3D Specimen Tomosynthesis Real-Time Assessment by Radiologists and Surgeons Potentially Decreases the Margin Re-excision Rate Following Breast-Conserving Surgery
by Attia M1*, Badawy K2*, SinhaA1,2, Jeffery H1,2, Garg Y1, Shifa B1, Manning R1, Karthigan R1, Kothari A1,2
1 Guy’s and St Thomas’ NHS Trust, Great Maze Pond, London SE1 9RT, UK.
2 King’s College London, Guy’s Campus, Great Maze Pond, London SE1 1UL, UK.
*Corresponding author: Attia M, Guy’s and St Thomas’ NHS Trust, Great Maze Pond, London SE1 9RT, UK.
Badawy K, King’s College London, Guy’s Campus, Great Maze Pond, London SE1 1UL, UK.
Received Date: 10 March, 2025
Accepted Date: 17 March, 2025
Published Date: 20 March, 2025.
Citation: Attia M, Badawy K, Sinha A, Jeffery H, Garg Y, et al. (2025) 3D Specimen Tomosynthesis Real-Time Assessment by Radiologists and Surgeons Potentially Decreases the Margin Re-excision Rate Following Breast-Conserving Surgery. J Oncol Res Ther 10: 10269. https://doi.org/10.29011/2574-710X.10269.
Abstract
Introduction: Clear margins are crucial in breast-conserving surgery (BCS). Intra-operative specimen X-rays have become standard practice. The primary outcome of this study was to determine that the correct use of the 3D specimen imaging system, compared to just 2D imaging, would have saved patients from further surgery. In addition, we analysed whether the margin re-excision rate would be lower if an experienced radiologist reviewed and reported the specimen X-rays intra-operatively. Methods: A retrospective review of the intra-operative specimen images was undertaken by a single experienced breast surgeon and two consultant breast radiologists blinded to histopathology. They used the 3D specimen tomosynthesis function and measured the width of the closest margin for patients who required re-operations for positive margins. These were patients for whom the operating surgeon had originally estimated margins as ‘clear’ intra-operatively. Sensitivity and specificity of the surgeon and radiologist reporting of margins was calculated compared to the histopathological assessment. Results: Fifty-four patients were included. Eighty-one out of 216 (37.5%) margins were positive on histology. Forty-four margins (54.3%) were assessed accurately as involved by the radiologists and 25 (30.9%) by the surgeon using the 3D tomosynthesis function. Eleven (20%) patients might have been saved from a second operation if the surgeon adequately assessed the images using the specimen 3D tomosynthesis, and 27 (50%) of patients if reported intra-operatively by a breast radiologist. Conclusion: Our study demonstrates that the intra-operative use of the 3D specimen tomosynthesis function could potentially save 20-50% of patients from a second operation. Without considering pass-through elements, re-excision of breast margins costed our hospital £211,004 (averaging £3,638 unit cost) for the 2022/2023 period. Those costs can be reduced significantly if the 3D specimen tomosynthesis function is used regularly intraoperatively.
Keywords: Wide local excision; Breast conserving surgery; 3D Specimen Tomosynthesis; Margin Re-excision; Breast Cancer; Margin Assessment.
Introduction
Worldwide, 2.3 million women were diagnosed with breast cancer in 2022, causing 670,000 deaths globally. Breast cancer is the most common cause of cancer in women in 157 countries, with only 0.5-1% of breast cancers occurring in men. High-income countries have dropped their age-standardised breast cancer mortality by 40% in the last 40 years, yet it remains the leading cause of cancerrelated deaths among women [1, 2].
Breast-conserving surgery (BCS) and adjuvant radiotherapy are the preferred surgical treatment for patients diagnosed with early-stage breast cancer [3-5]. BCS has a higher incidence of local recurrence compared to mastectomy; however, disease-specific survival rates are similar in both procedures. Negative surgical margins are essential to reduce local recurrence risk and disease-specific survival in BCS [6]. Further surgery due to positive margins varies according to institution, type of surgery undertaken and definition of a positive margin. Findings suggest that of the patients required to undergo re-excision, 65% have positive margins, with disease at the margins rather than close to the margins [7]. Centres across the UK show large variations in margin policy; however, one study’s mean national re-excision rates were 17.2%, with some units reporting rates up to 41% [7]. Only 4% of breast units follow the Society of Surgical Oncology and American Society for Radiation Oncology (SSO-ASTRO) guidelines. If these guidelines became standard practice, the UK re-excision rate would drop to 15.4% [7] . Some studies report margin re-excision as low as 4.3% amongst their cohorts when implementing SSO-ASTRO guidelines [8]. If the UK followed the 2015 ABS Consensus, re-excision rates would drop to 14.8% [7].
Further surgery can have far-reaching consequences, including psychological and social impacts, cosmetic outcomes, fat necrosis, mastectomy rates, theatre time and health economics [5, 7, 9].
Intra-operative specimen X-ray has become standard practice in the UK. These are obtained by sending specimens to the radiology department or by using purpose-built devices located within the operating rooms to capture intra-operative images. The Faxitron® (Hologic®, Arizona, USA) for example, provides standard 2 dimensional X-ray images of the specimen and or the Kubtec MOZART® System (KUB Technologies®, Stratford, Connecticut, USA), generates three-dimensional (3D images) using tomosynthesis, as well as a two-dimensional (2D images).
Common practice uses a conventional 2D imaging system, which may be inferior to 3D imaging. Previous studies have shown that assessment with 3D images can reduce the re-excision rate to 5% [10]. Our institution utilises the Kubtec MOZART® System; however, most surgeons at our institution use the superimposed 2D image, not utilising the 3D specimen tomosynthesis function. At our institute, surgeons review the radiological specimen images; a radiologist is not involved in the assessment, and surgeons accordingly act on taking further tissue if required. Our breast unit has five consultant surgeons and eight senior registrars. All specimens had an anterior margin at the mastectomy plane and a posterior margin at the pectoral fascia. The primary outcome of this study was to evaluate if the use of the Kubtec MOZART® System 3D specimen tomosynthesis function and radiological review would have reduced re-excision rates.
Methods
This study retrospectively reviewed specimen images that a consultant surgeon and senior registrar had intra-operatively examined and diagnosed to have radiological clear margins using the Kubtec MOZART® System, and that final histopathology identified a positive margin. Two surgeons reviewed each image with negative findings at the initial review intra-operatively. As is our practice, most of the initial reviews were performed using only the 2D function. During the retrospective review, an additional senior surgeon and two senior consultant radiologists assessed the four margins using the 3D tomosynthesis function. Upon reviewing the images, all investigators were blinded to the histopathology results. The breast surgeon used a standard highdefinition computer, whilst the radiologists used high-resolution dedicated mammography reporting workstations. All reviewers used the 3D specimen tomosynthesis function and documented if the tumour was close or involving each radial margin and whether a cavity shave would have been recommended.
This study included all patients undergoing BCS who required a return to theatre (Figure 1) for further surgery from July 2021 to June 2023, including patients with primary in-situ or invasive disease and patients who underwent primary surgery or surgery following neo-adjuvant systemic therapy. Patients who did not have intra-operative specimen imaging or had a positive margin diagnosed intra-operatively and were acted upon at the time were excluded from the study. Patients whose specimens had suboptimal orientation were also excluded.
The Kappa coefficient was calculated to assess agreement between the surgeon and the radiologist interpretation of positive radial margins based on the 3D images. Sensitivity and specificity were calculated for both surgeon and radiologist assessment of margins as compared with the gold standard of histopathological assessment. Additionally, sensitivity and specificity were calculated for a ‘dual assessment’ of the images by both the surgeon and radiologist, where a margin was considered positive if either the surgeon or the radiologist interpreted it as positive. All statistical analyses were conducted in Stata (version 18.0, StataCorp LLC, College Station, TX).
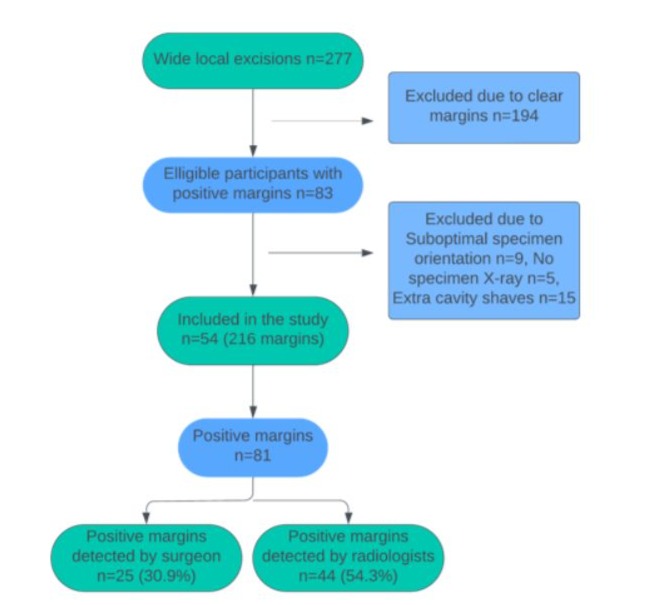
Figure 1: Flowchart diagram of patient recruitment for the study.
Results
Fifty-four specimens from 54 patients with at least one positive radial margin were included in the analysis. The median age was 60 (IQR 55-70). Patient tumour characteristics are shown in Table 1, both invasive (44/54, 81.5%) and pure ductal carcinoma insitu (DCIS) (10/54,18.5%) patients were included. The individual tumour size and type and any associated DCIS are shown in Table 2.
Eighty-one radial margins out of 216 (37.5%) were positive on histopathology. Forty-four (44/81, 54.3%) were assessed accurately as having involved margins on imaging by the radiologists and 25 (30.9%) by the surgeon using the 3D tomosynthesis function, (Figure 2). Table 3 shows the sensitivity and specificity of the surgeon, radiologist and dual assessment as compared with the histopathological gold standard. The radiologist reported margins with better sensitivity (54.3% vs 30.9%). There was a fair agreement between surgeon and radiologist interpretation (Kappa agreement 78.3%, Kappa coefficient 0.26, p<0.0001).
The surgeon was more likely to correctly classify a margin on 3D imaging in invasive cancer specimens than in pure DCIS specimens (76.7% vs 62.5%, p=0.064). The radiologist was also more likely to correctly classify a margin in invasive cancer specimens than pure DCIS specimens (82.4% vs 70.0%, p=0.077).
For invasive cancers, neither the surgeon nor the radiologist’s proportion of correctly identified margins was dependent on the grade of tumour, presence of associated DCIS, or type of tumour. The radiologist correctly classified 100% of margins in mucinous tumours, compared to the surgeon correctly classifying 66.7% however, this only accounted for twelve margins in three specimens.
Both surgeon and radiologist were least likely to correctly classify a margin in dense breast tissue (American College of Radiology breast density classification (ACR) D) and mixed density breast tissue. Radiologists were most likely to correctly classify a margin in fatty tissue (ACR A). Surgeons were most likely to correctly classify a margin in scattered fibroglandular tissue (ACR B). However, the sample size was too small to demonstrate statistically significant differences.
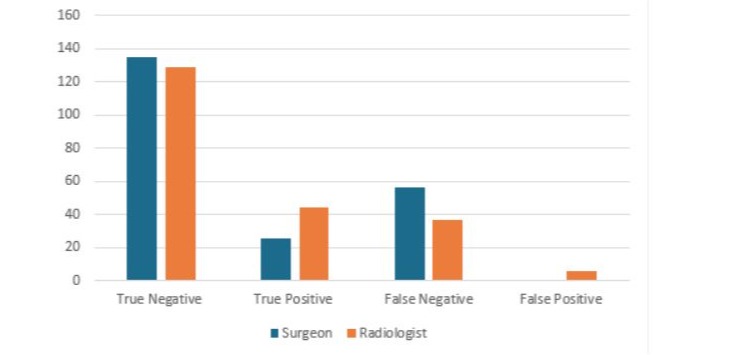
Figure 2: Number of margins correctly and incorrectly classified using the 3D images by the surgeon and the radiologist (n=216).
The proportion of patients who could have avoided re-excision was calculated as the number of patients in whom all margins that were positive on histopathology were correctly identified as positive on 3D specimen tomosynthesis by (a) the surgeon’s retrospective interpretation and (b) the radiologist’s retrospective interpretation. Overall, 11 patients (20%) could have avoided re-excision if all 3D images using tomosynthesis slicing function were adequately assessed by the surgeon in real-time. Twenty-seven (50%) patients could have avoided returning to theatre if the images had been reported intra-operatively by a breast radiologist.
Table 1: Patient demographics and operative details.
|
Parameter studied |
Value |
|
|
Age (years) |
||
|
Minimum |
36 |
|
|
Maximum |
84 |
|
|
Median, (IQR) |
60 (55,70) |
|
|
Size of largest lesion on imaging (mm) (mammogram/ultrasound/MRI) |
||
|
Minimum |
5 |
|
|
Maximum |
70 |
|
|
Median, (IQR) |
17 (11, 25) |
|
|
Total Size on histology (mm) |
||
|
Minimum |
0 |
|
|
Maximum |
51 |
|
|
Median, (IQR) |
20.5 (16,42) |
|
|
Breast density on imaging |
||
|
Fatty |
4 (7.4%) |
|
|
Scattered fibroglandular tissue |
3 (5.55%) |
|
|
Mixed density |
24 (44.44%) |
|
|
Dense |
13 (24%) |
|
|
Heterogeneously dense |
10 (18.5%) |
|
|
NAET/NACT |
||
|
Yes |
8 (14.8%) |
|
|
No |
46 (85.18%) |
|
|
Histological type |
||
|
NST |
36 (66.7%) |
|
|
Pure DCIS |
10 (18.5%) |
|
|
NST/encapsulated papillary cancer |
1 (1.8%) |
|
|
Mixed NST and Lobular |
1 (1.8%) |
|
|
ILC |
3 (5.6%) |
|
|
Mucinous |
3 (5.6%) |
|
|
Grade of invasive disease/ DCIS |
||
|
Invasive grade 1 |
5 (9.2%) |
|
|
Invasive grade 2 |
26 (48.1%) |
|
|
Invasive grade 3 |
13 (24.1%) |
|
|
Low grade DCIS |
3 (5.6%) |
|
|
Intermediate grade DCIS |
2 (3.7%) |
|
|
High grade DCIS |
3 (5.6%) |
|
|
Intermediate/High grade DCIS |
2 (3.7%) |
|
|
Localisation of lesions |
44/54 (81.5%) |
|
|
Operator grade |
||
|
Consultant |
39 (72.23%) |
|
|
Fellow |
15 (27.77%) |
|
|
Operation done |
||
|
Wide local excision |
49 (90.7%) |
|
|
Therapeutic mammoplasty |
5 (9.3%) |
|
|
Abbreviations; IQR – interquartile range. NAET – neoadjuvant endocrine therapy. NACT – Neoadjuvant chemotherapy. |
||
Table 2: Exhibiting patient’s tumour type and size.
|
Patient |
Grade |
Type |
ER [Allred] |
Her2 [Allred/FISH] |
Size (histology) in mm |
DCIS |
|
1 |
2 |
ILC |
+ |
- |
24 |
No |
|
2 |
2 |
NST |
+ |
- |
22 |
Yes |
|
3 |
2 |
ILC |
+ |
- |
13 |
No |
|
4 |
LG |
DCIS |
+ |
- |
11.5 |
Yes |
|
5 |
LG |
DCIS |
+ |
- |
2.2 |
Yes |
|
6 |
1 |
NST |
+ |
- |
24 |
No |
|
7 |
1 |
NST |
+ |
- |
18+45(DCIS) |
Yes |
|
8 |
2 |
Mucinous |
+ |
- |
21 |
Yes |
|
9 |
3 |
NST |
+ |
- |
18 |
No |
|
10 |
IG |
DCIS |
+ |
- |
29 |
Yes |
|
11 |
1 |
NST |
+ |
+ |
6+15(DCIS) |
Yes |
|
12 |
1 |
NST |
+ |
- |
7.5 |
Yes |
|
13 |
3 |
NST |
+ |
+ |
13 |
IG |
|
14 |
2 |
NST |
+ |
- |
20 |
Yes |
|
15 |
2 |
Mucinous |
+ |
- |
21 |
Yes |
|
16 |
3 |
NST |
+ |
- |
0 |
Yes |
|
17 |
2 |
NST |
+ |
- |
22.5 |
No |
|
18 |
2 |
NST |
+ |
- |
18 |
Yes |
|
19 |
2 |
ILC |
+ |
- |
19 |
Yes |
|
20 |
2 |
NST |
+ |
- |
10 |
Yes |
|
21 |
2 |
Mucinous |
+ |
- |
20 |
Yes |
|
22 |
IG/HG |
DCIS |
+ |
- |
41 |
Yes |
|
23 |
2 |
NST |
+ |
- |
16 |
No |
|
24 |
2 |
NST |
+ |
- |
18 |
Yes |
|
25 |
2 |
NST |
+ |
- |
14 |
Yes |
|
26 |
2 |
NST |
+ |
+ |
19 |
Yes |
|
27 |
2 |
NST |
+ |
- |
16 |
No |
|
28 |
2 |
NST |
+ |
- |
35 |
Yes |
|
29 |
2 |
NST |
+ |
- |
19 |
Yes |
|
30 |
2 |
NST/Lobular |
+ |
- |
17+6+1.8 |
Yes |
|
31 |
3 |
NST |
+ |
- |
24 |
Yes |
|
32 |
HG |
DCIS |
- |
- |
41 |
Yes |
|
33 |
3 |
NST |
- |
- |
27 |
No |
|
34 |
3 |
NST |
+ |
+ |
17 |
Yes |
|
35 |
1 |
NST |
+ |
- |
43 |
Yes |
|
36 |
LG |
DCIS |
+ |
- |
32 |
Yes |
|
37 |
IG |
DCIS |
+ |
- |
42 |
Yes |
|
38 |
3 |
NST |
+ |
- |
27 |
Yes |
|
39 |
3 |
NST |
+ |
- |
21 |
Yes |
|
40 |
HG |
DCIS |
+ |
- |
18 |
Yes |
|
41 |
2 |
NST |
+ |
- |
44 |
Yes |
|
42 |
3 |
NST |
+ |
- |
42 |
Yes |
|
43 |
3 |
NST |
+ |
- |
40 |
Yes |
|
44 |
IG/HG |
DCIS |
+ |
- |
51 |
Yes |
|
45 |
2 |
NST/Encapsulated papillary |
+ |
- |
25 |
Yes |
|
46 |
3 |
NST |
+ |
- |
13 |
Yes |
|
47 |
2 |
NST |
+ |
- |
11 |
Yes |
|
48 |
HG |
DCIS |
+ |
- |
21 |
Yes |
|
49 |
2 |
NST |
+ |
- |
16 |
Yes |
|
50 |
3 |
NST |
+ |
- |
32 |
Yes |
|
51 |
2 |
NST |
+ |
+ |
22.5 |
Yes |
|
52 |
3 |
NST |
+ |
- |
24 |
Yes |
|
53 |
2 |
NST |
+ |
- |
9 |
No |
|
54 |
2 |
NST |
+ |
- |
32 |
Yes |
|
Abbreviations: ILC -Invasive Lobular Carcinoma, NST – No Special Type, DCIS – Ductal carcinoma in situ, LG – Low Grade, IG- Intermediate Grade, HG – High Grade. |
||||||
Table 3: Sensitivity and specificity of radiologist and surgeon assessment of margins. For dual assessment, a margin is considered positive when either radiologist or surgeon assessed it as positive.
|
Radiologist |
Surgeon |
Dual |
|
|
Sensitivity (%) (95% CI) |
54.3 (47.7, 61.0) |
30.9 (24.7, 37.0) |
67.9 (61.7, 74.1) |
|
Specificity (%) (95% CI) |
95.6 (92.8, 98.3) |
100 (100, 100) |
95.6 (92.8, 98.3) |
|
Positive predictive value (%) (95% CI) |
88.0 (83.7, 92.3) |
100 (100, 100) |
90.2 (86.2, 94.1) |
|
Negative predictive value (%) (95% CI) |
77.7 (72.2, 83.3) |
70.7 (64.6, 76.6) |
83.2 (78.2, 88.2) |
Discussion
This study shows that intra-operative use of the 3D tomosynthesis slicing function can reduce the positive margin rate by 20% during margin assessments. In terms of patient outcomes this study shows that 50% of patients could have avoided re-excision if a breast radiologist assessed 3D images during surgery for negative margins. We found radiologists accurately assessed 54.3% of positive margins versus 30.9% by the surgeon using 3D specimen tomosynthesis. The 3D tomosynthesis specimen X-ray enables accurate interpretation of margins, as seen in Figure 3. UK guidelines mandate intra-operative specimen radiography for all impalpable lesions and that radiographs should be reported to or by the operating surgeon within 20 minutes. However, practices may vary across European countries where radiologist reporting may also be required [11].
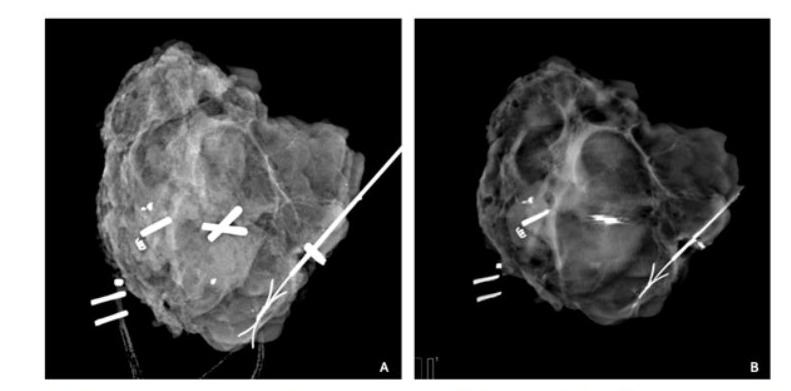
Figure 3: (A) 2D specimen mammogram displaying excised tissue with the irregular lesion. The margins appear adequate, with excision margins displayed and a satisfactory amount of healthy tissue between the mass and the specimen’s edge. (B) A single 1mm tomosynthesis slice 5mm from the anterior surface of the same specimen providing a view that is unobstructed by tissue above or below. It shows visible spicules extending laterally to the margins are subtle, yet a significant finding.
Although there is no gold standard for intra-operative specimen margin assessment (IMA) [12], meta-analyses data findings suggest cytology (CYT) and frozen section (FS) are found to be the most accurate modalities currently in use [13]. Compared to 3D specimen tomosynthesis, histopathological IMA techniques are time-consuming, costly, and need expert input. FS has a slow turnaround, disrupting surgical flow and increasing logistical challenges and resource demands [12]. Our data suggest that 3D specimen tomosynthesis reliably compares to histopathological methods while providing prompt diagnostic insights, saving time without compromising oncological safety.
Similar to our institute, Partain and colleagues employed surgeons—with no involvement from radiology—to assess both 2D and 3D specimen radiographs [10]. Re-excision rates fell from 9% to 5% when one surgeon utilised the 3D function, while most cases were in 2D. In 2021, Romanucci et al. investigated the accuracy of digital tomosynthesis in evaluating margins during BCS, finding it to be more accurate for tumour size evaluation. Their Pearson’s correlation coefficients for digital breast tomosynthesis and digital mammography to pathologically determined tumour-free margins were 0.92 and 0.79 in CC view, and 0.92 and 0.72 in LL view.
Re-excision is needed when final pathology indicates close or positive margins. Urano et al. established that the detectability of lesions of invasive cancers in BCS specimens using digital breast tomosynthesis was 97% when a radiologist interpreted images [14]. However, we propose that if surgeons receive adequate training they may be proficient to conduct most of the analysis, as indicated by Partain et al, allowing radiologists to be a safety net with a second opinion.
Implementing a protocol for radiologists to evaluate resection margins on post-specimen mammograms may significantly increase their workload. This assessment demands a careful evaluation of pre-operative imaging, a strong understanding of baseline disease, and response to neoadjuvant treatment to accurately determine tumour extent and its proximity to specimen edges. Additionally, the tumour’s homogeneity within dense breast tissue must be considered when comparing evaluation techniques. This detailed analysis requires extra time, especially in high-volume breast cancer units with frequent post-specimen mammography. Given radiologists’ existing diagnostic and screening duties, this new task may strain their capacity and negatively affect patient care if not properly supported.
Increased accountability is crucial as radiologists assess resection margins with proper orientation, directly affecting surgical decisions and patient outcomes. Demanding high accuracy in reporting raises error potential, especially with borderline margins. Discrepancies between radiological and pathological assessments may invite scrutiny, requiring robust quality assurance to mitigate risks. This added responsibility may necessitate dedicated training and standardisation for radiologists to handle this nuanced task effectively.
Given these challenges, combined with the evidence from other studies with MOZART®, we propose that it would generally be more efficient and effective to give surgeons adequate training and have them conduct most of the intraoperative analysis, allowing radiologists to be a safety net with a second opinion. Collaborating with the surgical team can streamline communication and clarify radiological reporting expectations, fostering a unified approach that benefits patient care while managing increased demands on radiology services.
Patient factors aside, preventing second surgeries offers cost benefits for institutes. At ours, re-excision costs an average of £3,638 (2022/23). Our dual assessment process may have saved 11 patients (20%) from additional surgeries. We perform about 140 wide local excisions annually, with a return to theatre rate of 19.3%. The Kubtec MOZART® System could potentially reduce returns by 50%, saving approximately £49,150 yearly, considering each return costs £3,638. Additionally, reducing re-operations alleviates the NHS burden, including waiting lists and delays in cancer treatment.
Limitations of this study include that specimen radiographs were reviewed retrospectively by a single surgeon. We aimed to simulate the intra-operative environment if the consultant breast surgeon performed the case with a less experienced colleague. The authors are now working on a prospective study where a consultant radiologist will report specimen radiographs in realtime during the operation to improve reporting accuracy and reduce re-excision rates. Moreover, the surgeons and radiologists reported sensitivity in our study is lower than what was reported in previous 3D specimen tomosynthesis studies, this could be due to the fact that almost 42.5% of patients included in our study had dense/heterogeanously dense breast tissue which could have affected image interpretation.
Implementing radiological assessment of resection margins in post-specimen mammograms greatly enhances patient outcomes. This improves margin evaluation accuracy, reducing incomplete excisions and the need for re-operations, thereby lessening the physical and emotional burden on patients. It streamlines multidisciplinary team discussions, as clearer initial evaluations lead to definitive decision-making, saving time and resources. Sharing margin assessment responsibility with surgeons fosters a collaborative team dynamic, strengthening professional relationships and promoting accountability for patient care. This enhanced role for radiologists improves workflow and fosters a sense of inclusion in the multidisciplinary team.
Conclusion
In conclusion, using the 3D specimen tomosynthesis slices intraoperatively could reduce re-excision rates by 20% compared to the 2D composite view when images are reviewed by the surgeon alone. If a breast radiologist had reported the specimen 3D images intraoperatively, 50% of patients could have avoided re-excision. This would lead to a significant improvement in patient outcomes and a reduction in the cost of healthcare provision. We propose that if surgeons receive adequate training they may become proficient to conduct most of the analysis, allowing radiologists to be more of a safety net with a second opinion, similar to practice in the U.S.
Declarations of interest: none.
References
- Breast Cancer. (2024). (WHO. Breast Cancer: World Health Organisation 2024.
- Siegel RL, Giaquinto AN, Jemal A (2024) Cancer statistics, 2024. CA Cancer J Clin 74:12-49.
- 3Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, et al. (2002) Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 347:1233-41.
- Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, et al. (2002) Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med 347:567-75.
- Ning J, Cheng G, Wu N (2024) A systematic review on the techniques, long-term outcomes, and complications of partial breast irradiation after breast-conserving surgery for early-stage breast cancer. Sci Rep 14:22283.
- Dongen JAV, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, et al. (2000) Long-term results of a randomized trial comparing breastconserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 92:1143-50.
- Tang SSK, Kaptanis S, Haddow JB, Mondani G, Elsberger B, et al. (2017) Current margin practice and effect on re-excision rates following the publication of the SSO-ASTRO consensus and ABS consensus guidelines: a national prospective study of 2858 women undergoing breast-conserving therapy in the UK and Ireland. Eur J Cancer 84:315-324.
- Urooj N, Abubakar M, Asghar K, Hassan M, Malik AA, et al. (2024) Impact of SSO-ASTRO Margin Guidelines on Re-excision Rate in Breast-conserving Surgery: A Single-center Experience. J Cancer Allied Spec 10:559.
- Matar-Ujvary R, Haglich k, Flanagan MR, Fuzesi S, Sevilimedu V, et al. (2023) The impact of breast-conserving surgery re-excision on patient-reported outcomes using the BREAST-Q. Ann Surg Oncol 30:5341-5349.
- Partain N, Calvo C, Mokdad A, Colton A, Pouns K, et al. (2020) Differences in Re-excision Rates for Breast-Conserving Surgery Using Intraoperative 2D Versus 3D Tomosynthesis Specimen Radiograph. Ann Surg Oncol 27:4767-4776.
- Parker S, Tomlins A (2016) Clinical guidelines for the management of breast cancer.
- Boughey JC, Hieken TJ, Jakub JW, Degnim AC, Grant CS, et al. (2014) Impact of analysis of frozen-section margin on reoperation rates in women undergoing lumpectomy for breast cancer: evaluation of the National Surgical Quality Improvement Program data. Surgery 156:190-7.
- St John ER, Al-Khudairi R, Ashrafian H, Athanasiou T, Takats Z, et al. (2017) Diagnostic Accuracy of Intraoperative Techniques for Margin Assessment in Breast Cancer Surgery: A Meta-analysis. Ann Surg 265:300-10.
- Urano M, Shiraki N, Kawai T, Goto T, Endo Y, et al. (2016) Digital mammography versus digital breast tomosynthesis for detection of breast cancer in the intraoperative specimen during breast-conserving surgery. Breast Cancer 23:706-11.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.