Molecular Weight Determination of Volatile Liquids using Tea Kettle Apparatus
Moges Abebe*
Department of Biological and Physical Sciences, Saint Augustine University, USA
*Corresponding author: Moges Abebe, Department of Biological and Physical Sciences, Saint Augustine University, USA
Received Date: 24 December, 2020; Accepted Date: 27 December, 2020; Published Date: 30 December, 2020
Citation: Abebe M, (2020) Molecular Weight Determination of Volatile Liquids using Tea Kettle Apparatus. Arch Nat Med Chem 5: 124. DOI: https://doi.org/10.29011/10.29011/2577-0195.000124
Abstract
In this paper, the determination of the gram-molecular weight of volatile liquids using a tea kettle apparatus is presented using the modified Dumas bulb method. We describe a tea kettle apparatus that can be used in the kitchen to study volatile liquids such as acetone, methanol, ethanol, and isopropyl alcohol. The method has been extended to perform sample purification, simple and fractional distillation as well as the separation of organic substances which have boiling points below 1000 C. Over 40 trials have been performed and the results can be duplicated by students at home. The apparatus and equipment described is low cost and can be purchased at local stores. The three experiments listed below will enliven remote learning by giving hands-on experiments to undergraduate students.
Introduction
The Dumas bulb method has been employed in many different laboratory experiments to measure the gram-molecular weight and other properties of various volatile liquids at higher temperatures. The accepted and accurate method of determining the molecular weight of liquids is mass spectroscope, but the cost and maintenance of the instruments make it unpractical for undergraduate education. Everyday use of volatile liquids in aerosol spray, paint thinners, and nail polish removers make these molecules worthwhile to study. The tea kettle experiment is very useful to teach simple experiments as well as organic, analytical, and physical chemistry investigations in undergraduate laboratories. In this report, a simplified version of a tea kettle apparatus was used to measure the properties of several volatile liquids at water boiling temperature. The procedure described below can be performed in residential kitchens and the materials are available at low cost in the local markets.
To determine the Gram-molecular Weight (GMW) of the samples the Ideal Gas law equation was manipulated to give the following derived equation: GMW = mRT/PV where m is the mass of the condensed liquid, T is the temperature in kelvin, P is the pressure in atmospheres, V is the volume of the kettle, and R is the gas constant. The volatile liquid was evaporated to fill the container, the vapor was cooled to condensation and the volume of the liquid was measured to calculate the gram-molecular weight. Measuring the volumes of the condensed liquids instead of the mass avoids problems associated with the unwanted weight droplets of water that could attach to the gas container and covering of the flask. Another problem that is avoided by volume measurement is the mass of the air in the flask before the volatile liquid was added [1].
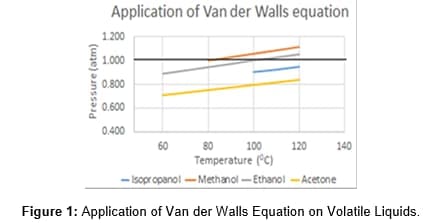
The selected liquids have boiling points between 56-83°C and a density of about 0.79 g/mL, possessing rapid evaporation characteristics at room temperature. Except for acetone, the other liquids have strong hydrogen bonding, higher vapor pressure and are polar substances. The volatile liquids did not emulate the ideal gas law and correction has been made to the vapor pressure. Figure 1 shows the plot of the temperature versus pressure for the volatile liquids used in this experiment. Van der Waals equation (VdW), (P+n2a/V2) (V-nb) = nRT is used to correct the volume and the pressure of the samples. The equation is designed to adjust for the volume of the vapor by subtracting the lost volume from the volume in the container the molecules are occupying. The collision between molecules prohibits the molecules to collide with the wall and the pressure is reduced. VdW equation compensates for the pressure by adding n2a/V2 to the pressure. Table 1 shows the constants in the Van der Waals equation as “a” and “b” that are specific to each of the volatile liquids. The dark line in the graph (Figure 1) is the expected result from the ideal gas equation. The temperature values above the boiling point of the volatile liquid to 1200C was reported. The four lines in the graph representing the volatile liquids show the deviation of the volatile liquid from the ideal gas law. This correction has been applied to the results reported in this paper. Other experiments that were recorded in this report are the simple and fractional distillation of volatile substances and the separation of mixtures of organic substances [2].
Materials
(The following items were found and purchased at the local stores and are reasonably priced. The entire apparatus is either plastic or stainless metal, as durability for undergraduate laboratory experimentation is highly beneficial.) Other items needed are a tea kettle, medicine dropper, 5L heating pot, 5L cooling pan, a graduated cylinder, a center drilled rubber stopper, and a thermometer. The stainless steel tea kettle is a polished metal container that can heat and cool quickly to enhance equilibrium between the water contained in the bath and the volatile liquid. The 2.5L volume kettle can contain up to 8ml of the vapor minimizing errors. The plastic tubing is a halfinch clear PVC flexible tubing and made of clear plastic for easy observation of the volatile liquid as it goes through phase changes. Atmospheric pressure is obtained from the weather bureau (Figure 2).
Chemicals
Acetone, ethanol, methanol, isopropyl alcohol, distilled water.
Experimental Procedure
The molecular weight determination of volatile liquids is performed using a 2.5 L tea kettle apparatus assembled as shown in Figure 2. The procedure listed below is to simulate a condition where a volatile liquid will be converted to a gas to fill up the kettle and when the temperature was cooled the vapor condenses back to the liquid state. This experiment is a modified version of the Dumas bulb experiment and when applied to a volatile liquid extra caution should be taken to avoid mistakes. The spout of the kettle is tightly fitted with metal tubing into a pre-drilled rubber stopper to prevent vapor loss. The opening in the outlet tubing with a constricted opening allowed the converted gas to escape. 10.0 ml of the volatile liquid was poured into the vessel and a thermometer was used to measure the temperature of the water bath. The kettle was immersed in a 1.5 L boiling water bath that had enough water to cover most of the kettle. The advantage of using boiling water for the experiment is the temperature will be the same for each trial and it avoids measuring the temperature several times during the experiment. The end of the tube can also be kept underwater to observe the excess vapors bubbles through the liquid. The extra volatile liquid escapes through the tubing as it’s converted to vapor. When no more bubbles are observed, the apparatus is heated for an additional minute. The kettle is removed and allowed to either air cool or is water-cooled to condense the vapor. As the vessel cools, the vacuum created by the cooling vapor can be heard as the air rushes into the kettle. The next step is to seal the tubing so that the vapor in the kettle will not be lost. The temperature of the boiling water bath is measured and recorded as being the temperature of the vapor in the vessel. The condensed liquid is collected by a dropper and the volume is measured using the density of volatile liquids. From this result, the mass of the condensed vapor was calculated. The molecular weight is calculated by using an excel program. The procedure is repeated using the same setup several times. Caution is taken to dry the kettle and not to allow any water to enter the kettle. A drop of water has a volume of about 0.05 ml and when converted to gas at STP, can take up 85 ml volume of the kettle. The kettle is cleaned after every use. Just in case the pressure increases, leakage from the lid will not affect the results [3].
Results
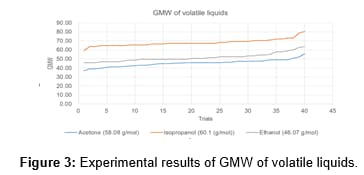
The results of the molecular weight determination of acetone, methanol, ethanol, and isopropyl alcohol are shown in Figure 3. The temperature of the boiling water was 373.15 K, the volume of the kettle was 2.54 L and the atmospheric pressure was in the range of 1.00-1.026 atm. A 10.0 mL sample was poured into the kettle and the volume of the condensed liquid was listed in the table. The mean values for the volume of the condensed liquid for acetone were 4.92 ± 0.407, for isopropyl alcohol was 7.34 ± 0.42, and ethanol 5.52 ± 0.48. The first experiment performed using the tea kettle apparatus was methanol and the results for the five attempted trials were promising. The values were evenly distributed between the accepted 32.04 grams/mole with an average GMW of 30.35 ± 0.08664 and an error bar of 5.286%. With that in mind, the experiment was continued and the results were as follows: the average gram-molecular weight for acetone was 45.31 ± 3.77 with an experimental error of 13.46%. Isopropyl alcohol had an average molar mass of 68.29 ± 4.08 with an experimental error of 13.6%. The percentage error for the molecular weight of acetone was 45.31 ± 3.77 with 98%. The red line in figure 3 is the graph for isopropyl alcohol and shows a higher gram-molecular weight from the accepted 60.10 g/mole with GMW of 52.3001 ± 4.5126 with an average percentage error of 12.07% (Figure 4).
Discussion
The three theories of volatile liquids, the Ideal Gas equation, Van der Waals equation, and intermolecular forces will be discussed in this section. The ideal gas equation was used to determine the possible sources of error in the experimental setup. In the GMW equations, R=0.08206 L•atm•mole-1•K-1 and the others have their units to match the gas constant. In this equation, the values in the numerators are directly related to the molar mass and an increase in their values will end up with an increase in the molar mass of the volatile liquids. The variables in the denominator are indirectly related to the molar mass and any error introduced in their measurement will affect the GMW of the liquids. Normal atmospheric pressure varies between 1.019 and 0.986 which will cause an error lower than 3%. The temperature of the vaporized liquid is in kelvin and an extreme measurement error of ± 250C can bring about a 7% error (columns 6 and 7). Since R is a constant, it doesn’t contribute to measurement errors. The tea kettle has a larger volume and measurement error will contribute up to 5% to the molar mass (columns 4 and 5). The major source of error comes from the mass of the condensed liquid (columns 8 and 9) which contributes to over 27% of the error. Caution was taken when measuring the volume of the condensed liquid.
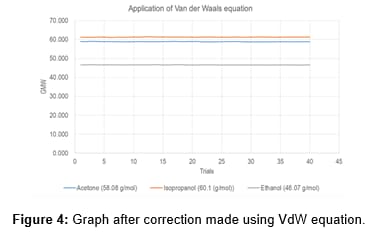
When the GMW of the volatile liquid was plotted against the experimental % error there was an increase in the experimental GMW results. Methanol, ethanol, and isopropyl alcohol have strong hydrogen bonding and display increased error while acetone doesn’t have hydrogen bonding and the error bar was higher than expected. The result in the graph showed that a correction has to be made to each graph. The best available pressure and volume correction were found in the Van der Waals equation and when it was applied the results showed great improvements. The figure on the right shows the plot with the applied correction for the volatile liquids (Table 2).
The kinetic molecular theory of gases has good insight into the behavior of these volatile liquids. Methanol, ethanol, and isopropyl alcohol have hydroxyl groups attached to their molecule making them highly polar. However, their expression as a volatile liquid, evaporating at a lower temperature shows that the molecules are far apart and require low energy to be separated so that they can be volatile. Methanol has a nonpolar methyl group attached to the polar side that will reduce the polar attraction between the molecules. Ethanol has two methyl groups attached to the polar end of the molecule and its boiling point as expected is higher than methanol. Isopropyl alcohol has two methyl groups covering the polar side the molecule and the boiling point of 82.60C matches its molecular weight of 60.10 g/mol. The stacking of the molecules of the Van der Waals intermolecular forces dominates the polar attractive forces that should have raised the boiling point. Acetone is the simplest and smallest ketone molecule with a carbonyl group in the middle and like isopropyl alcohol its two methyl groups isolate the polarity of the molecule. All four molecules are small, highly polar, with Van der Wall forces, and are still volatile and the reason for this volatile behavior must be the presence of the nonpolar methyl groups that reduce the strength of the polarity and decrease their boiling point [4].
Conclusion
It has been proven from this report that the tea kettle apparatus is a useful instrument for undergraduate chemistry experiments. Some of the major applications investigated in this report are GMW of volatile liquids, simple and fractional distillation of low boiling liquids and separation of mixtures in purification experiments. The Gram molecular weight determination of volatile liquids was conducted without calibration and the errors reported were due to deviation from ideal gas law. The sources of error were analyzed to determine which variable in the GMW equation had the greatest influence and it was found that the mass of the volatile liquid was the major source of error. The improvement made in this report was to use a large volume container and measuring the volume instead of the mass of the condensed liquid. The Van der Waals Equation was proved to reduce the error by less than 2%. There are several applications for this tea kettle apparatus in physical and analytical chemistry that will be useful in virtual and hands-on experiments. In analytical chemistry, the kettle will stand along with chromatography for volatile liquids. In physical chemistry, this apparatus can be employed in thermodynamics to investigate gas properties. This design can be used for volatile liquids in the perfume industry, aerosol sprays, and in pharmaceuticals.

|
Name |
Molar Mass (g/mol) |
Boiling Point© |
Density (g/cm3) |
a (L2bar/mol2) |
b (L/mol) |
|
Methanol |
32.04 |
64.7 |
0.79 |
9.476 |
0.0659 |
|
Ethanol |
46.06 |
78 |
0.79 |
12.18 |
0.08407 |
|
Isopropanol |
60.1 |
82.6 |
0.79 |
15.82 |
0.1109 |
|
Acetone |
58.08 |
56 |
0.79 |
16.02 |
0.1124 |
Table 1: Some of the properties of volatile liquids used in this experiment.
|
Acetone |
Isopropanol |
Methanol |
Ethanol |
|
|
M |
58.08 |
60.10 |
32.04 |
46.07 |
|
b (L/mol) |
0.1124 |
0.1109 |
0.0659 |
0.08407 |
|
a (L2bar/mol2) |
16.02 |
15.811 |
9.352 |
12.021 |
|
R (L•atm•mole-1•K-1) |
0.082 |
0.082 |
0.082 |
0.082 |
|
T (K) |
373.15 |
373.15 |
373.15 |
373.15 |
|
V (L) |
2.54 |
2.54 |
2.54 |
2.54 |
|
N (mol) |
0.0668 |
0.0757 |
0.0893 |
0.0945 |
|
M (g) |
3.88 |
4.55 |
2.86 |
4.3513 |
|
nRT/(V-nb) |
0.807 |
0.915 |
1.078 |
1.141 |
|
n2a/V2 |
0.011 |
0.014 |
0.012 |
0.017 |
|
P (atm) |
0.796 |
0.901 |
1.066 |
1.125 |
|
P (atm) |
0.796 |
0.901 |
1.066 |
1.125 |
|
V (L) |
2.54 |
2.54 |
2.54 |
2.54 |
|
T (K) |
373.15 |
373.15 |
373.15 |
373.15 |
|
R (L•atm•mole-1•K-1) |
0.08206 |
0.08206 |
0.08206 |
0.08206 |
|
mcondensed (g) |
3.88 |
4.55 |
2.86 |
4.35 |
|
N (mol) |
0.0668 |
0.0757 |
0.0900 |
0.0945 |
|
Molar mass (g/mol) |
58.76 |
60.88 |
32.34 |
46.64 |
|
% Error |
1.17 |
1.30 |
0.92 |
1.23 |
Table 2: Shows the results after adjustment made with VdW equation.
References
- Dumas JB (1826) Memoir on some points of atomistic theory. Ann chim phys 33: 337-391.
- Dumas JB (1832) Dissertation on the vapor density of some simple bodies. Ann chim phys 50: 170-178.
- Jensen WB (2015) Classical Molecular Weight Determinations. University of Cincinnati, USA.
- Weast RC (1972) Handbook of Chemistry and Physics. 53rd Edition, CRC Press, Cleveland.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.