Mature B-Cell Neoplasms with Immunophenotypically Distinct Dual B-Cell Clones: A Case Series
Feryal A Ibrahim1*, Dina S Soliman1-3, Samah A Kohla1,2, Afaf H AL Battah4, Suhair H El Ajez1, Somayya A Rahhal1, Susanna Akiki5, Mouhammad Sharaf Eldean6
1Department of Laboratory Medicine and Pathology/ haematopathology, Hamad Medical Corporation, Doha, Qatar 2Weill Cornell Medicine-Qatar, Doha, Qatar
3Department of Clinical Pathology, National Cancer Institute, Cairo
4Department of Hematology and Medical Oncology, National Center for Cancer Care and Research, Hamad Medical Corporation, Doha, Qatar
5Department of Laboratory Medicine and Pathology/ Molecular Genetics, Hamad Medical Corporation, Doha, Qatar
6Department of Laboratory Medicine and Pathology/ Anatomic Pathology, Hamad Medical Corporation, Doha, Qatar
*Corresponding author: Feryal A Ibrahim, Department of Laboratory Medicine and Pathology/ haematopathology, Hamad Medical Corporation, Doha, Qatar
Received Date: 24 February 2023
Accepted Date: 28 February 2023
Published Date: 03 March 2023
Citation: Ibrahim FA, Soliman DS, Kohla SA, Battah AHAL, El ajez SH, et al (2023) Mature B-Cell Neoplasms with Immunophenotypically Distinct Dual B-Cell Clones: A Case Series. Ann Case Report. 8: 1194. DOI: https://doi.org/10.29011/2574-7754.101194
Abstract
Mature B-cell neoplasms (MBCN) are generally believed to derive from the transformation of single monoclonal B-lymphocytes. However, reports of bi or multiclonality identified at diagnosis or during follow-up have not uncommonly been reported. Rare cases of low-grade mature B-neoplasms reported to have more than one clone with two-cell population having the same immunophenotype but with different LC expression. In such cases, the LC ratios by itself can be misleading without a comprehensive immunophenotyping. We report a series of four patients with two monotypic B-cell populations expressing different surface immunoglobulin light chain restrictions (one kappa and one lambda) including one case with two monoclonal B-lymphocytosis (MBL) clones (CLL-type), two cases of biclonal CLL, and one case with composite lymphoma of CLL and Mantle cell lymphoma (MCL). This series emphasizes the importance of comprehensive immunophenotyping together with cytomorphology/cytogenetic and molecular confirmation as part of a multimodality approach in the diagnostic work-up of haematological neoplasms and demonstrates our centre experience in the diagnosis and management of these cases.
Keywords: Flow Cytometry; Mature B-Cell Neoplasm; Bi-Clonal; Composite Lymphoma; Chronic Lymphocytic Leukaemia; Mantle Cell Lymphoma; Monoclonal B- Lymphocytosis
Introduction
Mature B-cell neoplasms (MBCN) are biologically and clinically heterogeneous group of neoplasms characterized by proliferation and accumulation of monotypic mature B-lymphocytes in peripheral blood (PB), bone marrow (BM), and/or lymphoid tissue. B-cell clonality is usually illustrated by flow cytometry (FCM) immunophenotyping that demonstrates kappa (K) or lambda (L) light chain (LC) restriction or less frequently lack of surface LC expression [1]. MBCN are in general believed to stem from the transformation of a single monoclonal B-lymphocyte, the expansion of which results in a clone of related cells having the same transforming mutation, identical immunoglobulin (Ig) heavy chain gene VDJ rearrangement, and identical Ig LC restriction. However, reports of cases with more than one clone identified at diagnosis or during follow-up have also been reported [2-7]. Although, the suspicion for the presence of two or more B-cell clones may be raised from the cytomorphologic or histopathologic examination, particularly in cases with the coexistence of two morphologically distinct B-cell neoplasms; however, most cases are diagnosed through multipara metric FCM immunophenotyping that allows the simultaneous characterization of multiple antigens expression on multiple cell population in the same specimen. Herein, we describe a series of 4 cases with dual monotypic B-cell population identified by FCM at Hamad Medical Corporate in Qatar.
Methods
Records of cases with more than one monotypic B-cells population with two different LC expression identified by FCM IPT on peripheral blood (PB) and/or bone marrow (BM) specimens at the Flow Cytometry Laboratory, Department of Laboratory Medicine and Pathology, Hamad Medical Corporate were retrieved. Relevant findings of PB, BM, flow cytometry, cytogenetics/molecular, treatment and outcome were collected.
The final diagnosis and sub classification were done according to WHO 2016 classification. The reported cases were detected on a prospective basis and was not a comprehensive retrospective screen; therefore, a reliable estimate of prevalence is not postulated. Multicolour FCM analysis was performed using a panel, composed of antibodies in a 3-5-color combination of (FITC/PE/ECD/PC5) and (FITC/PE/ECD/PC5/ PC7 or APC) fluorescent conjugates, as follows: T1: CD20/CD10/CD19/CD45, T2: FMC7/ CD23/CD19/ CD5, T3:CD103/CD11c/CD19/CD25, T4:CD23/CD79b/CD19/ CD38, T5:CD8/CD4/CD3/CD7, T6: CD45/CD43/-/CD19, T7: kappa/lambda/CD19/CD5, T8:kappa/lambda/CD19/CD5/ CD10, T9: kappa/lambda/-/CD19, T10: IgM/IgD/-/CD19 , T11:CD45/ CD19/CD3/ CD5/ CD200-APC. A cut-off of 20% over the isotype boundary was used to label a marker as positive. For Cases of CLL, modified Matutes scoring system was calculated [8]. In our series, CLL morphology was defined as predominantly small mature-looking lymphocytes with dense chromatin and a high nucleocytoplasmic ratio. FISH analysis for CLL panel: 11q22.4, t (11; 14) (q13; q32), 12p11.1-q11.1 and 17p13.1 / 17p11.1-q11.1, conventional karyotyping and molecular studies were done as appropriate. Invivoscribe Identic lone, B clonality assays were used to detect rearrangements of the immunoglobulin heavy chain and provide a clonal signature of lymphoproliferative disease. The kits provide standardized testing using multiple consensus primers sets validated in a collaborative study; the Eurocloality BIOMED2 Concerted Action study to identify lymphocyte populations derived from a single lymphoid cell by detecting the unique V-D-J gene rearrangements [9]. The primers used for PCR based analysis of genomic DNA prepared from blood or marrow target the conserved framework (FR) and joining (J) regions, which lie either side of the diversity region of the immunoglobulin heavy chain genes that are unique in length and sequence.
Results
During the specified period, four cases with two different monotypic B-cell populations expressing different type of surface light chain were identified. The Immunophenotype, cytogenetic/ molecular and Clinic-pathological findings are summarized in (Table 1a and 1b).
|
|
Phenotype of population 1 (% from total) compatible diagnosis |
Phenotype of population 2 (% from total) compatible diagnosis |
Cytogenetic |
Molecular |
Diagnosis |
|
1 |
Positive: CD19d, CD5, CD23, CD20p, CD43, CD200p, CD11cp, IgD, lambdad Negative: CD10, FMC7, CD79b, CD103, CD25, CD38, IgM. (22%) MBL-CLL type |
Positive: CD19, CD5d, CD23, CD20p, CD43, CD200, CD11cp, IgDp, kappad Negative: CD10, FMC7, CD79b, CD103, CD25, CD38, IgM. ( 5%) MBL CLL-type |
ND |
ND |
Two CLL-type MBL |
|
2 |
Positive: CD19, CD5, CD23, CD20h, CD43, CD200, IgD, CD11cp, lambdad Negative: CD10, FMC7, CD79b, CD103, CD25, CD38, IgM. (39%) CLL. |
Positive: CD19, CD5, CD23, CD20h, CD43, CD200, IgD, CD11cp, kappad Negative: CD10, FMC7, CD79b, CD103, CD25, CD38, IgM. (16%) CLL. |
KT: 46,XY,t(12;13) (p11.2;q14)[15]/46,XY[12] |
more than one clone |
biclonal CLL |
|
3 |
Positive: CD19, CD5p, CD23, CD20p, CD43, CD200, IgDd, CD11cp, Lambdad Negative: CD10, FMC7, CD49d, CD79b, CD38, CD25, CD103 and IgM. (58%) CLL. |
Positive: CD19, CD5, CD23, CD20 p, CD43, CD200, IgD d, CD11c p, kappa d Negative: CD10, FMC7, CD49d, CD79b, CD38, CD25, CD103 and IgM. (10%) CLL. |
Normal FISH |
more than one clone |
biclonal CLL |
|
4 |
Positive: CD19, CD5, CD23 p, CD20 b, CD79b, FMC7, IgD, IgM and, Lambda. Negative: CD10, CD103, CD25, CD43, CD38, CD200 CD11c. (58%) MCL. In BM: 59% |
Positive: CD19, CD5, CD23, CD20d, CD79bpd, CD43, CD200, CD38, CD11cp, , IgD, IgM, Kappa d Negative: CD10, CD103, CD25, FMC7. (12%) CLL. In BM: 10% |
46,XY,t(11;14)(q13;q32) [14]/55, XY,46[41.] |
more than one clone |
MCL/CLL |
Table 1a: immunophenotype, cytogenetic findings of group A (cases with two monotypic B-cell population with different sIg light chain restriction diagnosed from peripheral blood). p: partial, d: dim, b: bright, h: heterogeneous. CLL: chronic lymphocytic leukaemia, MBL: monoclonal B lymphocytosis, MCL: mantle cell lymphoma, ND: Not done.
|
|
Case 1 Two MBL, CLL-type |
Case 2 biclonal CLL |
Case 3 biclonal CLL |
Case 4 MCL/CLL |
|
Age/gender |
59/M |
61/M |
69/M |
57/M |
|
Incidental/symptomatic |
Incidental |
Incidental |
Cough & fever (COVID) |
abdominal pain and distention |
|
Adenopathy |
No |
No |
yes |
No |
|
Splenomegaly |
No |
No |
No |
yes |
|
Hepatomegaly |
Mild (18.4 cm) |
No |
No |
yes |
|
B symptoms |
No |
No |
fever |
No |
|
Hb g/dL |
15.0 |
14.8 |
11.7 |
13.5 |
|
Platelets x103/uL |
252 |
172 |
468 |
115 |
|
WBC x103/uL |
11.2 |
18.5 |
33.7 |
46.7 |
|
Lymphocytes x103/uL |
7.9 |
13 |
26.8 |
35.0 |
|
Monotypic population (1)% Monotypic population (2)% |
22 5 |
39 16 |
58 10 |
58 12 |
|
ECOG Performance status |
0 |
0 |
0 |
0-1 |
|
Stage |
NA |
Binet stage A |
Binet stage B |
stage IV, MIPI score 4 |
|
Treatment decision |
watch and wait |
watch and wait |
watch and wait |
Ibrutinib 560 mg daily |
|
Outcome (follow up period, month) |
Alive & well (22) |
Alive & well (17) |
Alive & well (18) |
Alive & well (72) |
Table 1b: Clinical and laboratory findings of group A (cases with two monotypic B- cell population with different light chain restriction). M: Male, F: Female. CLL: chronic lymphocytic leukaemia, MBL: monoclonal B lymphocytosis, MCL: mantle cell lymphoma, NA: not applicable.
Case1: Two MBL, CLL-type clone
A-59-years old male with an incidental finding of lymphocytosis, composed predominantly of small mature-looking forms. FCM revealed two distinct CLL- type MBL clones with different LC expression (K & L) comprising (5%) and (22%) respectively. The two populations differ as well by the intensity of some marker expression; slightly dimmer CD19 and CD200 on the L clone, while the smaller K clone show dimmer CD5 and IgD expression (Figure 1).
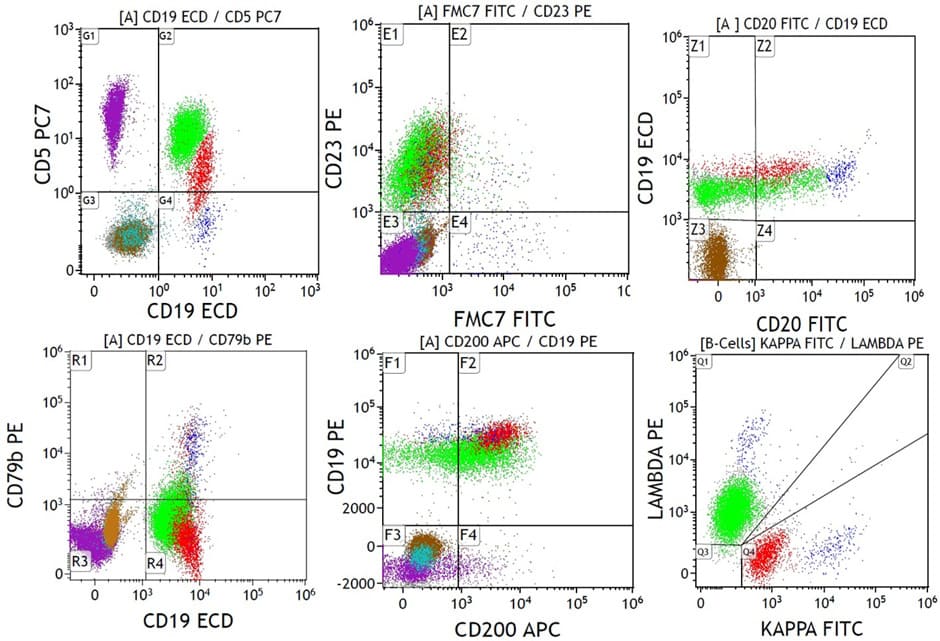
Figure 1: A case with two MBL, CLL type clone (Case no. 1, table 1a); both clones are positive for CD5, CD23, CD200 with partial dim CD20, negative for FMC7 and CD79b, one with dim kappa LC restriction (red colour) with slightly brighter CD19 and dimmer CD5 and one with dim lambda LC restriction (green colour.). A small polyclonal normal B-cell population is also seen (blue colour). Granulocytes (brown colour). T-lymphocytes (purple colour).
Case 2 &3: Biclonal CLL
Case no 2: A- 61-year-old male with an incidental finding of lymphocytosis. The lymphocytes were mostly small mature-looking lymphocytes with few clefted forms and few smudge cells with no increase in prolymphocytic. FCM analysis on PB showed two clones with the same IPT and were separated only by the different light chain restriction (K 16% and L 39%). FISH analysis on PB for CLL panel was normal. However, karyotype (KT) was abnormal: 46,XY,t(12;13)(p11.2;q14)(15)/46,XY(12). The diagnosis of biclonal chronic lymphocytic leukaemia/small lymphocytic lymphoma (CLL/SLL) with Modified Matutes score of 5, Binet stage A was concluded. Molecular analysis supported the presence of more than one B cell clone (Figure 2a, 2b & 2c).
Case no 3: A- 69- years old male, presented with cough and fever, found to have severe COVID-19 pneumonia. His CBC showed leucocytosis and lymphocytosis. Lymphocytes were mostly small mature- looking with rare clefted forms and some smudge cells with no increase in prolymphocytic. FCM analysis on PB showed biclonal CLL (Kappa 10% and Lambda 58%) with modified Matutes score of 5. The two clones share almost the same IPT and were separated by the different LC restriction and by the difference in the intensity of CD5 expression; the whole kappa clone was positive while, the lambda clone showed partial positivity. FISH for CLL panel was normal. The molecular analysis is consistent with the FCM analysis and supports the presence of major clone and minor B cell clones.
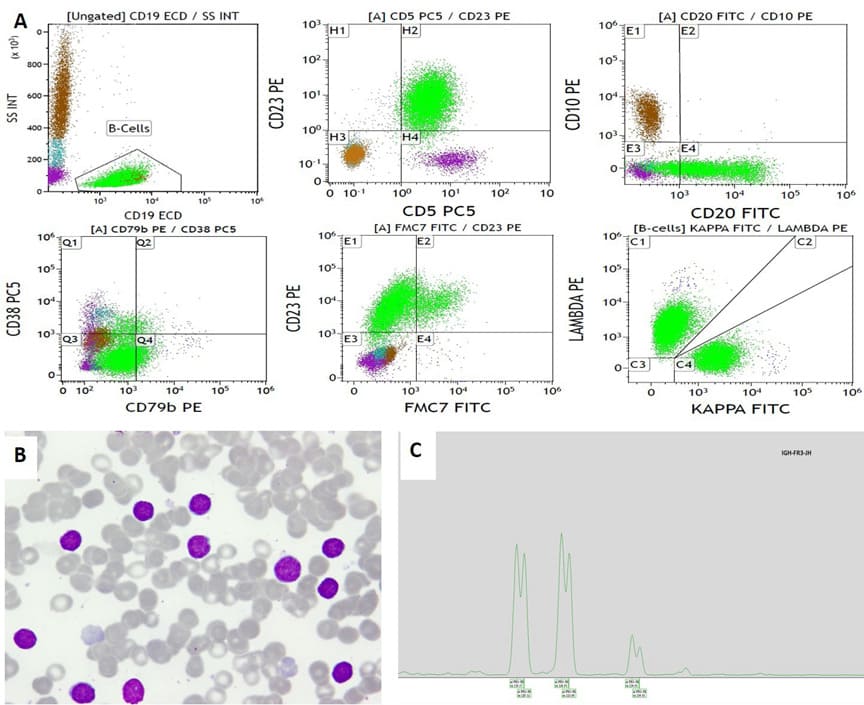
Figure 2: Example of biclonal CLL phenotype (Case no. 2, Table 1a). A. Flow cytometry; B-cell population (green colour) is positive for CD19, CD5, CD23, CD20 (heterogeneous), negative for CD10, CD79b, CD38 and majority is negative for FMC7. Surface light chain immunostaining showed one population positive for kappa (16%), and other population positive for lambda surface light chain (39%). A small polyclonal normal B-cell population is also seen (blue colour). Granulocytes (brown colour). T-lymphocytes (purple colour). B-Peripheral blood shows lymphocytosis; small mature in type. Wright stain1000x.C. PCR amplification of the immunoglobulin heavy chain gene conserved framework 3 (IGH-FR3) region showing two distinct peaks at 119bp, 125bp and 134bp supporting the presence of more than one B cell clone.
Case 4: Composite of CLL and MCL
A-57-year-old male patient, presented with four months history of recurrent abdominal pain, hepatosplenomegaly with no palpable lymphadenopathy. His CBC revealed leucocytosis with lymphocytosis. Lymphocytes were predominantly small mature looking with some small to medium cells with irregular nuclear contour and many smudge cells. FCM on PB revealed a major population of CD5positive Lambda B-cells (59%) and a smaller Kappa- population (10%) with CLL IPT. In this case, the BM was collected and showed increased lymphoid cells (66%) with similar morphology and IPT as seen in PB. BM biopsy was hyper cellular and show interstitially increased small B-lymphocytes; scattered and in clusters, and some with irregular nuclear contours. Large lymphoid aggregates were also noted. Most of the interstitial B-cells were positive for CD20, PAX5, CD5 and cyclin D1, with comparable degree of positivity for CD20 and PAX5 immunisations. Within the large lymphoid aggregates on the other hand, PAX5 positivity was more obvious than that for CD20 and CD79 and majority of the cells were negative for cyclin D1 with cyclin D1-positive cells located more at the margin of the aggregates. FISH analysis on PB revealed IGH/CCND1 rearrangement, t (11:14), in 65% of cells analysed. Conventional karyotype revealed two cell lines including 14 cells with t(11;14)(q13;q32) and 41 cells with normal karyotypes: 46,XY,t(11;14)(q13;q32)14/55, XY,46 in 41/55. A diagnosis of composite lymphoma of CLL and MCL (small cell variant), stage IV, MIPI score 4 was concluded, published case. [10]. Molecular analysis (conducted after publication) supports the presence of more than one B cell clone (Figure 3a,3b& 3c).
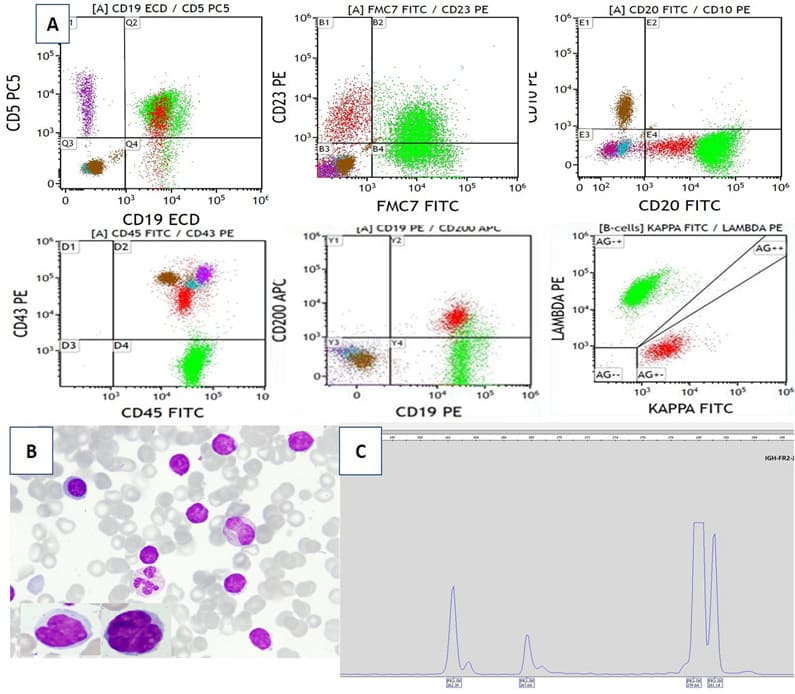
Figure 3: Composite of CLL and MCL (Case no. 4, Table 1a); A. FCM: both B-cell populations are positive for CD5; CLL population expressing CD23, dim CD20 with dim kappa light chain restriction, negative for FMC7 (red colour). MCL population with FMC7, bright CD20, partial CD23 and lambda light chain restriction (green colour). Granulocytes (brown colour). T-lymphocytes (purple colour). B. Peripheral blood shows lymphocytosis, mostly small mature with some having irregular nuclear (inserts). Wright stain 1000×. C. PCR amplification of the immunoglobulin heavy chain gene conserved framework 2 (IGH-FR2) region showing dominant peaks at 262b, 268bp and 280bp supporting the presence of more than one B cell clone.
Discussion & Conclusion
Despite the notion that most mature B-cell neoplasms are usually monoclonal, many reports of composite or biclonal cases have been published [2-7]. The two clones may be interpreted as two populations arising from a single primary transformed cell or denoting a coexistence of two distinct lymphoid neoplasms [3,4]. Definitive assessment of B-cell clonality or the presence of biclonality usually requires molecular study for IgH gene VDJ rearrangement. Nevertheless, immunophenotyping, particularly multicolour FCM approaches, have been widely recognized as a useful adjunct in detecting multiple clonal populations in samples, since multiple antigens can be analysed simultaneously on multiple populations of cells [11,12]. This was clearly demonstrated in Sanchez et al series [2], in which all cases with 2 phenotypically distinct B-cell populations were found to have 3 or more Ig gene rearrangement, while none of the cases with uniform IPT had more than 2 rearrangements, suggested the utility of immunophenotyping in the detection of B-cell bi or multiclonality. The incidence of more than one monotypic population in MBCN is estimated at around 5% as reported in the two large series by FCM on PB and BM [2] and on a more diverse specimen that included LN, body fluids, FNAs in addition to BM and PB [13]. The most frequent MBCN with more than one clonal population are those where the two populations show CLL immunophenotype (IPT), however, one is K and one is L restricted [2,3,13,14]. Another group would include cases with two morphologically and immunophenotypically distinct MBCN with one of the two has CLL IPT and the other population may have IPT of other MBCN such as hairy cell leukaemia (HCL), mantle cell lymphoma (MCL), or follicular lymphoma or a composite of two distinct MBCN [1518]. Normal naïve and reactive mature B-cells are a mixture of kappa or lambda light chain-expressing cells usually in ratio of 1-2:1. In contrast neoplastic mature B-cells population represent a single clone of cells that usually express single light chain [19]. However, rare cases of low-grade mature B-neoplasms reported to have more than one clone with two-cell population having the same immunophenotype but with different LC expression. In such cases, the LC ratios by itself can be misleading without a comprehensive immunophenotyping. This series include two CLL cases with two clones expressing different light chain. Biclonal CLL is a rare event with an incidence that varies between 0.7-3.4% among all CLL cases [14,20]. However, more frequently reported among atypical CLL (13.8%) [2]. Although it is possible to have two productive IGVH rearrangements in normal and malignant B-cells, only one of them will be translated into protein and expressed on the cell surface due to allelic exclusion. Therefore, lack of allelic exclusion was suggested as a possible cause for biclonality in CLL [21]. In comparison to patients with monoclonal CLL, patients with two or more clones present with lower WBC, lower lymphocyte count, and more frequent splenomegaly. However, despite the differences in the two groups, the overall survival rate was reported to be the same [2]. In practice, biclonal CLL cases represent a diagnostic challenge and emphasize the importance of comprehensive immunophenotyping for the evaluation of B-cells even in the setting of B-cells with seemingly normal kappa to lambda ratio. Not to say also the importance of distinguishing biclonal CLL from CLL coexisting with a second lymphoma (composite lymphoma), as different treatment modalities might be needed. Composite lymphoma (CL) implies the coexistence of 2 or more distinct types of lymphoma in a single tissue or organ at presentation [18]. In the MCL/CLL case, the MCL was of the small variant type, and hence, the morphology was suggestive of CLL and the CL was identified exclusively by FCM, which was confirmed by the detection of IGH/CCND1 rearrangement and was reflected by the presence of more than one clone by molecular analysis. The series includes one case with two MBL clones (CLL-type). While early studies suggested that cases of MBL (CLL- type) are predominantly monoclonal [22, 23], Nieto et al (2009), reported biclonal MBL in (19%) of cases [24]. In line with this, other studies, using sophisticated combined cell sorting and molecular studies conclusively showed that MBL cases often contain more than one B-cell clone [25]. The presence of more than one clone in the currently reported cases does not seem to adversely impact the clinical course of the patients, as during the follow up period (range 17- 22 months), the CBC count were almost stable for the biclonal MBL and the two cases of biclonal CLL and the patients were kept on watch and wait approach, The patient with composite lymphoma (MCL/CLL), maintained on Ibrutinib and is well and alive. In summary, this series emphasizes the importance of the multimodal approach for the diagnosis of B-cells neoplasm, integrating different ancillary techniques, including morphology, immunophenotyping, cytogenetic and molecular studies. It also illustrates that comprehensive immunophenotyping with an extended panel of markers, although cost more, however, crucial in the evaluation of B-cells for aberrant antigen expression, even in the setting of a normal kappa to lambda ratio, and critical for the detection of more than one monotypic B-cell population as well as in the diagnosis of composite lymphomas, as different treatment modalities might be needed.
Statement of Ethics: The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Written informed consent was obtained from each patient for publication of this case series and any accompanying images. The completed consent form is available to the editor if requested. The study was approved by HMC ethics committee on human research (Medical Research Centre-MRC). The study had ethical approval from IRB (Institutional research board) Reference # MRC-04-21-629.
Conflict of Interest Statement: The authors declare that they have no relevant financial interests.
Author Contributions: Ibrahim F: Created the methodology, performed data collection, wrote the manuscript. Soliman D: Shared in manuscript writing and submission. El ajez S and A Rahhal S: sample processing and analysis. Kohla S, Amer A: shared in data collection, Al Battah A, did the clinical examination follow-up, review and editing of the manuscript, El Akiki S provided the molecular analysis and reviewed the manuscript, Sharaf Eldean Provide anatomic pathology analysis.
Acknowledgement: The authors would like to acknowledge Qatar National library for funding this article
References
- Craig FE, Foon KA (2008) Flow cytometric immunophenotyping for hematologic neoplasms. Blood 111: 3941-3967.
- Sanchez ML, Almeida J, Gonzalez D, Gonzalez M, Garcia-Marcos MA, et al (2003) Incidence and clinicobiologic characteristics of leukemic B-cell chronic Lymphoproliferative disorders with more than one B-cell Blood 102: 2994-3002.
- Sanchez ML, Almeida J, lopez A, Sayagues JM, Rasillo A, et al (2006) Heterogeneity of neoplastic cells in B-cell chronic Lymphoproliferative disorders: Biclonality versus intra clonal evolution of a single tumor cell Hematologica 91: 331-339.
- Kuppers R, Ulrich D, Hansmann M (2014) Pathogenesis, diagnosis, and treatment of composite lymphoma. Lancet Oncol 15: e435-e446.
- Turbatu A, Stoian M, Brezean I, Stoica VC, Colita A, et al (2014) Composite diffuse large B-cell lymphoma and follicular B-cell lymphoma - case report and review of literature. Maedica (Bucur). 9: 204-209.
- Hussein S, Gill K, Baer LN, Hoehn D, Mansukhani M, et al (2015) Practical Diagnostic approaches to composite plasma cell neoplasm and low-grade B-cell lymphoma/clonal infiltrates in the bone marrow. Hematol Oncol 33: 31-41.
- Ghodke KA, Patkar NV, Subramanian PG, Gujral S, kadem PA, et al (2017) Biclonal chronic lymphocytic leukemia: A study of two cases and review of literature. Indian J Pathol Microbiol. 60: 84-86.
- Moreau EJ, Matutes E, A’Hern RP, Morilla AM< Morilla RM, et al (1997) Improvement of the chronic lymphocytic leukemia scoring system with the monoclonal antibody SN8 (CD79b). Am J Clin Pathol. 108: 378
- van Dongen, J., Langerak, A., Brüggemann, M. Evans PAS, Hummel M, et al (2003) Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: Report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 17:2257
- Ibrahim F, Al Sabbagh A, Amer A, Soliman DS, Sabah HA (2020) Composite Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma and Mantle Cell Lymphoma; Small Cell Variant: A Real Diagnostic Challenge. Case Presentation and Review of Literature. Am J Case Rep. 21: e921131.
- Porwit A (2013) Immunophenotyping of selected hematologic disorders focus on Lymphoproliferative disorders with more than one malignant cell population. Int Lab Hem 35: 275-282.
- Perkovic´ S, Basic´-Kinda S, Aurer I, Ugrina I, Lozic D, et al (2013) Multiparameter flow cytometry is necessary for detection, characterization and diagnostics of composite mature B cell lymphoproliferative neoplasms. Int J Hematol 98: 589-596.
- Mahdi T, Rajab A, Padmore R, Porwit A (2018) Characteristics of Lymphoproliferative Disorders with More Than One Aberrant Cell Population as Detected by 10-Color Flow Cytometry. Cytometry B Clin 94: 230-238.
- Kern W, Bacher U, Schnittger S, Dicker F, Alpermann T, et al (2014) Flow cytometric identification of 76 patients with biclonal disease among 5523 patients with chronic lymphocytic leukemia (B-cell) and its genetic characterization. Br J Haematol 164: 565-569.
- Giné E, Bosch F, Villamor N, Rozman M, Colomer D, et al (2002) Simultaneous diagnosis of hairy cell leukemia and chronic lymphocytic leukemia/small lymphocytic lymphoma: a frequent association? 16: 1454-1459.
- Hoeller S, Zhou Y, Kanagal-Shamanna R, Hoehn D, Bihl M, et al (2013) Composite mantle cell lymphoma and chronic lymphocytic leukemia/ small lymphocytic lymphoma: a clinicopathologic and molecular study. Hum Pathol. 44: 110-121.
- Jelloul FZ, Chen QH, Yang T, Haghi N, Brody J, et al (2018) Composite Small Lymphocytic Lymphoma/Chronic Lymphocytic Leukemia and Follicular Lymphoma: A Clinicopathological Study of Six Cases. Int J Surg Pathol. 26: 135-144.
- Gulati R, Zhou J (2020) Composite Lymphoma. In: Wang E., Lagoo A. (eds) Practical Lymph Node and Bone Marrow Pathology. Practical Anatomic Pathology. Springer, Cham.
- Seegmiller AC, His ED and Craig FE (2019) The Current Role of Clinical Flow Cytometry in the Evaluation of Mature B-Cell Neoplasms. Cytometry Part B (Clinical Cytometry). 96B: 20-29.
- González-Campos J, Ríos-Herranz E, De Blas-Orlando JM, martinNoya A, Berdejo RP, et al (1997) Chronic lymphocytic leukemia with two cellular populations: a biphenotypic or biclonal disease. Ann 74: 243-246.
- Langerak AW, Davi F, Ghia P, Murray F, Potter KN, et al (2011) European Research Initiative on CLL (ERIC). Immunoglobulin sequence analysis and prognostication in CLL: guidelines from the ERIC review board for reliable interpretation of problematic cases. 25: 979-984.
- Rawstron AC, Bennett FL, O’Connor SJ, Kwok M, Fenton JAL, et al (2008) Monoclonal B-cell lymphocytosis and chronic lymphocytic N Engl J Med 359: 575-583.
- Dagklis A, Fazi C, Sala C, Cantarelli V, Scielzo C, et al (2009) The immunoglobulin gene repertoire of low-count chronic lymphocytic leukemia (CLL)-like monoclonal B lymphocytosis is different from CLL: Diagnostic implications for clinical monitoring. Blood 114: 26-32.
- Nieto WG, Almeida J, Romero A, Teodosio C, Lopez A, et al (2009) Increased frequency (12%) of circulating chronic lymphocytic leukemia-like B-cell clones in healthy subjects using a highly sensitive multicolor flow cytometry approach. Blood 114: 33–37.
- Klinger M, Zheng J, Elenitoba-Johnson KS, Perkins SL, Faham M, et al (2016) Next-generation IgVH sequencing CLL-like monoclonal B-cell lymphocytosis reveals frequent oligoclonality and ongoing Leukemia. 30: 1055-1061.
© by the Authors & Gavin Publishers. This is an Open Access Journal Article Published Under Attribution-Share Alike CC BY-SA: Creative Commons Attribution-Share Alike 4.0 International License. Read More About Open Access Policy.