figure 2: Case 2: A 48-year-old male with a full-thickness contact burn on the right hand. The right hand on the day of injury (A); intra-operative right hand post-excision revealing full-thickness surgical wound (B); intra-operative hydrated and secured BDCM on right hand (C); right hand post-hydrosurgical debridement (D); right hand on post-operative day 14 from 1:1 and 2:1 mSTSG with ASCS (E).
Discussion
For patients with deep wounds that are not ready for immediate autografting, dermal matrices serve to supplement missing dermal tissue and enhance autografting results. Typically, this process is conducted in two stages for full-thickness wounds: application of the dermal matrix after surgical excision and subsequently autografting. However, between these two phases, there is typically a window of 2-4 weeks for tissue integration into the dermal matrices and the wound bed to become well-vascularized. The two-staged approach can lead to better tissue integration, enhanced autograft take rates, and reduction in complications. [8,11] On the contrary, the longer duration between stages may cause increased pain, infection, and scarring. [6,7] Ideally, reducing the duration to definitive wound closure between stages may address the potential downsides to using dermal matrices. One should consider utilization of a dermal matrix which has been optimized for rapid tissue integration, vascularization, and consistency [8].
In our experience of 2 cases, the BDCM resulted in fast integration and wound bed vascularization. Patients had robust wound beds between 5 and 10 days post-application. With other dermal matrices we did not observe a robust wound bed until at least two weeks post-application and others can take much longer at four weeks, which is in accordance with the literature. [3,5] Compared to our previous experience with other dermal matrices, the BDCM sped up the time to autografting, achieving more timely definitive wound closure for two-stage procedures. These were our first 2 cases at our burn center; however, we have since started more widespread utilization with different wound etiologies to facilitate faster healing and reduction in length of stay.
This initial retrospective observational study provides valuable early clinical experience on the use of the BDCM in practice. One of the limitations was the small sample size, so future research on a larger study population is warranted to generalize these results. In addition, as a descriptive study, no direct comparison was made to other dermal matrices. Ideally, it would have been preferred to follow these patients’ extended course to report on long-term aesthetic and functional outcomes.
Conclusion
This case series suggests that the young bovine dermal collagen matrix (BDCM) may support earlier clinical autografting by promoting a well-vascularized wound bed within 5-10 days. Initial outcomes indicate that the BDCM is a safe and effective option, warranting further studies to assess graft readiness, healing, complications, long-term outcomes, and potential health economic benefits.
Acknowledgements: None
Ethical Guidelines: This was a Retrospective review, and all patients provided release of information for research and photo consent.
Conflicts of Interest: Rachel Penny is an employee of AVITA Medical.
References
- Gupta S, Moiemen N, Fischer JP (2024) Dermal Regeneration Template in the Management and Reconstruction of Burn Injuries and Complex Wounds: A Review. Plast Reconstr Surg Glob Open 12: e5674.
- Stefanelli V, Lombardi J, Ferrer J, Gardocki-Sandor M (2025) Vascularization of Human Acellular Dermal Matrices: A Comparative Study in a Nonhuman Primate Model. Tissue Eng Part A 31: 419-432.
- Chang DK, Louis MR, Gimenez A, Reece EM (2019) The Basics of Integra Dermal Regeneration Template and its Expanding Clinical Applications. Semin Plast Surg 33: 185-189.
- Zhong SP, Zhang YZ, Lim CT (2010) Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2: 510-525.
- Olsen T, Ali-Khan S, Bell D (2024) Comparative Analysis of AnimalDerived vs Fully Synthetic Acellular Dermal Matrices in Reconstructive Surgery: An Examination of Clinical, Aesthetic, and Economic Measures. Ann Plast Surg 92: S172-S178.
- Finnerty CC, Jeschke MG, Branski LK, Barret JP, Dziewulski P, et al. (2016) Hypertrophic scarring: the greatest unmet challenge after burn injury. Lancet 388: 1427-1436.
- Chipp E, Charles L, Thomas C, Whiting K, Moiemen N, et al. (2017) A prospective study of time to healing and hypertrophic scarring in paediatric burns: every day counts. Burns Trauma 5: 3.
- Bush KA, Nsiah BA, Jay JW (2025) Bovine Dermal Collagen Matrix Promotes Vascularized Tissue Generation Supporting Early Definitive Closure in Full-Thickness Wounds: A Pre-clinical Study. Cureus 17: e81517.
- Mathew-Steiner SS, Roy S, Sen CK (2021) Collagen in Wound Healing. Bioengineering (Basel) 8: 63.
- Bružauskaitė I, Bironaitė D, Bagdonas E, Bernotienė E (2016) Scaffolds and cells for tissue regeneration: different scaffold pore sizes-different cell effects. Cytotechnology 68: 355-369.
- Shahrokhi S, Arno A, Jeschke MG (2014) The use of dermal substitutes in burn surgery: acute phase. Wound Repair Regen 22: 14-22.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.