Rare Malignant Peripheral Nerve Sheath Tumor with Perineurial Differentiation - A Clinical Case with a Literary Review
Authors: Lena Marinova1*, Bistra Yordanova2, Vaska Vasileva3, Petranka Troyanova4 , Viktor Petrov5
*Corresponding Author: : Lena Marinova, Medical oncology clinic, Radiation and metabolic brachytherapy department, UMHAT „Queen Joanna“ Sofia, Bulgaria
1, 3, 5Medical oncology clinic, Radiation and metabolic brachytherapy department, UMHAT „Queen Joanna“ Sofia, Bulgaria
2Complex oncology center pathochistological department, Ruse, Bulgaria
4Medical oncology clinic, Chemotherapy department; UMHAT „Queen Joanna“ Sofia, Bulgaria
Received Date: 28 May 2022
Accepted Date: 1 June 2022
Published Date: 3 June 2022
Citation: Marinova L, Yordanova B, Vasileva V, Troyanova P, Petrov V (2022) Rare Malignant Peripheral Nerve Sheath Tumor with Perineurial Differentiation - A Clinical Case with a Literary Review. Ann Case Report 7: 859. DOI: https://doi.org/10.29011/2574-7754.100859
Abstract
Malignant peripheral nerve sheath tumor (MPNST) is a rare form of sarcoma. Extremely rare is the pathohistological diagnosis of malignant perineurioma, which is a rare subset of MPNSTs. We present a 60 year old man with an extremely aggressive nondifferentiated tumor, whose pathohistological and immunohistochemical characteristics correspond to a rarely diagnosed Malignant perineurioma. Four operations were performed for 6 months. The diagnostics is extremely difficult and requires a broad immunohistochemical analysis to refine tumor histogenesis. We presented a broad immunohistochemical analysis that supports the rare pathochistological diagnosis. After the first surgery, no postoperative radiation therapy was performed, which is why the tumor continues to persist and aggressively infiltrates adjacent muscle groups. Given the infiltrative nature of the malignant perineurioma and the major difficulties to perform radical surgery with clean resection lines, postoperative radiotherapy is required and recommended.
Keywords: Malignant perineurioma; Perineurial malignant peripheral nerve sheath tumor; Malignant Triton tumor; Peripheral nerve sheath tumors; Immunohistochemical analysis; Postoperative radiotherapy
Introduction
Malignant peripheral nerve sheath tumor (MPNST) is a rare form of sarcoma arising from Schwann cells or pluripotent cells of the neural crest [1,2]. MPNST is a neoplasm that arises primarily in the peripheral nerves [3] and accounts for 5-10% of all soft tissue sarcomas [4]. MPNSTs are aggressive soft tissue sarcomas with nerve sheath differentiation and a tendency to metastasize [5]. Malignant triton tumor (MTT) is a subtype of MPNST with a component of malignant rhabdomyoblasts in addition to malignant Schwann cells [6,7]. The term MTT is used for tumors exhibiting the features of an MPNST and containing rhabdomyoblastic elements [8,9]. MPNST with both heterologous rhabdomyosarcomatous differentiation (malignant Triton tumor), and glandular epithelial differentiation is exceedingly rare [10,11]. Malignant perineurioma is a rare subset of malignant peripheral nerve sheath tumors (MPNSTs) with ultrastructural and immunohistochemical features of perineurial differentiation, distinguishing it from other MPNSTs, which typically demonstrate Schwannian features [12, 13]. MPNST of perineurial origin are exceedingly uncommon [12,14]. We present a 60 year old man with an extremely aggressive undifferentiated tumor whose pathochistological and immunohistochemical characteristics corresponds to a rarely diagnosed Malignant perineurioma.
A Clinical Case: We present a 60 year old man with a complaint of bumps in the left inguinofemoral region with a limitation of 4 months, which gradually increased, became painful and limited the movements of the hip joint. In January 2022 the patient was emergently hospitalezed in the surgical department. From the CT
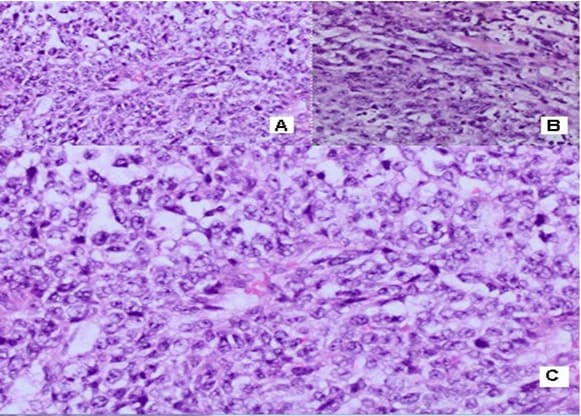
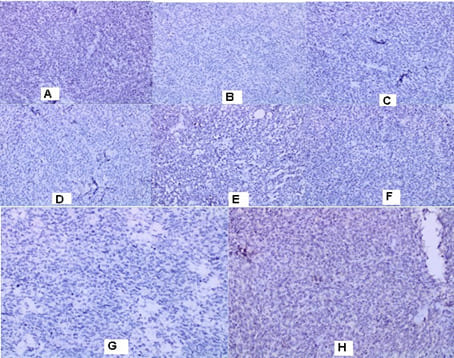
in the left inguinal region, two subcutaneous nodular formations with a heterogeneous structure and a maximum size in the axial plan of 58mm/43 mm are visualized. The borderline fat is deleted against m. Sartorius. No data on enlarged pelvic lymph nodes. Surgery/05.01.2022: When opening the subcutaneous fat, the tissues are thickened with the leakage of purulent secretions. A formation was found with a diameter of about 5cm/5.5 cm with the soft consistency, tightly coated with the underlying tissues and vessels and with decay data. Tumor excision and necrectomy were performed. Histological Result: Macroscopic description irregularly shaped material, crumbly, 5cm/3cm/3cm, with soft consistency, a colorful cut surface with brownish gray-rounded areas. Morphological Result: Soft tissue infiltrated by a solid tumor with highly atypical cells with polymorphic nuclei with coarse chromatin, visible nucleoli, frequent mitotic figures, scarce predominantly eosinophilic cytoplasm. Consultation of an experienced pathohistologist: Soft tissue material with tumor infiltration with a predominantly solid construction, focal with pseudopapillary growth around blood vessels, necrotic areas and bleeding. The tumor is made up of cells with moderate to pronounced nuclear polymorphism with prominent nucleoli, eosinophilic cytoplasm, high mythotic activity, stroma richly vascularized with the presence of mature lymphocytes and lymphoid tissue around the periphery (Figure 1). Immunohistochemical (IHC) Analysis: HMB45 (-), CK7 (-), CK20 (-), GATA (-), CD30 (-), PLAP (-), PAX8 (-), PSAP (-), TTF (-), Podoplanin D2-40 (-), Oct3/4 (-), Alfa FP (-), HCG (-). CD117 focal positivity, CD20 (+) in lymphoid tissue. IHC analysis excluded malignant melanoma, metastasis from adrenal carcinoma, germinative cell tumors of the testis, renal cell carcinoma, prostate and urinary carcinoma, lymphoma, lung carcinoma metastases, hepatocellular carcinoma metastases. Conclusion: It is about tumor infiltration from an extremely undifferentiated malignant tumor. After 1 month of the CT with contrast, in the left inguinal region a subcutaneous nodular heterogeneous formation with positioned dren and single air collections in the structure are visualized. Maximum size 66/57 mm. Deleted border adipose tissue compared to m. Sartorius, peripheral nodular lesions with a slight increase in density through the phases of examination, with increased density of adipose tissue. No data on enlarged pelvic lymph nodes. Surgery/28.02.2022: A 7cm/8cm tumor, tightly fastened with fascia Lata and Sartorius muscle and Adductor longus muscle was established. The fascia Lata was cut to the top of the Scarpa triangle. The whole preparation, along with the fascia and part of the muscle fibers of the two muscles, were prevented to the presentation of the artery and vein femoralis.
The whole preparation, along with the adipose tissue, fascia and surface and deep lymph nodes, are removed. Histological Result: Macroscopic skin 13 cm/4.5 cm with underlying soft tissues. In the subcutaneous fat, a tumor formation of 8cm/6.5cm/3 cm is found. The tumor has extensive zones of necrosis and hemorrhage, with focal areas of brownish pigmentation on the periphery, solid construction with a grayish color, lobulated, relatively distinguished from the surrounding tissue. The resection line at the bottom of the removed material is focally engaged. Morphological Result: Soft tissues with infiltration of hypercellular
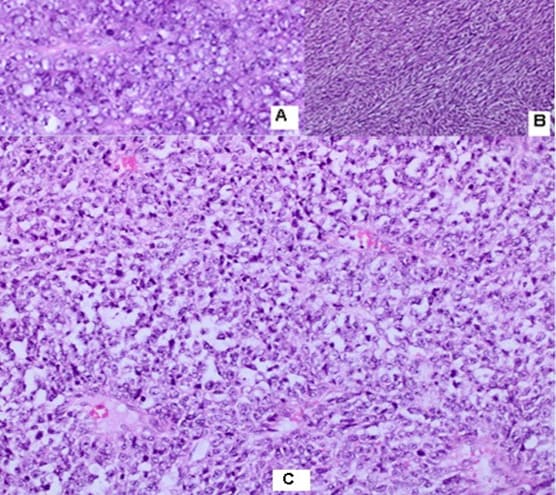
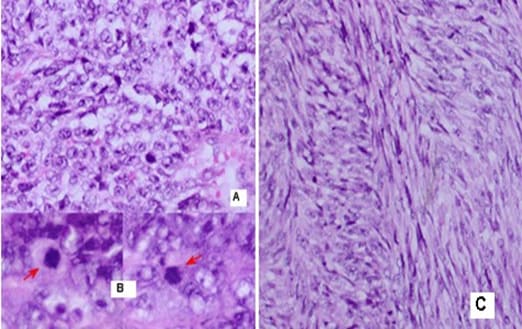
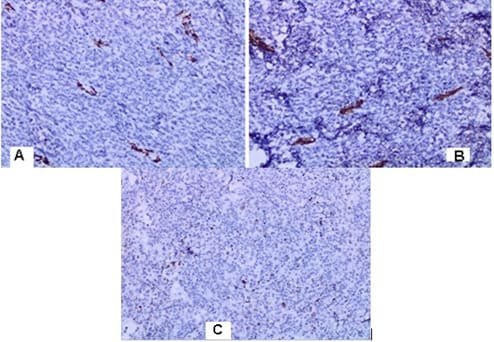
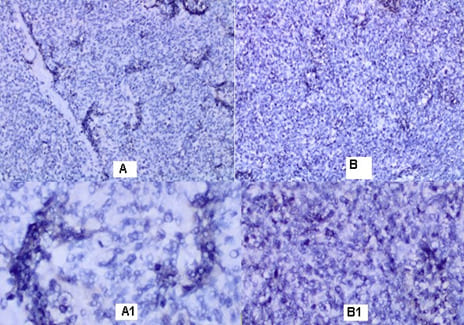
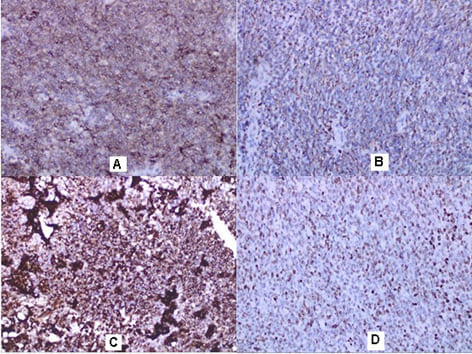
malignant tumor with fascicular structure, in places with the arrangement of „fish bone“, with hemorrhage and geographical type of necrosis, perivascular hypercellularity (Figure 2). Cytologically, the tumor is represented by spindle shaped cells with hyperchromic and wavy nuclei, coarse dispersed chromatin, identified nucleoli, eosinophilic cytoplasm, ranging in quantity to extremely scarce, high mitotic index 46 mitoses per 10 (Figure 3). Eight lymph nodes without metastases. The bottom of the resected specimen fascia with adjacent blood and peripheral nerves adjacent to the soft tissues with infiltration from the tumor described above. Resection boundaries are casual, but close, with the tumor at a distance of 0.6 mm from the bottom of the resected specimen. IHC Study: SMA and CD34 positive IHC expression in the blood vessels against the background of a negative tumor population; P53-focal positive expression in some of the tumor nuclei (Figure 4). Negative IHC expression to Melanosome; CK7; Chromogranin; GATA3; Myogenin; Synaptophysin; TTF1; CK20 negative expression in tumor cells with focal positivity in lymphoid tissue (Figure 5). Desmin and S100 Protein slightly focally positive IHC expression in separate groups of tumor cells at a per high power field x 400 (Figure 6). Positive IHC expression to CD117; CK AE1/AE3; Vimentin Strong positive expression, KI 67-High Mitotic Index/46 mitoses per 10 HPF (Figure 7). Diagnosis: Based on morphological characteristics and immunofenotype, taking into account the finding of imaging studies and previous biopsy a diagnosis is Malignant Peripheral Nerve Sheath Tumor with a high degree of malignancy (MPNST; High Grade). It is reported slightly focal Desmin and S100 protein expression, which are characteristic of Malignant Triton Tumor (MTT), a subtype of MPNST with a component of malignant rhabdomyoblasts. The positive CD117 expression also corresponds to the rhabdomyosarcoma characteristic of this high malignant tumor with a high mitotic index-46 mitoses per 10 HPF. Strong Vimentin and CK AE1/AE3 expression defines malignant perineurioma, which is a rare subset of MPNSTs with immunohistochemical features of perineurial differentiation.
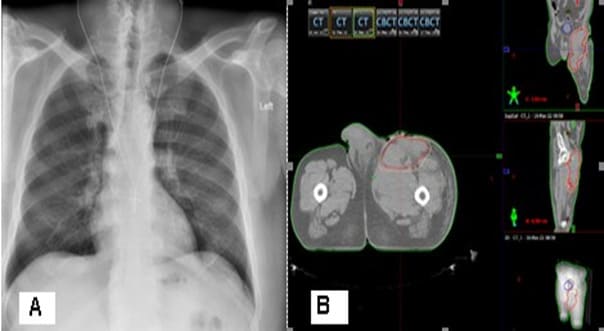
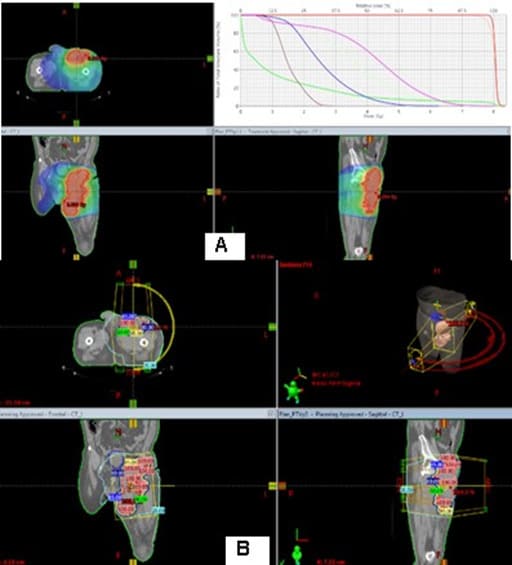
After the second surgery, the patient is not referred for postoperative radiotherapy (RT). Due to the expansion of a painful flushed bump in the upper pole of the operative cicatrix, covering the entire upper half of the thigh with the lisa of plastic and purulent secretions, in May a new operation was performed to maximize the evacuation of necrotic tumor masses and the purulent collection, which passes a submuscular and subfascicular, reaching a vascular nerve bundle. The patient is directed for postoperative RT, which we have considered that due to pain syndrome and available bleeding, should be performed in a palliative aspect. The radiography of the lung is without pathological changes.The pelvic and left lower limb CT reports a large soft tumor, relatively well restricted by the muscles with infiltration of the left inguinal region and the inner part of the left thigh (Figure 8). A decompressive hemostatic Intensity Modulated RT (IMRT) with the technique VMAT up to total dose 20 Gy with daily dose 4 Gy/5 fractions was carried out (Figure 9).
After two weeks of the RT completion, the patient reported a decrease in swelling and pain syndrome, as well as to improve the movement of the limb. After 2 months, it is necessary to carry out a control CT with a contrast to the final assessment of the tumor response and the subsequent therapeutic behavior.
Discussion
MPNSTs are uncommon spindle cell sarcomas that appear in the setting of neurofibromas or schwannomas and are associated with peripheral nerves [1]. According to the Fifth Edition of the World Health Organization (WHO)’s Classification of Tumors Soft Tissue and Bone Tumors [15], nerve sheath tumors are divided into those which are benign (e.g., schwannomas, neurofibromas including plexiform neurofibromas, perineuriomas, etc.) and those which are malignant, of which MPNSTs form a major subset [16]. MPNSTs are a form of sarcoma that is, a tumor arising from cells of mesenchymal origin that have undergone malignant transformation. Mesenchymal cells display at least partial differentiation towards a connective tissue lineage a broad term that includes, among others, muscle, adipose, bone, cartilage, vascular, and nervous tissue [5]. The neoplasm was predominantly composed of spindle cells arranged in interlacing fascicles, whorls and a palisiding pattern [1]. The fasciculated, spindle cell growth pattern may cause confusion with leiomyosarcoma, fibrosarcoma, or monophasic synovial sarcoma [17]. The AJCC staging system utilizes the size, depth, and invasiveness of the tumor (T), the presence/absence of lymph node involvement (N), and the presence/absence of distant metastasis (M) to assign a stage from 1 to 4, some of which include multiple sub-stages. In addition, tumor histologic grade is a key consideration and is included in both the eighth edition of the AJCC staging system and ARST0332 risk group assignments [18,19]. The pathochistological characteristic of MPNST are uniform spindle cells with hyperchromatic, thin, wavy, or focally buckled nuclei and typically have diffuse and strong expression of S100 and SOX10 [3]. The capacity of MPNSTs to undergo focal mesenchymal (or even epithelial) differentiation is well known. Epithelial areas may be histologically benign; however, mesenchymal differentiation is sarcomatous in nature and histologically malignant [20]. MPNST with both heterologous rhabdomyosarcomatous differentiation (malignant Triton tumor/ MTT), and glandular epithelial differentiation is exceedingly rare [10]. Rhabdomyoblastic differentiation (MTT) associated with aggressive behavior by positive IHC expression to the S100 protein and Desmin was reported [2,6,8]. The pathogenesis of MTT is unknown, the presence of both neural cells and rhabdomyoblasts have led some to hypothesize that both cellular components derive from less differentiated neural crest cells that have both mesodermal and ectodermal potential and others points out direct evidence for the potential of schwannoma cells to exhibit myogenic differentiation [21]. The cytoplasm is typically light staining and indistinct. The overall architecture may be either diffuse or arranged in alternating hypocellular and densely cellular areas. High-grade tumors usually contain necrosis and increased mitotic activity [17]. When MTT develops over Neurofibromatosis type I (NF-1), the diagnosis can be confirmed based on morphologic grounds supported by an immunostain such as focal positivity immunostains for S-100 protein. Desmin, myo-D1 and myogenin, by which the rhabdomyoblasts are identified [22,23]. Peripheral nerve sheath tumors (PNST) include schwannomas that arise from Schwann cells; neurofibromas comprising schwann cells, fibroblasts and endoneurial cells and perineuriomas that display perineurial differentiation [24]. Diagnostic criteria and differential diagnosis for the major categories of nerve sheath tumors are proposed, including neurofibroma, schwannoma, and perineurioma [25]. Whereas the malignant counterpart, namely MPNST display variable schwannian differentiation, few MPNSTs with perineurial differentiation/perineurial MPNSTs/malignant perineuriomas have been documented, including in the setting of multiple soft tissue perineuriomas [26,27]. The diagnostics is extremely difficult and requires a broad IHC analysis to refine tumor histogenesis. Hirose et al. [28], documented seven perineurial MPNSTs with EMA positivity that showed perineurial differentiation on ultrastructure [29]. Spindle cells showed hyperchromatic nuclei with indistinct pale cytoplasm and increased mitotic activity (6 per high power field). A few round plump cells with brightly eosinophilic cytoplasm and hyperchromatic nuclei were seen admixed with neoplastic spindle cells [1]. This pathochistological picture corresponds to our pathohistological finding (Figure 1-3). Immunohistochemically, perineurial differentiation was indicated by the diffuse expression of epithelial membrane antigen and focal reactivity for CD34 [27]. The immunophenotypic profile consisted of only vimentin and epithelial membrane antigen (EMA) positivity, while antibodies to S-100, CD34, smooth muscle actin, and pankeratins were negative [30]. Tumor cells were strongly immunoreactive for epithelial membrane antigen, CD56 (N-CAM), and vimentin, but were negative for S-100 protein and other lineage-specific epithelial, mesenchymal, hematolymphoid, and reticulo-histiocytic markers [31]. The single epithelioid case was diffusely and strongly positive for S-100 protein [32]. In a clinical case presented, it concerns for Malignant Peripheral Nerve Sheath Tumor with a high degree of malignancy (MPNST; High Grade). Cytologically, the tumor is represented by spindle shaped cells with hyperchromic and wavy nuclei, coarse dispersed chromatin, identified nucleoli, eosinophilic cytoplasm, ranging in quantity to extremely scarce, high mitotic index-46 mitoses per 10 ( Figure 1-3). IHC report SMA and CD34 positive IHC expression in the blood vessels against the background of a negative tumor population; p53 focal positive expression in some of the tumor nuclei (Figure 4); Negative IHC expression to Melanosome; CK7; Chromogranin; GATA3; Myogenin; Synaptophysin; TTF1; CK20- negative expression in tumor cells with focal positivity in lymphoid tissue (Figure 5) ; Desmin and S100 Protein Slightly focally positive IHC expression in separate groups of tumor cells at a high power field x 400 (Figure 6); Positive IHC expression to CD117; CK AE1/AE3;Vimentin Strong positive expression, KI 67-High Mitotic Index/46 Mitoses per 10 HPF (Figure 7). It is reported focal Desmin and S100 protein expression, which are characteristic of Malignant Triton Tumor (MTT), a subtype of MPNST with a component of malignant rhabdomyoblasts [1]. The positive CD117 expression also corresponds to the rhabdomyosarcoma characteristic [33] of this high malignant tumor with a high mitotic index 46 mitoses per 10 HPF. Strong Vimentin and CK AE1/AE3 expression defines malignant perineurioma, which is a rare subset of MPNSTs with immunohistochemical features of perineurial differentiation. The positive Vimentin expression is characteristic of the soft tissue mesenchymal tumors [34]. Immunohistochemical CK AE1/AE3 is positive at Epitheloid and Synovial Sarcomas [35].
Resectability is strongly related to outcomes, and survival depends upon wide resection [36,37]. Some literature would suggest that patients with localized soft tissue sarcomas may benefit from undergoing re-resection if there was an apparent macroscopic tumor left in the tumor bed. This approach decreases local tumor burden in anticipation of or following local RT [38,39]. In a clinical case presented after the first surgery, no postoperative RT was performed, which is why the tumor continues to persist and aggressively infiltrate adjacent muscle groups. Given the infiltrative nature of the malignant perineurioma and the major difficulties to perform radical surgery with clean resection lines, postoperative RT is required and recommended. The literature generally suggests that RT is most useful in patients with large (e.g., >5 cm), high-grade tumors, and/or those with positive margins at resection [40-42]. In a clinical case presented, a decompressive hemostatic IMRT with the technique VMAT up to total dose 20 Gy with daily dose 4 Gy/5 fractions was carried out (Figure 9). After two weeks of the RT completion, the patient reported a decrease in swelling and pain syndrome, as well as to improve the movement of the limb.
Conclusion
We presented a 60 year old man with an extremely aggressive nondifferentiated tumor, whose pathohistological and immunohistochemical characteristics correspond to a rarely diagnosed malignant perineurioma. For 6 months, 4 surgical interventions were carried out. The diagnostics is extremely difficult and requires a broad immunohistochemical analysis to refine tumor histogenesis. After the first surgery, no postoperative radiotherapy was performed, which is why the tumor continues to persist and aggressively infiltrate adjacent muscle groups. Given the infiltrative nature of the malignant perineurioma and the major difficulties to perform radical surgery with clean resection lines, postoperative radiotherapy is required and recommended.
Figures
References
- Suresh T N, Harendra Kumar M L, Prasad C.S.B.R, Kalyani R, Borappa K (2009) Malignant peripheral nerve sheath tumor with divergent differentiation. Indian J Pathol Microbiol. 52: 74-6.
- Merter A, Başarır K, Yıldız Y, Sağlık Y (2018) Malignant triton tumor of the gluteal region in a patient unaffected by neurofibromatosis: A case Acta Orthop Traumatol Turc. 52: 236-239.
- Kao E, Mantilla JG (2022) Malignant peripheral nerve sheath tumor (MPNST).
- Enzinger F.M., Weiss S.W. (1988) 2nd ed. C.V. Mosby Company; St. Louis: Soft Tissue Tumors; 745-756.
- Knight SWE, Knight TE, Santiago T, Murphy AJ, Abdelhafeez AH (2022) Malignant Peripheral Nerve Sheath Tumors-A Comprehensive Review of Pathophysiology, Diagnosis, and Multidisciplinary Management. Children (Basel). 9: 38.
- Ram R, Gardner J, Alapati S, Jambhekar K, Pandey T (2017) Malignant Triton Tumor (Malignant Peripheral Nerve Sheath Tumor with Rhabdomyoblastic Differentiation) Occurring in a Vascularized Free Flap Reconstruction Graft. Int J Surg Pathol. 25: 462-467.
- Malerba M, Garofalo A (2003) A rare case of nerve-sheath sarcoma with rhabdomyoblastic differentiation (malignant triton tumor) Tumori. 89: 246-50.
- Brooks JSJ, Freeman M, Enterline HT (1985) Malignant “Triton” tumors: natural history and immunohistochemistry of nine cases with literature review. Cancer. 55: 2543-2549.
- Woodruff JM, Chernik NL, Smith MC, Millet WB, Foote FW (1973) Peripheral nerve tumors with rhabdomyosarcomatous differentiation (malignant “Triton” tumors). Cancer. 32: 426-439.
- Thway K, Hamarneh W, Miah AB, Fisher C (2015) Malignant Peripheral Nerve Sheath Tumor with Rhabdomyosarcomatous and Glandular Elements: Rare Epithelial Differentiation in a Triton Tumor. Int J Surg 23: 377-383.
- Karpuz V, Letovanec N, Hochstetter AV, Joris F (2000) Malignant peripheral nerve sheath tumor with rhabdomyoblastic differentiation and glandular component. Ann Pathol. 20: 62-65.
- Everson MC, Pendleton C, Jack MM, Smith BW, Carter JM, et al. (2021) Sporadic Malignant Perineurioma: A Rare Diagnosis Among Malignant Peripheral Nerve Sheath Tumors, World Neurosurgery. 149: 36-41.
- Rekhi B. (2013) Perineurial malignant peripheral nerve sheath tumor in the setting of multiple soft tissue perineuriomas: A rare presentation of an uncommon tumor. J Can Res Ther. 9: 131-134.
- Rosenberg AS, Langee CL, Stevens GL, Morgan MB (2002) Malignant peripheral nerve sheath tumor with perineurial differentiation: „malignant perineurioma“ J Cutan Pathol. 29: 362-7.
- WHO Classification of Tumours Editorial Board. (2020) Soft Tissue and Bone Tumours. International Agency for Research on Cancer (IARC); Lyon, France.
- Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F (2020) WHO Classification of Tumours of Soft Tissue and Bone. International Agency for Research on Cancer (IARC); Lyon, France.
- Stasik CJ, Tawfik O (2006) Malignant Peripheral Nerve Sheath Tumor with Rhabdomyosarcomatous Differentiation (Malignant Triton Tumor). Arch Pathol Lab Med. 130: 1878-1881.
- Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, et al. (2002) AJCC Cancer Staging Manual. Springer; New York, NY, USA. Purposes and Principles of Staging: 3-8.
- Spunt SL, Million L, Chi YY, Anderson J, Tian J, et al. (2020) A RiskBased Treatment Strategy for Non-Rhabdomyosarcoma Soft-Tissue Sarcomas in Patients Younger than 30 Years (ARST0332): A Children’s Oncology Group Prospective Study. Lancet Oncol. 21: 145-161.
- Scheithauer BW, JM Woodruff, RA Erlandson (2000) Primary malignant tumors of the peripheral nerve. In: Tumors of the Peripheral Nervous System. Washington, DC: Armed Forces Institute of Pathology; 336340. Atlas of Tumor Pathology; 3rd series, fascicle 11.
- AYu N, Lennartz K, Pozharisski KM, Rajewsky MF (1991) Rat model of the human “triton” tumor: direct genetic evidence for the myogenic differentiation capacity of schwannoma cells using the mutant neu gene as a cell lineage marker. Differentiation. 48: 33-42.
- Brooks JSJ (1999) Disorders of soft tissue. In: Sternberg SS, editor. Diagnostic Surgical Pathology. ed 3. Philadelphia: Lipincott Williams and Wilkins. 131-221.
- Brooks JS, Freeman M, Enterline HT (1985) Malignant triton tumors: Natural history and immunohistochemistry of nine new cases with literature review. Cancer. 55: 2543-2549.
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC (2007) The 2007 WHO classification of tumours of the central nervous Acta Neuropathol. 114: 97-109
- Rodriguez FJ, Folpe AL, Giannini C, Perry A (2012) Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathologica. 123: 295-319.
- Zámecník M, Michal M (1999) Malignant peripheral nerve sheath tumor with perineurial cell differentiation (malignant perineurioma) Pathol Int. 49: 69-73.
- Rosenberg AS, Langee CL, Stevens GL, Morgan MB (2002) Malignant peripheral nerve sheath tumor with perineurial differentiation: „malignant perineurioma“. J Cutan Pathol. 29: 362-7.
- Hirose T, Scheithauer BW, Sano T (1998) Perineurial malignant peripheral nerve sheath tumor (MPNST): A clinicopathologic, immunohistochemical, and ultrastructural study of seven cases. Am J Surg Pathol. 22: 1368-1378.
- Hornick JL, Fletcher CD (2005) Soft tissue perineurioma: Clinicopathologic analysis of 81 cases including those with atypical histologic features. Am J Surg Pathol. 29: 845-858.
- Rosenberg AS, Langee CL, Stevens GL, Morgan MB (2002) Malignant peripheral nerve sheath tumor with perineurial differentiation: „malignant perineurioma“. J Cutan Pathol. 29: 362-7.
- Agaimy A, Wuensch PH (2005) Perineurioma of the stomach: A rare spindle cell neoplasm that should be distinguished from gastrointestinal stromal tumor. Pathology Research and Practice. 201: 463-467.
- Allison KH, Patel RM, Goldblum JR, Rubin BP (2005) Superficial malignant peripheral nerve sheath tumor: a rare and challenging Am J Clin Pathol. 124: 685-92.
- Frauenfeld L, Schürch CM (2022) CD117.
- Ohashi R (2022) Vimentin.
- Pernick N. (2022) Cytokeratin AE1/AE3.
- Ferrari A, Casanova M, Bisogno G, Mattke A, Meazza C, et al. (2002) Clear Cell Sarcoma of Tendons and Aponeuroses in Pediatric Patients: A Report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Cancer. 94: 3269-3276.
- Gachiani J, Kim D, Nelson A, Kline D (2007) Surgical Management of Malignant Peripheral Nerve Sheath Tumors. Neurosurg. Focus. 22:
- Angelov L, Davis A, O’Sullivan B, Bell R, Guha A (1998) Neurogenic Sarcomas: Experience at the University of Toronto. Neurosurgery. 43: 56-64.
- Pedro MT, Antoniadis G, Scheuerle A, Pham M, Wirtz CR, et al. (2015) Intraoperative High-Resolution Ultrasound and Contrast-Enhanced Ultrasound of Peripheral Nerve Tumors and Tumorlike Lesions. Neurosurg Focus.39:
- Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo, et al. (2008) A Systematic Meta-Analysis of Randomized Controlled Trials of Adjuvant Chemotherapy for Localized Resectable Soft-Tissue Cancer. 113: 573-581.
- Sharif S, Ferner R, Birch JM, Gillespie JE, Gattamaneni HR, et al. (2006) Second Primary Tumors in Neurofibromatosis 1 Patients Treated for Optic Glioma: Substantial Risks After Radiotherapy. J Clin 24: 2570-2575.
- Kahn J, Gillespie A, Tsokos M, Ondos J, Dombi E, et al. (2014) Radiation Therapy in Management of Sporadic and Neurofibromatosis Type 1-Associated Malignant Peripheral Nerve Sheath Tumors. Front 4: 324.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.